LETTER
As of 4 July 2013, there have been 134 confirmed cases of human infection and 43 fatalities with a new avian influenza A virus (H7N9) (1). H7N9 is currently contained in Southeastern China, and the majority of human cases of infection have reported direct exposure to poultry (2). While no sustained human-to-human transmission has been reported, there have been cases of family clusters of infection (3), suggesting limited person-to-person transmissibility, which, in light of recent findings that H7N9 can be transmitted via aerosol in ferrets (4), suggests the potential for an H7N9 pandemic. Several broadly neutralizing antibodies against influenza A viruses have been isolated, including FI6 that binds to all group 1 and 2 subtypes of influenza A virus (5). We previously demonstrated that adeno-associated virus (AAV) vector serotype 9 expressing the FI6 antibody at the nasopharyngeal mucosa of mice and ferrets provided complete protection against clinical isolates of several pandemic strains of H5N1 and H1N1 influenza (6). We evaluated this strategy in mice for efficacy against a clinical isolate of H7N9 (A/Anhui/01/2013 [A/H1]) (7).
H7N9 A/H1 was isolated in China on 31 March 2013, 1 month after the first reports of H7N9 infection (Fig. 1C) (7). An aliquot of this virus arrived on 23 April at the National Microbiology Laboratory of the Public Health Agency of Canada, and within 4 weeks of receipt, the virus was amplified and the 50% lethal dose (LD50) was established in mice. Transport, handling, and disposal of H7N9 A/H1 during these experiments complied with published international and national recommendations, and the animal experiments were performed in accordance with the regulations of the Canadian Human Pathogens and Toxins Act (8–10). On 30 May, BALB/c mice were intranasally administered 1011 genome copies of AAV9.FI6, and 15 days later, AAV9.FI6-treated and naïve mice were challenged with 100 LD50 of H7N9 A/H1. Figure 1 presents the outcome of these experiments in terms of the weights of the mice (Fig. 1A) and percent survival (Fig. 1B). The control animals (i.e., no vector treatment but H7N9 A/H1 challenge; n = 10) showed a rapid and progressive weight loss (Fig. 1A), requiring that all animals be euthanized by day 9 (Fig. 1B). Animals treated with AAV9.FI6 showed improved outcome. Treated mice began to lose weight at the same rate as naïve mice, although the severity of clinical symptoms (i.e., ruffled fur and rapid breathing) was less, and 6/9 mice eventually recovered and survived. Effectiveness of the vaccine was also assessed by necropsy of some animals 5 days postchallenge and measuring viral load in lung homogenates as described previously (6) in naïve (n = 3) and vector-treated (n = 5) mice. We observed a nonsignificant trend toward reduced viral load in the lungs of the AAV9.FI6-treated mice (data not shown).
Fig 1.
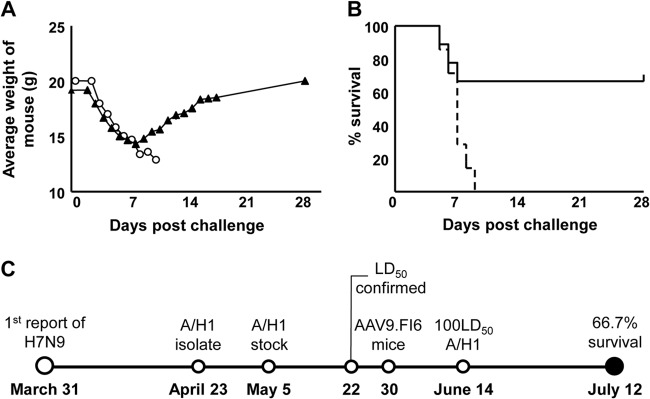
Delivery of AAV9.FI6 to airway confers partial protection against H7N9 A/H1. (A) Weights of mice following challenge with H7N9 A/H1. Open circles, control mice (n = 10); closed triangles, AAV9.FI6-treated mice (n = 14). (B) Survival of challenged mice. Mice were euthanized when they appeared in distress or their body weight declined ≥30%. Dashed lines, control mice; solid lines, AAV9.FI6-treated mice. (C) Timeline of the H7N9 A/H1 human infection report to animal vaccination and vector-mediated protection.
The emergence of H7N9 A/H1 as a human pathogen provided a unique opportunity to evaluate our AAV technology platform and the potential use of the described vectored neutralizing antibody during a mounting influenza outbreak. We demonstrated partial efficiency of AAV9.FI6 in a relevant model 4.5 months after the initial report of an infection in a human being.
ACKNOWLEDGMENTS
This work was supported by internal funding sources to J.M.W., a grant from the Public Health Agency of Canada (531252), and a grant from the Canadian Institutes of Health Research (246355) to G.K.
J.M.W. is a consultant to ReGenX Holdings and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings. J.M.W. holds a patent on adeno-associated virus (AAV) clades (U.S. patent application 7,906,111B2) with pending continuation (U.S. patent application 13/023,918). J.M.W. has a pending application on AAV-mediated passive immunization of airborne pathogens (PCT/US2012/034355). M.P.L. has a pending application on AAV-mediated passive immunization of airborne pathogens (PCT/US2012/034355). All other authors declare no competing financial interests.
Footnotes
Published ahead of print 16 October 2013
REFERENCES
- 1.World Health Organization 2013. Human infection with avian influenza A(H7N9) virus—update. Disease outbreak news. WHO, Geneva, Switzerland: http://www.who.int/csr/don/2013_07_20/en/index.html [Google Scholar]
- 2.Zhou J, Wang D, Gao R, Zhao B, Song J, Qi X, Zhang Y, Shi Y, Yang L, Zhu W, Bai T, Qin K, Lan Y, Zou S, Guo J, Dong J, Dong L, Wei H, Li X, Lu J, Liu L, Zhao X, Huang W, Wen L, Bo H, Xin L, Chen Y, Xu C, Pei Y, Yang Y, Zhang X, Wang S, Feng Z, Han J, Yang W, Gao GF, Wu G, Li D, Wang Y, Shu Y. 2013. Biological features of novel avian influenza A (H7N9) virus. Nature 499:500–503 [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Zhou L, Zhou M, Chen Z, Li F, Wu H, Xiang N, Chen E, Tang F, Wang D, Meng L, Hong Z, Tu W, Cao Y, Li L, Ding F, Liu B, Wang M, Xie R, Gao R, Li X, Bai T, Zou S, He J, Hu J, Xu Y, Chai C, Wang S, Gao Y, Jin L, Zhang Y, Luo H, Yu H, Gao L, Pang X, Liu G, Shu Y, Yang W, Uyeki TM, Wang Y, Wu F, Feng Z. 24 April 2013. Preliminary report: epidemiology of the avian influenza A (H7N9) outbreak in China. N. Engl. J. Med. [Epub ahead of print.] 10.1056/NEJMoa1304617 [DOI] [Google Scholar]
- 4.Zhang Q, Shi J, Deng G, Guo J, Zeng X, He X, Kong H, Gu C, Li X, Liu J, Wang G, Chen Y, Liu L, Liang L, Li Y, Fan J, Wang J, Li W, Guan L, Li Q, Yang H, Chen P, Jiang L, Guan Y, Xin X, Jiang Y, Tian G, Wang X, Qiao C, Li C, Bu Z, Chen H. 2013. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 341:410–414 [DOI] [PubMed] [Google Scholar]
- 5.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A. 2011. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333:850–856 [DOI] [PubMed] [Google Scholar]
- 6.Limberis MP, Adam VS, Wong G, Gren J, Kobasa D, Ross TM, Kobinger GP, Tretiakova A, Wilson JM. 2013. Intranasal antibody gene transfer in mice and ferrets elicits broad protection against pandemic influenza. Sci. Transl. Med. 5:187ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 368:1888–1897 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization 2013. Laboratory biorisk management for laboratories handling human specimens suspected or confirmed to contain avian influenza A (H7N9) virus causing human disease: interim recommendations. WHO, Geneva, Switzerland: http://www.who.int/influenza/human_animal_interface/influenza_h7n9/InterimRecLaboratoryBioriskManagementH7N9_10May13.pdf [Google Scholar]
- 9.Centers for Disease Control and Prevention 2013. Interim risk assessment and biosafety level recommendations for working with influenza A (H7N9) Viruses. CDC, Atlanta, GA: http://www.cdc.gov/flu/avianflu/h7n9/risk-assessment.htm [Google Scholar]
- 10.Government of Canada 2009. Human pathogens and toxins act. Government of Canada, Ottawa, Ontario, Canada: http://lois-laws.justice.gc.ca/eng/acts/H-5.67/index.html [Google Scholar]


