Abstract
Diagnosing canine visceral leishmaniasis (CVL) is a critical challenge since conventional immunoserological tests still present some deficiencies. The current study evaluated a prototype flow cytometry serology test, using antigens and fluorescent antibodies that had been stored for 1 year at 4°C, on a broad range of serum samples. Noninfected control dogs and Leishmania infantum-infected dogs were tested, and the prototype test showed excellent performance in differentiating these groups with high sensitivity, specificity, positive and negative predictive values, and accuracy (100% in all analyses). When the CVL group was evaluated according to the dogs' clinical status, the prototype test showed outstanding accuracy in all groups with positive serology (asymptomatic II, oligosymptomatic, and symptomatic). However, in dogs which had positive results by PCR-restriction fragment length polymorphism (RFLP) but negative results by conventional serology (asymptomatic I), serological reactivity was not observed. Additionally, sera from 40 dogs immunized with different vaccines (Leishmune, Leish-Tec, or LBSap) did not present serological reactivity in the prototype test. Eighty-eight dogs infected with other pathogens (Trypanosoma cruzi, Leishmania braziliensis, Ehrlichia canis, and Babesia canis) were used to determine cross-reactivity and specificity, and the prototype test performed well, particularly in dogs infected with B. canis and E. canis (100% and 93.3% specificities, respectively). In conclusion, our data reinforce the potential of the prototype test for use as a commercial kit and highlight its outstanding performance even after storage for 1 year at 4°C. Moreover, the prototype test efficiently provided accurate CVL serodiagnosis with an absence of false-positive results in vaccinated dogs and minor cross-reactivity against other canine pathogens.
INTRODUCTION
Canine visceral leishmaniasis (CVL) is considered one of the most important canine protozoan diseases of zoonotic concern (1). Various species of Phlebotomus and Lutzomyia sandflies are the potential vectors for the pathogenic agent Leishmania infantum (2). In some European, Asian, and African countries and in America, infection in dogs is associated with a risk of human disease (3–5). In Brazil, the Ministry of Health, through the Visceral Leishmaniasis Control and Surveillance Program (VLCSP), has instituted specific measures to reduce morbidity and case fatality rates, including treating human cases, instituting vector control, and, an action that is unique in the world, sacrificing all seropositive/infected dogs and prohibiting the treatment of CVL (6).
During the last decade, the criteria for eliminating infected animals were based on enzyme-linked immunosorbent assays (ELISAs) for screening and indirect immunofluorescence antibody tests (IFATs) for the confirmatory diagnosis of CVL (6, 7). That these tests may lead to false-positive results due to cross-reactivity with other parasitic diseases is well known (8, 9). Recently, this approach was modified, and testing is now based on a dual-path platform (DPP) for screening and an ELISA for confirmation (10). However, Grimaldi et al. (11) evaluated the DPP test for the serodiagnosis of CVL and showed that it does not perform well in detecting asymptomatic dogs from areas where canine disease is endemic.
It has been shown that vaccination with Leishmune may lead to seroconversion in healthy dogs (10). The vaccination of dogs has increasingly become a common practice in areas in Brazil where CVL is endemic; recently, in addition to the Leishmune vaccine, the Leish-Tec vaccine has become available commercially, and new candidates, such as the LBSap vaccine, are being studied (12–15). In this sense, seroconversion has become an important problem for surveillance/control programs that employ conventional methodologies in their seroepidemiological surveys, because it can lead to the unnecessary euthanasia of healthy dogs. Nevertheless, the role of vaccination in the diagnosis of CVL still has not been studied sufficiently.
Because serological methods still represent the most realistic and applicable tools for epidemiological surveys and for CVL diagnosis, the development of novel serological tests and the validation of alternative methodologies are urgently needed. Toward these ends, several studies have focused on applying flow cytometry technology to serological analyses of leishmaniasis in humans and canines (16–20).
To complement the good performance of flow cytometry-based methodologies in serological approaches, we recently developed a protocol for antigen preparation and optimal antigen preservation conditions, which improved the long-term quality and efficiency of the antigens, which in turn allows for the routine use of this tool for laboratory CVL diagnosis (21). The goal of the present study was to use antigens and conjugate antibodies that were stored for 1 year at 4°C to evaluate a prototype test based on flow cytometry serology for CVL diagnosis. For this purpose, we conducted serological analyses on a broad range of serum samples obtained from L. infantum-infected dogs with different clinical statuses, dogs vaccinated against visceral leishmaniasis, and dogs infected with other major canine pathogens.
MATERIALS AND METHODS
Study animals.
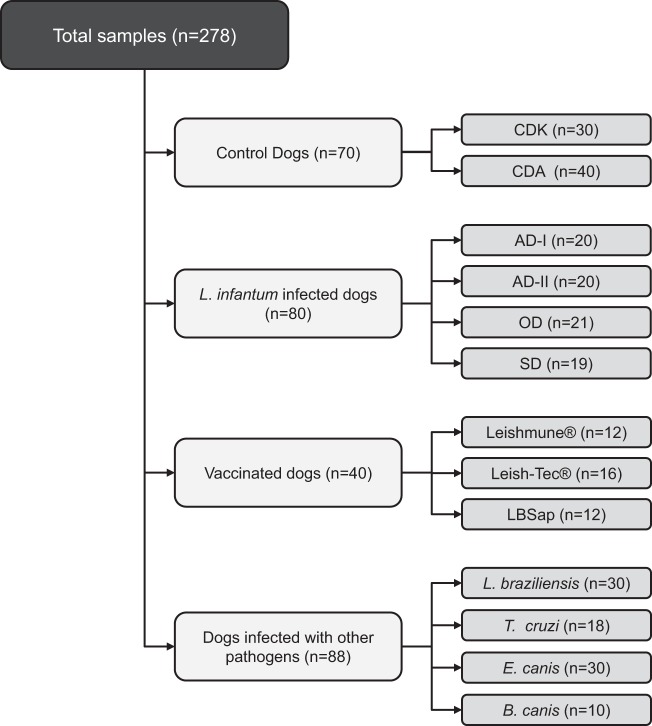
Serum samples obtained from 278 mongrel dogs of either gender were used (Fig. 1). Seventy samples from noninfected dogs were included as a control group. This group was composed of sera (n = 30) from a subset of control dogs born in a kennel in the animal facility of the Federal University of Ouro Preto (Minas Gerais, Brazil) and sera (n = 40) from a subset of control dogs from a cross-sectional study conducted in 2008 in Belo Horizonte, Brazil, where CVL is endemic (22). The control dogs were characterized by negative parasitological and PCR-restriction fragment length polymorphism (RFLP) results for L. infantum and by seronegative results for Leishmania spp. by IFATs and ELISAs.
Fig 1.
Experimental design employed in the prototype flow cytometry serological test. The control dog group included dogs from a kennel (CDK) and dogs from an area of endemicity (CDA). The L. infantum-infected dogs were stratified according to their statuses as asymptomatic I dogs (AD-I), asymptomatic II dogs (AD-II), oligosymptomatic dogs (OD), or symptomatic dogs (SD). Vaccinated dogs and dogs infected with other pathogens constitute the other two subsets.
The CVL group (n = 80) was determined according the dogs' serological reactivity in ELISAs and IFATs and also by the PCR-RFLP results. The PCR-RFLP assays were previously performed on the buffy coat from blood samples, according to the method described by Coura-Vital et al. (22). The CVL group was divided into four subgroups according to clinical status, as proposed by Mancianti et al. (36) and reviewed by Coura-Vital et al. (22): two asymptomatic groups (asymptomatic I [n = 20] and asymptomatic II [n = 20]), an oligosymptomatic group (n = 21), and a symptomatic group (n = 19). The asymptomatic I dogs were seronegative by the IFATs and ELISAs but positive by the PCR-RFLP molecular assays. The other three groups (asymptomatic II, oligosymptomatic, and symptomatic) were characterized by having two positive serological tests (IFAT and ELISA).
In addition to the groups described above, the study also used 40 adult mongrel dogs of either gender that were maintained in the kennel of the Federal University of Ouro Preto and vaccinated with a commercial vaccine, either Leishmune (n = 12) or Leish-Tec (n = 16), or a potential candidate vaccine, LBSap (n = 12). All animals received three doses of the vaccines used in this study, with an interval of 21 days between each dose. The immunizations were conducted according to the instructions of the manufacturer of the commercial vaccine or the proposed protocol for the candidate LBSap vaccine (15).
To further characterize the degree of cross-reactivity and specificity by flow cytometry serology, we also investigated 88 serum samples from dogs naturally infected with Leishmania braziliensis (n = 30), dogs experimentally infected with Trypanosoma cruzi (n = 18), and dogs with common tick-borne infections such as Ehrlichia canis (n = 30) and Babesia canis (n = 10). These samples were from the serum bank of the Clinical Research Laboratory of Pharmacy School from the Federal University of Ouro Preto and were kindly provided by different laboratories. Each infection was previously characterized by specific serology (ELISA)- and PCR-positive results, and samples were PCR negative for L. infantum.
Sample collection.
Peripheral blood samples were collected by an intravenous puncture of the radial vein of each dog using a disposable 5-ml syringe and a vacuum vial (Vacuette; Campinas, SP, Brazil). The serum samples obtained were stored at −20°C in 1.8-ml sterile cryogenic vials (Sarstedt, Newton, NC) until required for the assays.
For the bone marrow cultures, dogs were sedated with an intravenous dose (8 mg/kg body weight) of sodium thiopental (Thionembutal; Abbott Laboratories, São Paulo, Brazil), and bone marrow fluid was removed from the ventral region of the sternum or from the iliac crest using a sterile syringe. Then, bone marrow aspirates were transferred to sterile tubes containing Novy-MacNeal-Nicolle–liver infusion tryptose (NNN-LIT) medium supplemented with 10% fetal bovine serum (FBS) (23).
Dogs seroreactive to ELISA and IFAT were euthanized by the Zoonotic Disease Control Center of Belo Horizonte (Minas Gerais, Brazil). After the euthanasia, biopsy specimens from the ear skin and spleen were collected using sterile scalpels. The tissue fragments were placed onto microscope slides and stained with Giemsa for parasitological exams.
This study was approved by the Universidade Federal de Ouro Preto Committees of Ethics in Animal Experimentation (protocol no. 083/2007).
Design of the prototype flow cytometry test.
The prototype test described in this study is registered at the Instituto Nacional da Propriedade Industrial (Brazil) under patent number BR 1020120047420, deposited on 2 March 2012. The antigen preparation and reaction conditions were as previously described by Ker et al. (21).
For this experiment, the L. infantum antigen had been preserved in 0.5% formaldehyde, and the IgG-labeled antibody had been stored at 4°C for 1 year. Briefly, the antigen suspensions (5.0 × 105 parasites/well) were incubated at 37°C for 30 min in the presence of 50 μl of diluted serum samples at a 1:4,096 dilution using a 96-well U-bottom plate (BD Falcon). Following the incubation, the parasite suspension was washed twice with 150 μl of phosphate-buffered saline (PBS) with 3% FBS (1,000 × g for 10 min at 4°C) and reincubated in the dark for 30 min at 37°C in the presence of 50 μl of previously diluted 1:1,000 anti-canine IgG fluorescein isothiocyanate (FITC)-labeled antibody (catalog number A40-105F; Bethyl Laboratories Inc., Montgomery, TX). After being incubated (at 37°C for 30 min) and washed twice with 150 μl of PBS with 3% FBS (1,000 × g for 10 min at 4°C), the stained parasites were fixed with fluorescence-activated cell sorter (FACS) fix solution and maintained for at least 30 min at 4°C in the dark prior to the flow cytometric data acquisition. Internal controls, for which the parasites were incubated in the absence of dog serum but in the presence of the FITC-labeled secondary reagent, were included for all the experiments to monitor nonspecific binding. Flow cytometric measurements were performed on a FACScan flow cytometer (Becton, Dickinson, San Jose, CA) interfaced to an Apple FACStation, and the CellQuest software package was used for data acquisition and storage. The analysis was performed with FlowJo software (FlowJo, Ashland, OR). IgG reactivity was expressed as the percentage of fluorescence-positive parasites, and the cutoff value was obtained through a receiver operating characteristic curve according to the method described by Ker et al. (21).
Gold standards.
Two parasitological methods were used as the gold standards for diagnosis: investigation of amastigotes on tissue smears of skin and spleen on Giemsa-stained slides and examination of promastigote forms in bone marrow cultures.
Statistical analysis.
The data analyses were conducted using Stata software (version 11.0; Stata Corporation, College Station, TX), and the flow cytometry serology performance was assessed by percentages. The prototype test was evaluated by its sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy, using the results of the parasitological tests as reference standards (at the 95% confidence interval). The overall performance of the prototype test was calculated using the noninfected control dogs as truly negative and the dogs with parasitological exams positive for L. infantum as truly positive. Moreover, the groups of animals infected with other pathogens were used as negative samples for individual calculations of specificity.
RESULTS
The prototype flow cytometry serology test performed well for discriminating noninfected from L. infantum-infected dogs with different clinical forms.
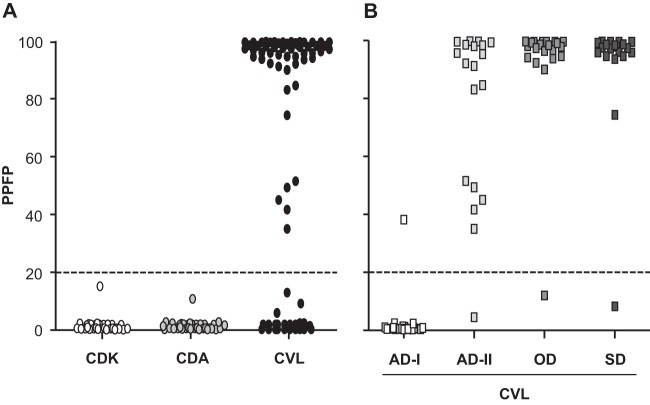
Results of the evaluation of the performance of the prototype flow cytometry serology test for CVL diagnosis are shown in Fig. 1. We observed that 58/80 (72.5%) CVL dogs had a positive result, and none of the dogs in the control group showed reactivity (Fig. 2A). To assess the performance of flow cytometry serology in diagnosing different clinical statuses, dogs classified as asymptomatic I, asymptomatic II, oligosymptomatic, and symptomatic were analyzed; positive results were observed in 1/20 (5%), 18/19 (94.7%), 20/21 (95.2%), and 19/20 (95%) dogs, respectively (Fig. 2B).
Fig 2.
Flow cytometry serology employing antigens and IgG-labeled antibody stored for 1 year to discriminate noninfected control dogs from L. infantum-infected dogs presenting different clinical forms. (A) The results are expressed as percentages of positive fluorescent parasites (PPFP) for individual samples at a serum dilution of 1:4,096 from noninfected control dogs from a kennel (white circles), control dogs from an area of endemicity (CDA) (gray circles), and L. infantum-infected dogs (CVL) (black circles). (B) The CVL dogs were stratified according to their clinical statuses as asymptomatic I dogs (AD-I) (white squares), asymptomatic II dogs (AD-II) (light-gray squares), oligosymptomatic dogs (OD, medium-gray squares), or symptomatic dogs (SD) (dark-gray squares). The dotted lines represent the cutoffs between the negative and positive results.
The prototype flow cytometry serology test showed a high capacity to discriminate reactivity of Leishmania-vaccinated dogs and minimize cross-reactivity with other canine pathogens.
We performed an analysis of serologic reactivity in the serum of dogs vaccinated with either of the two vaccines against CVL (Leishmune and Leish-Tec) that are commercially available in Brazil or a potential vaccine candidate against CVL (LBSap). Our data demonstrate that none of the serum from vaccinated dogs showed seroreactivity in the prototype flow cytometry serology test (Fig. 3A).
Fig 3.
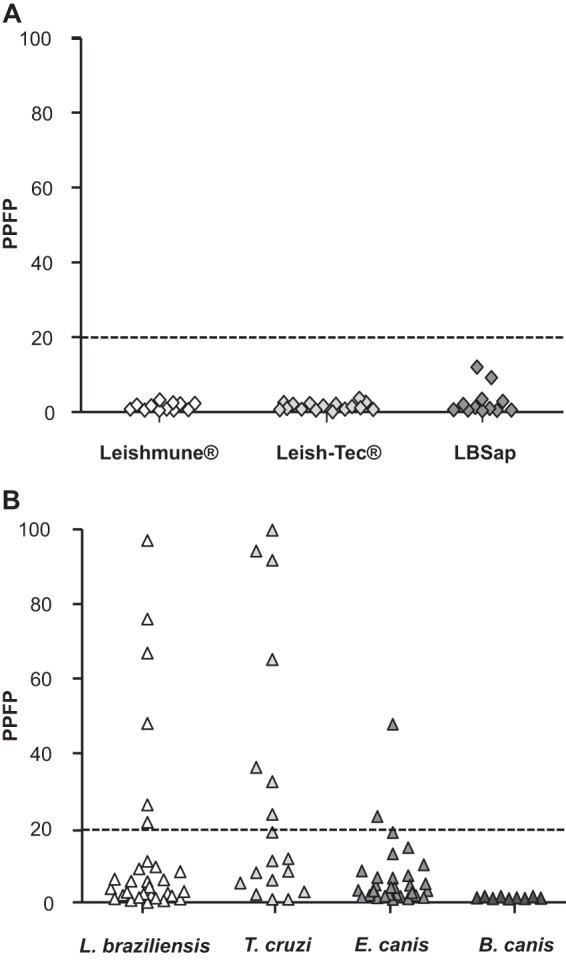
Flow cytometry serology employing antigens and IgG-labeled antibody stored for 1 year to discriminate the reactivity of the dogs vaccinated against Leishmania and also the cross-reactivity with other canine pathogens. (A) The results are expressed as percentages of positive fluorescent parasites (PPFP) for individual samples at a serum dilution of 1:4,096 from the dogs vaccinated with Leishmune (white diamonds), Leish-Tec (light-gray diamonds), or LBSap (dark-gray diamonds). (B) We also tested dogs with other relevant pathogens: L. braziliensis (white triangles), T. cruzi (light-gray triangles), E. canis (medium-gray triangles), or B. canis (dark-gray triangles). The dotted lines represent the cutoffs between the negative and positive results.
The prototype test showed medium cross-reactivity when sera from the dogs infected with L. braziliensis (6/30; 20%) or T. cruzi (7/18; 38.9%) were tested. Furthermore, the dogs infected with E. canis showed low cross-reactivity (2/30; 6.6%), and B. canis-infected samples provided false-positive results (Fig. 3B).
The prototype test resulted in outstanding performance indices in the serological diagnosis of CVL.
This study included an analysis of the sensitivity, specificity, positive and negative predictive values, and accuracy of the prototype test using 36 of the 80 dogs with CVL as reference dogs with positive parasitological exams; these dogs were considered confirmed positive cases. The 70 dogs from the control group had negative results on the parasitological exams and were considered to be confirmed negative cases. Data analysis demonstrated that the prototype test had high sensitivity (100%), high specificity (100%), a high PPV (100%), and a high NPV (100%). Furthermore, analysis of the accuracy confirmed excellent performance of the prototype test (100%) in CVL diagnosis (Table 1).
Table 1.
Performance indices of flow cytometry serology for detection of anti-Leishmania IgG antibodies in canine sera
| Sample type | Results (% [95% CI])a |
||||
|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| CVL | 100.0 (90.4–100.0) | 100.0 (83.3–99.4) | 100.0 (86.2–99.5) | 100.0 (88.3–100.0) | 100.0 (91.9–99.7) |
| L. braziliensis | 80.0 (62.7–90.5) | 85.7 (72.2–93.3) | 100.0 (86.2–100.0) | 90.9 (81.6–95.8) | |
| T. cruzi | 55.6 (33.7–75.5) | 81.8 (68.1–90.5) | 100.0 (72.3–100.0) | 85.2 (73.4–92.3) | |
| B. canis | 100.0 (70.1–100.0) | 100.0 (90.4–100.0) | 100.0 (70.1–100.0) | 100.0 (92.1–100.0) | |
| E. canis | 93.3 (78.7–98.2) | 94.7 (82.7–98.5) | 100.0 (87.9–100.0) | 97.0 (89.6–99.2) | |
CI, confidence interval.
To assess the performance indices of the prototype flow cytometry serology test in animals infected with other pathogens, the animals infected with T. cruzi, L. braziliensis, E. canis, or B. canis were evaluated. The specificity obtained for T. cruzi-infected serum samples was the lowest for all groups assessed (55.6%), followed by that for L. braziliensis-infected samples (80%). The prototype test had high specificities for E. canis- and B. canis-infected samples (93.3% and 100%, respectively). Furthermore, the NPVs were 100% for all groups. The PPVs were 85.7%, 81.8%, 94.7%, and 100%, and the accuracies were 90.9%, 85.2%, 97%, and 100% for T. cruzi, L. braziliensis, E. canis, and B. canis, respectively, confirming the excellent performance of the prototype test (Table 1).
DISCUSSION
Serological testing has been a basic and essential tool for diagnosing and controlling many infectious diseases (24). Flow cytometry is becoming an increasingly useful tool in both health care and research laboratories because it is a rapid, accurate, and reproducible method of analysis (25). Although there is still a substantial cost for operational support in experiments involving flow cytometry, the creation of a shared resource laboratory model to enhance the scope and quality of the scientific research that applies the flow cytometry-based methodologies was recently described (26, 27). In the same context, through the Oswaldo Cruz Foundation, the Brazilian government implemented the Network Technology Platforms Program for Technological Development in Health Supplies to enhance research perspectives in flow cytometry approaches, which is also suitable for diagnostic services in public health. This platform model offers new perspectives for the use of flow cytometry as a diagnostic tool for neglected tropical diseases such as visceral leishmaniasis.
In the previous studies that employed L. infantum antigens prepared just before the serological reaction, it was observed that flow cytometry serology provided outstanding performance for the diagnosis of CVL (19, 28). In the current study, using a standard antigen preparation, we observed excellent performance for the prototype test, which had high sensitivity (100%) and specificity (100%) for detecting IgG in CVL-infected dogs. Moreover, our data demonstrate a high PPV (100%) and a high NPV (100%), indicating that the prototype flow cytometry test is highly reliable for detecting positive CVL samples and also for excluding CVL in noninfected dogs. The high accuracy (100%) observed in this prototype test points toward precise diagnosis. Therefore, our results reinforce the indication that the flow cytometry serology assay is a very useful tool for the diagnosis of CVL.
For the tests in different CVL groups, our data demonstrate that the prototype flow cytometry serology test had outstanding performance for identifying the asymptomatic II, oligosymptomatic, and symptomatic dogs. These findings certify that the prototype test employing the described conditions was capable of providing excellent performance for CVL diagnosis, even after 1 year of storage of the antigen preparation and the IgG-labeled antibody. However, we observed that only one infected dog from the asymptomatic I group was detected. These animals have high prevalence and incidence rates in areas where CVL is endemic, and CVL is not detected by conventional serology methods (22, 29) or by flow cytometry serology, as demonstrated in the current study. We believe that the low sensitivity observed in detecting CVL in this group is due to the immunological profile shown by these dogs that were characterized by having low production of Ig antibodies (IgG, IgG1, IgG2, IgM, IgA, and IgE), which occurs in the early stages of infection (10, 18, 28). During such periods, B lymphocytes do not secrete polyclonal antibodies, and consequently, serological methods are less sensitive at this stage of infection (30, 31). Moreover, it has been observed that these dogs are more likely to seroconvert than are PCR-negative dogs (32).
Vaccines against CVL have been promoted as an important tool and a cost-effective strategy for controlling the disease (33). Thus, knowledge about the performance of diagnostic methods for vaccinated dogs is urgently needed to avoid false-positive results, which can lead to the unnecessary euthanasia of noninfected dogs. Andrade et al. (19) described the ability of flow cytometry serology to exclude seroreactivity from Leishmune-vaccinated dogs. Extending this research, we investigated the performance of a prototype flow cytometry test in dogs vaccinated with the Leishmune, Leish-Tec, and LBSap vaccines (14, 15). The novel finding obtained in the present study is that the prototype flow cytometry serology test had extraordinary performance with regard to excluding reactivity in the animals vaccinated with commercial vaccines and also in the dogs immunized with a potential candidate vaccine.
Different pathogens from the same family, such as the Trypanosomatidae (Leishmania spp. and T. cruzi), share similar antigenic repertoires of epitopes that can lead to cross-reactive antibodies in immunodiagnostic tests. Use of the conventional serological methods for the diagnosis of CVL may lead to cross-reactivity with other canine infections, mainly in dogs infected with T. cruzi, L. braziliensis, E. canis, or B. canis (7–9). In our study, despite the fact that the prototype flow cytometry serology test exhibited the lowest specificities in the T. cruzi- and L. braziliensis-infected samples, the results obtained were superior to those observed for other serological tests which assessed the cross-reactivity of these pathogens using conventional methods (8, 34). Nevertheless, in a previous flow cytometry serology study, Andrade et al. (19) verified that a higher dilution (1:8,192) of serum can reduce cross-reactivity in dogs infected with T. cruzi or L. braziliensis with no change in the diagnostic performance for CVL.
With regard to canine tick-borne infections, ehrlichiosis and babesiosis are highly prevalent in Brazil and represent a challenge to veterinarians and public health workers (35). Considering that these vector-borne diseases affect dogs with concomitant CVL in areas of endemicity, we analyzed for the first time the cross-reactivity of a prototype flow cytometry serology test in dogs naturally infected with B. canis and E. canis. This test demonstrated high specificity, positive and negative predictive values, and accuracy, emphasizing its excellent performance in the diagnosis of CVL, even if the animals were infected with one of those pathogens.
The performance of diagnostic tests is greatly limited by the antigen used in the technique. In this study, we showed that the long-term efficacy and robustness of the L. infantum antigens used in the prototype flow cytometry test can be maintained by employing a cheap preservative and storing it at a controlled temperature, which points to the potential commercial use of this prototype test. In this context, our findings strengthen the usefulness of flow cytometry serology on a wider scale, especially in areas of CVL endemicity, and for animals with potential coinfections and those that have been vaccinated. Thus, to validate this test prospectively, we intend to use the prototype test in a large number of dogs from an urban area of Brazil where CVL is endemic.
ACKNOWLEDGMENTS
This work was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Brazil (FAPEMIG grant CBB-APQ-3073-4.01/07), the Programa de Pesquisa para o SUS (PPSUS/MS/CNPq/FAPEMIG/SES-MG grant CBB-APQ-00356-10), the Conselho Nacional de Pesquisa (CNPq grant 472554/2007-7), and the Departamento de Ciência e Tecnologia do Ministério da Saúde (DECIT/MS/CNPq/BR grant 576062/2008-1). A.B.R., C.M.C., A.T.-C., O.A.M.-F., and R.C.G. thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and W.C.-V., N.D.D.M., and D.D.S.L. thank CAPES/PNPD for fellowships.
Footnotes
Published ahead of print 9 October 2013
REFERENCES
- 1.Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. 2008. Canine leishmaniosis—new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 24:324–330 [DOI] [PubMed] [Google Scholar]
- 2.Killick-Kendrick R. 1999. The biology and control of phlebotomine sand flies. Clin. Dermatol. 17:279–289 [DOI] [PubMed] [Google Scholar]
- 3.Nunes CM, Pires MM, da Silva KM, Assis FD, Goncalves Filho J, Perri SH. 2010. Relationship between dog culling and incidence of human visceral leishmaniasis in an endemic area. Vet. Parasitol. 170:131–133 [DOI] [PubMed] [Google Scholar]
- 4.Gavgani AS, Mohite H, Edrissian GH, Mohebali M, Davies CR. 2002. Domestic dog ownership in Iran is a risk factor for human infection with Leishmania infantum. Am. J. Trop. Med. Hyg. 67:511–515 [DOI] [PubMed] [Google Scholar]
- 5.Faye B, Banuls AL, Bucheton B, Dione MM, Bassanganam O, Hide M, Dereure J, Choisy M, Ndiaye JL, Konate O, Claire M, Senghor MW, Faye MN, Sy I, Niang AA, Molez JF, Victoir K, Marty P, Delaunay P, Knecht R, Mellul S, Diedhiou S, Gaye O. 2010. Canine visceral leishmaniasis caused by Leishmania infantum in Senegal: risk of emergence in humans? Microbes Infect. 12:1219–1225 [DOI] [PubMed] [Google Scholar]
- 6.Ministério da Saúde 2006. Manual de vigilância e controle da leishmaniose visceral, 1st ed. Secretaria de Vigilância em Saúde, Brasília, Brazil: http://portal.saude.gov.br/portal/arquivos/pdf/manual_leish_visceral2006.pdf [Google Scholar]
- 7.Lira RA, Cavalcanti MP, Nakazawa M, Ferreira AG, Silva ED, Abath FG, Alves LC, Souza WV, Gomes YM. 2006. Canine visceral leishmaniosis: a comparative analysis of the EIE-leishmaniose-visceral-canina-Bio-Manguinhos and the IFI-leishmaniose-visceral-canina-Bio-Manguinhos kits. Vet. Parasitol. 137:11–16 [DOI] [PubMed] [Google Scholar]
- 8.Ferreira EC, de Lana M, Carneiro M, Reis AB, Paes DV, da Silva ES, Schallig H, Gontijo CM. 2007. Comparison of serological assays for the diagnosis of canine visceral leishmaniasis in animals presenting different clinical manifestations. Vet. Parasitol. 146:235–241 [DOI] [PubMed] [Google Scholar]
- 9.Oliveira TM, Furuta PI, de Carvalho D, Machado RZ. 2008. A study of cross-reactivity in serum samples from dogs positive for Leishmania spp., Babesia canis and Ehrlichia canis in enzyme-linked immunosorbent assay and indirect fluorescent antibody test. Rev. Bras. Parasitol. Vet. 17:7–11 [DOI] [PubMed] [Google Scholar]
- 10.Ministério da Saúde 2011. Esclarecimento sobre substituição do protocolo diagnóstico da leishmaniose visceral canina. Nota técnica conjunta No. 01/2011-CGDT-CGLAB/DEVIT/SVS/MS. Ministério da Saúde, Brasilia, Brazil [Google Scholar]
- 11.Grimaldi G, Jr, Teva A, Ferreira AL, dos Santos CB, Pinto IS, de-Azevedo CT, Falqueto A. 2012. Evaluation of a novel chromatographic immunoassay based on Dual-Path Platform technology (DPP CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 106:54–59 [DOI] [PubMed] [Google Scholar]
- 12.Borja-Cabrera GP, Santos FN, Bauer FS, Parra LE, Menz I, Morgado AA, Soares IS, Batista LM, Palatnik-de-Sousa CB. 2008. Immunogenicity assay of the Leishmune vaccine against canine visceral leishmaniasis in Brazil. Vaccine 26:4991–4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes AP, Costa MM, Coelho EA, Michalick MS, de Freitas E, Melo MN, Luiz Tafuri W, Resende DDM, Hermont V, Abrantes CDF, Gazzinelli RT. 2008. Protective immunity against challenge with Leishmania (Leishmania) chagasi in beagle dogs vaccinated with recombinant A2 protein. Vaccine 26:5888–5895 [DOI] [PubMed] [Google Scholar]
- 14.Giunchetti RC, Correa-Oliveira R, Martins-Filho OA, Teixeira-Carvalho A, Roatt BM, de Oliveira Aguiar-Soares RD, de Souza JV, das Dores Moreira N, Malaquias LC, Mota e Castro LL, de Lana M, Reis AB. 2007. Immunogenicity of a killed Leishmania vaccine with saponin adjuvant in dogs. Vaccine 25:7674–7686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roatt BM, Aguiar-Soares RD, Vitoriano-Souza J, Coura-Vital W, Braga SL, Correa-Oliveira R, Martins-Filho OA, Teixeira-Carvalho A, de Lana M, Gontijo NF, Marques MJ, Giunchetti RC, Reis AB. 2012. Performance of LBSap vaccine after intradermal challenge with L. infantum and saliva of Lu. longipalpis: immunogenicity and parasitological evaluation. PLoS One 7:e49780. 10.1371/journal.pone.0049780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocha RD, Gontijo CM, Eloi-Santos SM, Teixeira Carvalho A, Correa-Oliveira R, Marques MJ, Genaro O, Mayrink W, Martins-Filho OA. 2002. Anti-live Leishmania (Viannia) braziliensis promastigote antibodies, detected by flow cytometry, to identify active infection in American cutaneous leishmaniasis. Rev. Soc. Bras. Med. Trop. 35:551–562 (In Portuguese.) [DOI] [PubMed] [Google Scholar]
- 17.Pissinate JF, Gomes IT, Peruhype-Magalhaes V, Dietze R, Martins-Filho OA, Lemos EM. 2008. Upgrading the flow-cytometric analysis of anti-Leishmania immunoglobulins for the diagnosis of American tegumentary leishmaniasis. J. Immunol. Methods 336:193–202 [DOI] [PubMed] [Google Scholar]
- 18.Garcia LM, Coelho-Dos-Reis JG, Peruhype-Magalhaes V, Teixeira-Carvalho A, Rocha RD, Araujo MS, Gomes IT, Carvalho SF, Dietze R, Lemos EM, Andrade MC, Martins-Filho OA. 2009. Anti-fixed Leishmania chagasi promastigotes IgG antibodies detected by flow cytometry (FC-AFPA-IgG) as a tool for serodiagnosis and for post-therapeutic cure assessment in American visceral leishmaniasis. J. Immunol. Methods 350:36–45 [DOI] [PubMed] [Google Scholar]
- 19.Andrade RA, Silva Araujo MS, Reis AB, Gontijo CM, Vianna LR, Mayrink W, Martins-Filho OA. 2009. Advances in flow cytometric serology for canine visceral leishmaniasis: diagnostic applications when distinct clinical forms, vaccination and other canine pathogens become a challenge. Vet. Immunol. Immunopathol. 128:79–86 [DOI] [PubMed] [Google Scholar]
- 20.de Andrade RA, Reis AB, Gontijo CM, Braga LB, Rocha RD, Araujo MS, Vianna LR, Martins-Filho OA. 2007. Clinical value of anti-Leishmania (Leishmania) chagasi IgG titers detected by flow cytometry to distinguish infected from vaccinated dogs. Vet. Immunol. Immunopathol. 116:85–97 [DOI] [PubMed] [Google Scholar]
- 21.Gama Ker H, Dian de Oliveira Aguiar-Soares R, Mendes Roatt B, das Dores Moreira N, Coura-Vital W, Martins Carneiro C, Teixeira-Carvalho A, Martins-Filho OA, Cordeiro Giunchetti R, da Silveira-Lemos D, Barbosa Reis A. 2013. Effect of the preservative and temperature conditions on the stability of Leishmania infantum promastigotes antigens applied in a flow cytometry diagnostic method for canine visceral leishmaniasis. Diagn. Microbiol. Infect. Dis. 76:470–476 [DOI] [PubMed] [Google Scholar]
- 22.Coura-Vital W, Marques MJ, Veloso VM, Roatt BM, Aguiar-Soares RD, Reis LE, Braga SL, Morais MH, Reis AB, Carneiro M. 2011. Prevalence and factors associated with Leishmania infantum infection of dogs from an urban area of Brazil as identified by molecular methods. PLoS Negl. Trop. Dis. 5:e1291. 10.1371/journal.pntd.0001291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camargo EP. 1964. Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosomes in liquid media. Rev. Inst. Med. Trop. Sao Paulo 6:93–100 [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention 1999. Achievements in public health, 1900-1999: control of infectious diseases. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 25.Jaroszeski MJ, Radcliff G. 1999. Fundamentals of flow cytometry. Mol. Biotechnol. 11:37–53 [DOI] [PubMed] [Google Scholar]
- 26.Moore J, Roederer M. 2009. The flow cytometry shared resource laboratory: best practices to assure a high-quality, cost-effective partnership with biomedical research laboratories. Cytometry A 75:643–649 [DOI] [PubMed] [Google Scholar]
- 27.Monti F, Rosetti M, Masperi P, Tommasini N, Dorizzi RM. 2012. Shared resource laboratories: impact of new design criteria to consolidate flow cytometry diagnostic service. Int. J. Lab. Hematol. 34:533–540 [DOI] [PubMed] [Google Scholar]
- 28.Carvalho Neta AV, Rocha RDR, Gontijo CMF, Reis AB, Martins-Filho OA. 2006. Flow cytometry used in canine visceral leishmaniasis diagnosis. Arq. Bras. Med. Vet. Zootec. 58:480–488 (In Portuguese.) [Google Scholar]
- 29.Coura-Vital W, Reis AB, Reis LE, Braga SL, Roatt BM, Aguiar-Soares RD, Marques MJ, Veloso VM, Carneiro M. 2013. Canine visceral leishmaniasis: incidence and risk factors for infection in a cohort study in Brazil. Vet. Parasitol. 197:411–417 [DOI] [PubMed] [Google Scholar]
- 30.Oliveira GG, Santoro F, Sadigursky M. 1993. The subclinical form of experimental visceral leishmaniasis in dogs. Mem. Inst. Oswaldo Cruz 88:243–248 [DOI] [PubMed] [Google Scholar]
- 31.Coura-Vital W, Marques MJ, Giunchetti RC, Teixeira-Carvalho A, Moreira ND, Vitoriano-Souza J, Vieira PM, Carneiro CM, Correa-Oliveira R, Martins-Filho OA, Carneiro M, Reis AB. 2011. Humoral and cellular immune responses in dogs with inapparent natural Leishmania infantum infection. Vet. J. 190:e43–e47. 10.1016/j.tvjl.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 32.Coura-Vital W, Reis AB, Fausto MA, Leal GG, Marques AMJ, Veloso VM, Carneiro M. 2013. Risk factors for seroconversion by Leishmania infantum in a cohort of dogs from an endemic area of Brazil. PLoS One 8:e71833. 10.1371/journal.pone.0071833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gramiccia M, Gradoni L. 2005. The current status of zoonotic leishmaniases and approaches to disease control. Int. J. Parasitol. 35:1169–1180 [DOI] [PubMed] [Google Scholar]
- 34.da Costa CA, Genaro O, de Lana M, Magalhaes PA, Dias M, Michalick MS, Melo MN, da Costa RT, Magalhaes-Rocha NM, Mayrink W. 1991. Canine visceral leishmaniasis: evaluation of the serologic method used in epidemiologic studies. Rev. Soc. Bras. Med. Trop. 24:21–25 (In Portuguese.) [DOI] [PubMed] [Google Scholar]
- 35.Dantas-Torres F. 2008. Canine vector-borne diseases in Brazil. Parasit. Vectors 1:25. 10.1186/1756-3305-1-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancianti F, Gramiccia M, Gradoni L, Pieri S. 1988. Studies on canine leishmaniasis control. I. Evolution of infection of different clinical forms of canine leishmaniasis following antimonial treatment. Trans. R. Soc. Trop. Med. Hyg. 82:566–567 [DOI] [PubMed] [Google Scholar]