Abstract
Mycobacterium avium subsp. paratuberculosis causes Johne's disease (JD) in ruminants. Proteomic studies have shown that M. avium subsp. paratuberculosis expresses certain proteins when exposed to in vitro physiological stress conditions similar to the conditions experienced within a host during natural infection. Such proteins are hypothesized to be expressed in vivo, are recognized by the host immune system, and may be of potential use in the diagnosis of JD. In this study, 50 recombinant maltose binding protein (MBP)-M. avium subsp. paratuberculosis fusion proteins were evaluated using serum samples from sheep infected with M. avium subsp. paratuberculosis, and 29 (58%) were found to be antigenic. Among 50 fusion proteins, 10 were evaluated in MBP fusion and factor Xa-cleaved forms. A total of 31 proteins (62%) were found to be antigenic in either MBP fusion or factor Xa-cleaved forms. Antigenicity after cleavage and removal of the MBP tag was marginally enhanced.
INTRODUCTION
Johne's disease (JD) in ruminants is a chronic infection of the intestines caused by Mycobacterium avium subsp. paratuberculosis. Economic losses arise due to culling, reduced production of milk and wool, and mortalities (1, 2). The disease is characterized by a long incubation period, and subclinical infection creates a potential source of infection for uninfected animals. The most common method of detection of JD is by measuring immune responses in the infected host. The two widely used assays to measure cell-mediated and antibody-mediated immune responses are the gamma interferon (IFN-γ) assay and the enzyme-linked immunosorbent assay (ELISA), respectively. These depend on M. avium subsp. paratuberculosis-specific antigens for stimulating IFN-γ from memory T cells or detecting specific antibodies in blood samples. Currently used antigens in these assays are French-pressed proteins or purified protein derivatives (PPDs) derived from whole cells of M. avium subsp. paratuberculosis, containing a large number of antigens. The diagnostic specificity of commercial antibody ELISAs is generally high, but sensitivity is poor (3, 4). The accuracy of an ELISA also can be adversely affected by potential cross-reactions due to exposure of the host to environmental mycobacteria. The specificity of an assay can be enhanced by absorbing the serum against Mycobacterium phlei proteins (5, 6). However, it is difficult to enhance ELISA sensitivity due to the poor Th2 responses during the lengthy latent period of the disease and the large population of infected animals with latent subclinical infections in an exposed flock or herd. Therefore, to enhance assay performance, new M. avium subsp. paratuberculosis-specific antigens that are expressed during latency need to be identified.
Evidence of dormancy in M. avium subsp. paratuberculosis when the organism was present in the soil/pasture environment was reported (7). In vitro studies simulating the stress conditions of natural infection reported dormancy-associated proteins in M. avium subsp. paratuberculosis (8–10). These findings led to a hypothesis that M. avium subsp. paratuberculosis expresses stress/dormancy-related proteins during infection of the host. The use of M. avium subsp. paratuberculosis proteins that are expressed in vivo following pathogen entry into the host as diagnostic antigens may be of value in the detection of an early stage of M. avium subsp. paratuberculosis infection. Indeed, some of the M. avium subsp. paratuberculosis proteins known to be differentially regulated under stress conditions were found to be antigenic in serum collected from sheep infected with M. avium subsp. paratuberculosis, and these findings support the hypothesis that stress proteins expressed in vitro are also expressed in vivo (11–13).
A large number of recombinant M. avium subsp. paratuberculosis antigens have been investigated for their diagnostic potential in cell- and antibody-mediated assays (11, 13–22). Some of these M. avium subsp. paratuberculosis proteins were from groups of proteins that were differentially regulated under physiological stress conditions. Although many proteins were found to be antigenic, no obvious candidate has yet been identified as having suitable diagnostic sensitivity and specificity.
A major limitation for characterization of recombinant M. avium subsp. paratuberculosis proteins is their expression as inclusion bodies or insoluble proteins, especially when prepared using histidine (His) as an affinity purification tag (13). Production of antigens from insoluble proteins involves processes that may be detrimental to biological activity. Expression of maltose binding protein (MBP) fusion proteins facilitates maintenance of the solubility, structure, and functions of recombinant proteins through downstream processing (23, 24). Several recombinant MBP-M. avium subsp. paratuberculosis fusion proteins (MBP fusion proteins) were found to be antigenic in sheep, cattle, and mice infected with M. avium subsp. paratuberculosis (15, 16, 25). However, MBP alone, with a molecular mass of about 42.5 kDa, is known to have a small amount of seroreactivity; because of this, it must be used as a control in ELISAs (23). Furthermore, it is not known if the MBP protein masks the immune recognition of a protein of interest. Therefore, cleavage of the MBP tag from the recombinant M. avium subsp. paratuberculosis proteins may be beneficial for their use.
Factor Xa is a protease that specifically cleaves after the arginine residue in its preferred site Ile-(Glu or Asp)-Gly-Arg sequence (26) and can be used to separate the MBP affinity purification tag from the protein of interest following expression and purification (27, 28). This protease was used in the current study for removal of the MBP purification tag. The aims of this study were to evaluate the antigenicity of M. avium subsp. paratuberculosis recombinant proteins hypothesized to be upregulated under stress conditions and to investigate their potential use in early diagnosis. These proteins were examined with and without the MBP tag to determine if the proteins cleaved of MBP had better antigenicity, and some were compared with the corresponding His-tagged recombinant protein.
MATERIALS AND METHODS
Antigens.
The M. avium subsp. paratuberculosis proteins in this study (Table 1) were selected based on their expression in response to in vitro physiological stress conditions (8–10). Fifty M. avium subsp. paratuberculosis recombinant proteins used in this study were produced as MBP fusion proteins at the Bacterial Diseases of Livestock Research Unit, USDA-ARS Agricultural Research Service National Animal Disease Center (NADC) (Ames, IA), and one (MAP1272c) was produced as a His-tagged recombinant M. avium subsp. paratuberculosis protein (29). The MBP fusion proteins were produced as described previously (30). Briefly, the full-length coding sequence of the M. avium subsp. paratuberculosis protein was amplified using gene-specific primers and was cloned into the pMAL-c2 translational fusion expression vector. The vector and amplified products were digested with XbaI and HindIII, and the ligated products were transformed into Escherichia coli DH5α cells. The overexpressed proteins were extracted and purified by affinity chromatography with amylose resin columns (New England BioLabs). Three His-tagged recombinant M. avium subsp. paratuberculosis proteins (MAP2698c, MAP2487c, and MAP3567) were produced at the Faculty of Veterinary Science, University of Sydney (Sydney, Australia), as previously described (12). Briefly, gene-specific primers were designed to include attB1 and attB2 sites at the 5′ end of each sequence. The complete open reading frames of each gene were amplified by PCR using Gateway technology (Invitrogen, Australia). Amplified and purified PCR products were cloned into the donor vector pDONR221 (Invitrogen, Australia) and transformed into One Shot TOP10 chemically competent E. coli cells (Invitrogen, Australia) to produce an entry clone. Purified entry clones were subcloned into the destination vector pET160-DEST with an N-terminal 6×His and Lumio tag (Champion pET160 Gateway expression kit with Lumio technology; Invitrogen, Australia) and transformed into One Shot TOP10 chemically competent E. coli cells to produce an expression clone. One Shot BL21 Star (DE3) cells (Invitrogen, Australia) were transformed with the purified expression clone. The transformed culture was induced with 1 mM isopropyl-β-1-thiogalactopyranoside to express the recombinant proteins. Recombinant proteins were extracted and purified by affinity liquid chromatography (AKTApurifier system; GE Healthcare). The four His-tagged recombinant M. avium subsp. paratuberculosis proteins (1 from the NADC and 3 from the University of Sydney) were compared with the corresponding MBP fusion proteins.
Table 1.
Evaluation and comparison of recombinant M. avium subsp. paratuberculosis proteins (MBP fusion and His-tagged proteins)
| Protein type and function | Protein(s) |
|---|---|
| MBP fusion proteins | |
| Phosphate metabolism | MAP0435c |
| Cold shock protein | MAP0810 |
| Putative virulence factor | MAP1272c |
| Universal stress proteins | MAP1339 |
| Cell wall synthesis | MAP2058c |
| Signal recognition | MAP3968 |
| ATP and purine biosynthesis | MAP2450c, MAP3393c |
| Cell division | MAP0068, MAP1889c |
| Heat shock protein chaperone | MAP3268, MAP3701c |
| Response regulators | MAP0834c, MAP3200 |
| Protein synthesis | MAP1027c, MAP4125 |
| Proteolysis | MAP1834c, MAP2280c, MAP2281c |
| Amino acid metabolism | MAP1293, MAP1297, MAP1846c, MAP2864c |
| Antioxidant enzymes | MAP1588c, MAP1589c, MAP1653, MAP4340 |
| Hypothetical protein | MAP0593c, MAP0184c, MAP1586, MAP3555, MAP3864 |
| Fatty acid metabolism | MAP0508, MAP0516c, MAP1017c, MAP2698c, MAP2872c, MAP3190, MAP3577, MAP3651c |
| Cellular processes | MAP0187c, MAP0540, MAP1560, MAP1885c, MAP2411, MAP2487c, MAP2705c, MAP3007, MAP3538, MAP3567 |
| His-tagged proteins | |
| Putative virulence factor | MAP1272c |
| Fatty acid metabolism | MAP2698c |
| Cellular processes | MAP2487c, MAP3567 |
Proteolytic cleavage of MBP fusion proteins.
A pilot study was performed with MBP fusion proteins (MAP0435c, MAP1846c, and MAP1017c) to determine the optimal time for enzymatic cleavage of the M. avium subsp. paratuberculosis protein from the MBP tag. Factor Xa (Amersham Biosciences) was reconstituted to a final concentration of 1 unit/μl in nuclease-free water at 4°C. MBP fusion proteins were diluted to 1 mg/ml in phosphate-buffered saline (PBS). Factor Xa (1 μl) was added to the MBP fusion protein (100 μl) in a 1.5-ml screw-cap tube and mixed briefly with a vortex mixer. Reaction buffer (100 μl) was added to the mixture, which was vortex mixed briefly and incubated for 16 to 40 h at room temperature (RT) (22 to 24°C). Fifteen-microliter aliquots were collected after 16, 18, 20, 25, 30, 35, and 40 h of incubation for SDS-PAGE analysis. The optimal cleavage time was determined for each protein based on the appearance of two bands that corresponded to the expected molecular masses of MBP and the relevant M. avium subsp. paratuberculosis protein.
Due to the limited volume (500 μl) of MBP fusion proteins, only 22 proteins with volumes and concentrations adequate for cleavage and antigenicity evaluation were available. A reaction mixture containing 1 ml of MBP fusion protein (1 mg), 10 μl (10 units) of factor Xa, and 1 ml of reaction buffer in a 5-ml screw-cap tube was prepared, vortex mixed briefly, and incubated for up to 40 h at RT. Aliquots of 15 μl were collected at various incubation times (16, 18, 20, 25, 30, and 40 h) and examined by SDS-PAGE.
SDS-PAGE analysis.
SDS-PAGE analyses of proteins were performed using 12% precast polyacrylamide gels (Mini-PROTEAN TGX precast gel, product no. 456-1043; Bio-Rad). Briefly, a 15-μl aliquot of each protein sample (MBP-LacZ, MBP-M. avium subsp. paratuberculosis fusion protein, factor Xa-cleaved M. avium subsp. paratuberculosis protein, purified MBP, or purified M. avium subsp. paratuberculosis protein) was placed in a 1.5-ml screw-cap tube. The protein samples were mixed with 3 μl of reducing sample buffer and heated for 5 min in a boiling water bath. Protein samples (18 μl) were loaded onto a precast gel, and electrophoresis was performed at a constant voltage of 180 V (SmartPower 4000 power pack) for 65 min or until the visible line of bromphenol blue reached the bottom of the gel, using a Mini-PROTEAN 3 cell system (Bio-Rad). Protein bands were stained with Coomassie brilliant blue (0.1% Coomassie brilliant blue G-250, 3% ortho-phosphoric acid, 10% ammonium sulfate, and 20% methanol). The protein bands were visualized with a Geldoc system (Bio-Rad).
Affinity chromatography purification of cleaved proteins.
Purification of cleaved MBP fusion proteins was performed with an AKTApurifier fast performance liquid chromatography (FPLC) system (GE Healthcare) using a MBPTrap HP dextran-Sepharose high performance column (5 ml; GE Healthcare), following the manufacturer's manual. Briefly, the column was equilibrated with 5 column volumes (CVs) of binding buffer at a flow rate of 2 ml/min. A cleaved protein sample solution (1 ml) was applied using a 1-ml sample loop, followed by washing with 5 CVs of binding buffer to remove unbound protein (M. avium subsp. paratuberculosis protein), at a flow rate of 5 ml/min. The flowthrough protein peaks were collected in 2-ml fractions. The bound MBP was eluted with 5 CVs of elution buffer at a flow rate of 5 ml/min, and protein peaks were collected in 2-ml fractions. The column was reequilibrated with 5 CVs of binding buffer at a flow rate of 5 ml/min. The cleaved and purified M. avium subsp. paratuberculosis proteins were dialyzed against PBS overnight at 4°C using a 12-kDa-cutoff membrane tube. Proteins were concentrated by centrifuging the dialyzed protein solution at 2,000 × g for 10 min at 4°C (AllegraX-12R; Beckman Coulter), using Amicon Ultra-15 centrifugal filter units (nominal molecular mass limit of 10 kDa). The protein yield in the retentate was estimated using a spectrophotometer (NanoDrop 1000; Thermo Scientific) set at 280 nm.
Serum samples.
A total of 46 sheep serum samples were obtained from the serum archive maintained at the Faculty of Veterinary Science, University of Sydney. Twenty-three serum samples were from unexposed/uninfected sheep from Western Australia (31) that were certified to be free of JD based on the negative test results for their flocks. Another 23 serum samples were obtained from exposed/infected sheep that either tested positive by tissue/fecal culture or had histopathological lesions consistent with ovine JD. Serum samples obtained from infected animals also tested positive with the Institut Pourquier ELISA (32), with a sample-to-positive ratio of >70%, and were categorized as strong reactors (n = 8), medium reactors (n = 8), or low reactors (n = 7). The infected sheep were fecal culture positive (shedder; n = 15) or negative (nonshedder; n = 8). Histopathological lesions were categorized according to a previously described method (33), as follows: no or low-grade lesion (grade 0 to 2; n = 4), paucibacillary lesion (grade 3a plus grade 3c; n = 2 + 8), or multibacillary lesion (grade 3b; n = 9).
Positive- and negative-control sheep sera were obtained from sheep from the University of Sydney that were in an independent experimental infection trial (34). The negative-control serum was obtained from a sheep not exposed to M. avium subsp. paratuberculosis that had tested negative by fecal culture, biopsied tissue culture, histopathological analysis, and ELISA. Positive-control serum was obtained from a Gudair vaccine-immunized sheep, and the serum was determined to be positive by the Institut Pourquier ELISA.
ELISA methodology.
Antibody ELISA was performed to evaluate the seroreactivity of MBP fusion proteins, cleaved M. avium subsp. paratuberculosis proteins, and His-tagged recombinant M. avium subsp. paratuberculosis proteins, using serum samples from M. avium subsp. paratuberculosis-infected and uninfected sheep. Briefly, 50 μl/well of antigen at the specified final concentrations of MBP fusion antigens (see Tables 3 and 4), cleaved M. avium subsp. paratuberculosis antigens (5 μg/ml) (see Table 4), or His-tagged M. avium subsp. paratuberculosis antigens (see Table 5) in carbonate buffer (0.1 M carbonate buffer [pH 9.6]) were coated on 96-well flat-bottom microplates (Nunc MaxiSorp; Nunc) and incubated overnight at 4°C. The plates were machine washed 5 times with purified reverse-osmosis (RO) water with Tween 20. ELISA plates coated with MBP fusion proteins and MBP-LacZ were blocked with 4% skim milk, and plates coated with cleaved M. avium subsp. paratuberculosis proteins and His-tagged M. avium subsp. paratuberculosis proteins were blocked with 1% (vol/vol) fetal calf serum (FCS) for 30 min each.
Table 3.
Antigenicity evaluation of MBP fusion proteins
| Protein | Protein concn (μg/ml) | OD450 (n) for: |
Pc | AUCROC | |
|---|---|---|---|---|---|
| Unexposed samples (23) | Exposed samples (23) | ||||
| MAP0068a | 5 | 0.133 | 0.218 | 0.002 | 0.71 |
| MAP0184ca,b | 10 | 0.215 | 0.355 | 0.002 | 0.745 |
| MAP0187c | 5 | 0.236 | 0.31 | 0.118 | |
| MAP0435c | 20 | 0.278 | 0.291 | 0.797 | |
| MAP0508a,b | 6 | 0.3 | 0.501 | 0.004 | 0.713 |
| MAP0516ca,b | 5 | 0.257 | 0.439 | <0.001 | 0.758 |
| MAP0540 | 7 | 0.298 | 0.36 | 0.238 | |
| MAP0810 | 10 | 0.187 | 0.241 | 0.111 | |
| MAP0834ca,b | 5 | 0.141 | 0.216 | 0.002 | 0.742 |
| MAP1027c | 10 | 0.176 | 0.234 | 0.083 | |
| MAP1272ca,b | 10 | 0.26 | 0.451 | 0.005 | 0.703 |
| MAP1293a,b | 5 | 0.186 | 0.292 | 0.012 | 0.717 |
| MAP1297a,b | 10 | 0.172 | 0.321 | 0.002 | 0.744 |
| MAP1339 | 5 | 0.204 | 0.274 | 0.067 | |
| MAP1560 | 10 | 0.252 | 0.273 | 0.51 | |
| MAP1586 | 5 | 0.285 | 0.311 | 0.515 | |
| MAP1589c | 10 | 0.183 | 0.269 | 0.02 | 0.677 |
| MAP1653b | 10 | 0.195 | 0.263 | 0.045 | 0.683 |
| MAP1834ca,b | 9 | 0.262 | 0.44 | 0.002 | 0.765 |
| MAP1889c | 5 | 0.215 | 0.203 | 0.652 | |
| MAP2058ca,b | 6 | 0.265 | 0.442 | 0.005 | 0.703 |
| MAP2281c | 7 | 0.29 | 0.281 | 0.773 | |
| MAP2411 | 10 | 0.265 | 0.298 | 0.481 | |
| MAP2450ca,b | 8 | 0.202 | 0.293 | 0.045 | 0.677 |
| MAP2487c | 8 | 0.307 | 0.331 | 0.576 | |
| MAP2705c | 10 | 0.208 | 0.228 | 0.541 | |
| MAP2864ca,b | 8 | 0.336 | 0.455 | 0.035 | 0.658 |
| MAP2872ca,b | 8 | 0.166 | 0.289 | <0.001 | 0.765 |
| MAP3007a,b | 7 | 0.211 | 0.357 | 0.003 | 0.74 |
| MAP3200b | 10 | 0.216 | 0.339 | 0.008 | 0.699 |
| MAP3268 | 10 | 0.272 | 0.229 | 0.386 | |
| MAP3393c | 10 | 0.261 | 0.291 | 0.497 | |
| MAP3567a,b | 1 | 0.137 | 0.234 | 0.002 | 0.728 |
| MAP3577 | 7 | 0.194 | 0.215 | 0.465 | |
| MAP3651ca,b | 5 | 0.208 | 0.323 | 0.01 | 0.695 |
| MAP3701c | 10 | 0.166 | 0.198 | 0.274 | |
| MAP3864b | 10 | 0.208 | 0.289 | 0.048 | 0.642 |
| MAP3968a,b | 7 | 0.154 | 0.277 | 0.002 | 0.719 |
| MAP4125 | 10 | 0.284 | 0.292 | 0.847 | |
| MAP4340b | 10 | 0.194 | 0.296 | 0.006 | 0.732 |
Proteins able to produce significantly higher OD values for serum samples obtained from sheep with paucibacillary infections than for serum samples from unexposed sheep.
Proteins able to produce significantly higher OD values for serum samples that were low reactors in the Institut Pourquier ELISA than for serum samples from unexposed sheep.
Mean OD values for the exposed group are significantly higher than those for the unexposed group (P < 0.05).
Table 4.
Antigenicity evaluation of MBP fusion and factor Xa-cleaved proteins
| Protein | Protein concn (μg/ml) | Data for MBP fusion proteins |
Data for factor Xa-cleaved proteins |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OD450 (n) for: |
Pc | AUCROC | OD450 (n) for: |
Pc | AUCROC | ||||
| Unexposed samples (23) | Exposed samples (23) | Unexposed samples (23) | Exposed samples (23) | ||||||
| MAP0593c | 20 | 0.262 | 0.301 | 0.295 | 0.182 | 0.253 | 0.005 | 0.75 | |
| MAP1017c | 10 | 0.203 | 0.266 | 0.046 | 0.687 | 0.206 | 0.314 | 0.005 | 0.753 |
| MAP1588cb | 10 | 0.167 | 0.243 | 0.027 | 0.702 | 0.153 | 0.206 | 0.033 | 0.662 |
| MAP1846cb | 10 | 0.158 | 0.239 | 0.022 | 0.702 | 0.178 | 0.23 | 0.029 | 0.708 |
| MAP1885c | 10 | 0.237 | 0.261 | 0.659 | 0.166 | 0.169 | 0.864 | ||
| MAP2280cb | 10 | 0.196 | 0.279 | 0.027 | 0.702 | 0.239 | 0.344 | 0.002 | 0.786 |
| MAP2698c | 4 | 0.207 | 0.268 | 0.15 | 0.197 | 0.239 | 0.007 | 0.7 | |
| MAP3190a,b | 10 | 0.222 | 0.327 | 0.007 | 0.708 | 0.165 | 0.224 | 0.03 | 0.668 |
| MAP3538b | 10 | 0.172 | 0.247 | 0.022 | 0.703 | 0.188 | 0.264 | 0.001 | 0.792 |
| MAP3555a,b | 10 | 0.272 | 0.448 | 0.002 | 0.736 | 0.203 | 0.311 | 0.002 | 0.738 |
Proteins able to produce significantly higher OD values for serum samples obtained from sheep with paucibacillary infections than for serum samples from unexposed sheep.
Proteins able to produce significantly higher OD values for serum samples that were low reactors in the Institut Pourquier ELISA than for serum samples from unexposed sheep.
Mean OD values for the exposed group are significantly higher than those for the unexposed group (P < 0.05).
Table 5.
Antigenicity evaluation of His-tagged proteins
| Protein | Protein concn (μg/ml) | OD450 for: |
Pa | AUCROC | |
|---|---|---|---|---|---|
| Unexposed samples | Exposed samples | ||||
| MAP1272c | 5 | 0.140 | 0.318 | <0.0001 | 0.90 |
| MAP2487c | 4 | 0.160 | 0.297 | 0.004 | 0.70 |
| MAP2698c | 2 | 0.149 | 0.285 | 0.003 | 0.685 |
| MAP3567 | 0.5 | 0.271 | 0.443 | 0.009 | 0.751 |
Mean OD values for the exposed group are significantly higher than those for the unexposed group (P < 0.05).
The serum samples were diluted (1:100) in a diluent (0.1% [vol/vol] FCS in PBS with Tween 20 [PBST]) containing 1.3 mg/ml heat-killed M. phlei protein (Elizabeth Macarthur Agricultural Institute, New South Wales, Australia) and were absorbed overnight at 4°C, with constant end-to-end shaking. The absorbed serum was centrifuged at 2,500 × g for 10 min at RT to separate the supernatant from the particulate M. phlei. The absorbed serum supernatant (50 μl) was added to the required wells and incubated for 1 h at RT. The plate was machine washed 5 times with RO water with Tween 20 prior to the addition of horseradish peroxidase-labeled mouse anti-sheep IgG monoclonal antibody conjugate (50 μl, 1:40,000, clone GT-34; Sigma, New South Wales, Australia) in diluent (0.1% [vol/vol] FCS in PBST) and then incubated for 1 h at RT. The plate was machine washed as described above, and 100 μl of 3,3′,5′,5′-tetramethylbenzidine (TMB) substrate was added. The plate was incubated for 20 min in the dark, after which the chromogenic reaction was stopped with the addition of stop solution (50 μl of 2 M sulfuric acid). The optical density at 450 nm (OD450) was measured using a plate reader (Multiskan Ascent; Thermo Scientific, Victoria, Australia). Serum samples were also tested with MBP-LacZ (5 μg/ml) to examine seroreactivity to MBP. The ELISA results are presented as mean OD450 values.
Data analysis.
Statistical analysis was performed using GenStat 12.1 (VSN International Ltd., United Kingdom) and GraphPad Prism 4.0 (GraphPad Software Inc., La Jolla, CA) software. The seroreactivities of MBP fusion antigens, factor Xa-cleaved M. avium subsp. paratuberculosis antigens, and His-tagged M. avium subsp. paratuberculosis antigens between groups were compared by analysis of variance (ANOVA) with a Bonferroni correction for multiple comparisons, as described previously (35). The area under the receiver operating characteristic curve (AUCROC) was calculated for the ability of an assay using each antigen to discriminate between uninfected and M. avium subsp. paratuberculosis-infected sheep. An assay with an AUCROC value of 1.0 is considered to be perfect, and one with a value of 0.5 is considered worthless (36).
RESULTS
Cleavage of MBP fusion proteins on a pilot scale.
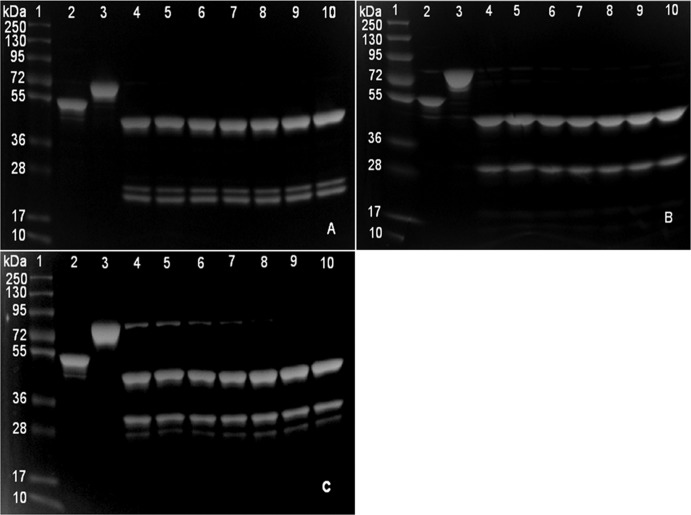
Three MBP fusion proteins were cleaved by factor Xa protease in a pilot study and analyzed by SDS-PAGE (Fig. 1A to C). Factor Xa was able to cleave MBP-MAP0435c and MBP-MAP1017c after 16 h of incubation at RT. Factor Xa was able to cleave MBP-MAP1846 completely only after 30 h of incubation. Cleavage of all three fusion proteins revealed the expected band sizes for MBP (42.5 kDa) and M. avium subsp. paratuberculosis proteins. However, multiple bands were observed for the proteins MAP0435c and MAP1017c. The pilot study showed that different proteins required different cleavage times.
Fig 1.
SDS-PAGE analysis of pilot scale factor Xa cleavage of MBP fusion proteins. (A) MBP-MAP0435c; (B) MBP-MAP1017c; (C) MBP-MAP1846. Lanes 1, molecular mass markers (PageRuler Plus prestained protein ladder); lanes 2, MBP-LacZ (54 kDa); lanes 3, MBP-M. avium subsp. paratuberculosis fusion protein before cleavage; lanes 4 to 10, MBP-M. avium subsp. paratuberculosis fusion proteins cleaved at different time points, i.e., 16 h (lanes 4), 18 h (lanes 5), 20 h (lanes 6), 25 h (lanes 7), 30 h (lanes 8), 35 h (lanes 9), and 40 h (lanes 10).
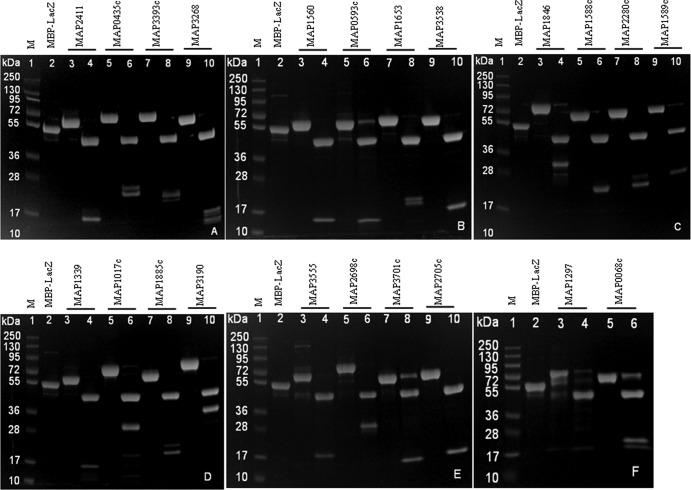
Cleavage of MBP fusion proteins on a large scale.
Twenty-two MBP fusion proteins were cleaved by factor Xa at different incubation times. Eleven proteins were completely cleaved with 16 h of incubation, six proteins required 20 h, and five proteins required 40 h for complete cleavage (Table 2). The SDS-PAGE analyses of cleaved products are shown in Fig. 2A to F. For each MBP fusion protein, the MBP tag was separated from the M. avium subsp. paratuberculosis protein of expected molecular mass. Four proteins, i.e., MBP-MAP0593c, MBP-MAP3701c, MBP-MAP1297, and MBP-MAP0068c, showed weak bands of undigested fusion proteins even with 40 h of incubation. Fusion proteins such as MBP-MAP0435c, MBP-MAP3393c, MBP-MAP3268, MBP-MAP1653, MBP-MAP1846, MBP-MAP2280c, MBP-MAP1885c, and MBP-MAP0068c were found to be cleaved, but there were multiple bands of M. avium subsp. paratuberculosis proteins.
Table 2.
Expected molecular masses of MBP fusion and cleaved proteins
| Cluster | Protein | CTa (h) | Molecular mass (kDa) of: |
|
|---|---|---|---|---|
| Fusion proteinb | M. avium subsp. paratuberculosis protein | |||
| Amino acid metabolism | MAP1297 | 40 | 67.8 | 25.3 |
| MAP1846c | 40 | 73 | 30.5 | |
| Antioxidant enzymes | MAP1588c | 20 | 61.3 | 18.8 |
| MAP1589c | 16 | 64.1 | 21.6 | |
| MAP1653 | 16 | 58.9 | 16.4 | |
| ATP biosynthesis | MAP3393c | 20 | 60 | 17.5 |
| Cell division | MAP0068 | 40 | 60 | 17.5 |
| Cellular processes | MAP1560 | 16 | 57.7 | 15.2 |
| MAP1885c | 16 | 60.9 | 18.4 | |
| MAP2411 | 16 | 58 | 15.5 | |
| MAP2705c | 20 | 56.4 | 13.9 | |
| MAP3538 | 16 | 58.4 | 15.9 | |
| Fatty acid metabolism | MAP1017c | 16 | 70.3 | 27.8 |
| MAP2698c | 20 | 73.9 | 31.4 | |
| MAP3190 | 16 | 75.8 | 33.3 | |
| Heat shock protein | MAP3268 | 16 | 58.9 | 16.4 |
| MAP3701c | 40 | 58.7 | 16.2 | |
| Hypothetical protein | MAP0593c | 40 | 57.3 | 14.8 |
| MAP3555 | 16 | 61.3 | 18.8 | |
| Phosphate metabolism | MAP0435c | 16 | 61.1 | 18.6 |
| Proteolysis | MAP2280c | 20 | 65.7 | 23.2 |
| Universal stress proteins | MAP1339 | 20 | 57.9 | 15.4 |
CT, cleavage time.
Fusion protein mass is the total mass of MBP (42.5 kDa) and the M. avium subsp. paratuberculosis protein.
Fig 2.
SDS-PAGE of factor Xa-cleaved M. avium subsp. paratuberculosis proteins. Lanes 1, molecular mass markers (PageRuler Plus prestained protein ladder); lanes 2, MBP-LacZ; lanes 3, 5, 7, and 9, MBP-M. avium subsp. paratuberculosis fusion proteins; lanes 4, 6, 8, and 10, factor Xa-cleaved MBP and M. avium subsp. paratuberculosis proteins.
SDS-PAGE analysis of cleaved and purified proteins.
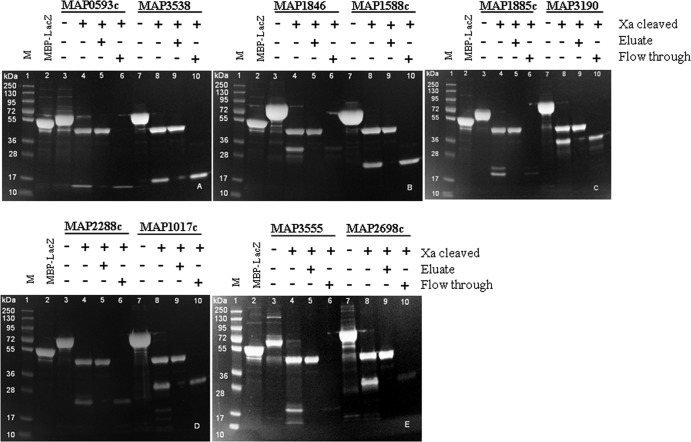
Due to the limited volumes of MBP fusion proteins received from the USDA Agricultural Research Service (500 μl each), only 22 proteins were used for factor Xa cleavage experiments. Of 22 cleaved proteins, only 10 proteins had adequate volumes for affinity liquid chromatography purification.
The flowthrough (cleaved M. avium subsp. paratuberculosis proteins) and eluate (cleaved MBP tag) fractions were verified by SDS-PAGE analysis (Fig. 3A to E). The expected molecular masses are shown in Table 2 and Fig. 3A to E. All of the cleaved M. avium subsp. paratuberculosis proteins were obtained with >95% purity, based on the band appearance on SDS-PAGE analysis. The yields of purified and concentrated M. avium subsp. paratuberculosis proteins were approximately 20 μg/ml (MAP2698c and MAP3555), 30 μg/ml (MAP1846, MAP1885c, and MAP2280c), 40 μg/ml (MAP1017c and MAP0593c), 60 μg/ml (MAP3538), or 70 μg/ml (MAP1588c and MAP3190). Separated bands for cleaved M. avium subsp. paratuberculosis proteins MAP1846, MAP1885c, and MAP3555 were faint, due to the low protein concentrations.
Fig 3.
SDS-PAGE of factor Xa-cleaved and purified MBP fusion proteins. Lanes 1, molecular mass markers (PageRuler Plus prestained protein ladder) (M); lanes 2, MBP-LacZ; lanes 3 to 10, proteins shown as MBP fusion protein and factor Xa-cleaved protein, eluate, and flowthrough fraction.
Antigenicity of MBP fusion proteins.
Fifty MBP fusion proteins were evaluated, and 29 (58%) were found to be detected by antibodies in sera obtained from M. avium subsp. paratuberculosis-infected sheep (Tables 3 and 4). The remaining 42% (21/50) of the proteins were not able to differentiate infected from uninfected animals. The seroreactivity of the control MBP-LacZ was not significantly different in the infected and uninfected groups (P > 0.05). The greatest ability to differentiate the infected group from the uninfected group was observed for the MAP0516c protein, which is encoded by echA20 and is involved in fatty acid metabolism (AUCROC = 0.758, P = 0.001), MAP2872c, which is encoded by fabG5 and is a 3-ketoacylreductase (AUCROC = 0.765, P = 0.001), and MAP1834c, which is encoded by prcA and is a proteome subunit protein (AUCROC = 0.765, P = 0.002).
Among the MBP fusion proteins evaluated, 19/50 (38%) were able to produce significantly higher OD values in serum samples obtained from sheep with paucibacillary infections, compared with sera from unexposed sheep (P < 0.05). Similarly, 26/50 proteins (52%) were able to produce significantly higher OD values in serum samples that were low reactors in the Institut Pourquier ELISA, in comparison with sera from unexposed sheep (P < 0.05) (Tables 3 and 4). However, none of the proteins was able to detect infections that were associated with no or low-grade lesions.
Antigenicity of cleaved and MBP fusion proteins.
Successfully cleaved and purified M. avium subsp. paratuberculosis proteins were evaluated and ELISA results were compared with those for fused forms (Table 4). The OD values of infected sheep sera were significantly higher than those of the uninfected sheep sera for all factor Xa-cleaved proteins except MAP1885c. The MBP fusion proteins MAP0593c and MAP2698c were not able to differentiate between infected and uninfected sheep, but when they were evaluated as cleaved proteins purified from the MBP fusion proteins, the OD values of the infected group were significantly higher (P < 0.05) than those of the uninfected group. Protein MAP1885c was not able to differentiate between the infected and uninfected groups in either the fused or cleaved forms. Based on AUCROC values, factor Xa cleavage did not enhance the immunoreactivity of four proteins (MAP1846, MAP1588c, MAP1885c, and MAP3555). The M. avium subsp. paratuberculosis-specific immunoreactivity of two proteins (MAP2698c and MAP0593c) was enhanced while that of three proteins (MAP3538, MAP1017c, and MAP2280c) was marginally enhanced by cleavage of MBP. In general, the ability of cleaved proteins to differentiate the infected and uninfected groups was enhanced in only 50% of the proteins (5/10 proteins).
Antigenicity evaluation of His-tagged recombinant M. avium subsp. paratuberculosis proteins.
All four His-tagged recombinant M. avium subsp. paratuberculosis proteins were able to differentiate between the infected and uninfected sheep sera (P < 0.05) (Table 5). The order of most antigenic to least antigenic proteins was MAP1272c > MAP3567 > MAP2487c > MAP2698c, with AUCROC values of 0.90, 0.75, 0.70, and 0.69, respectively.
The seroreactivity of two different protein expression systems (His tag and MBP fusion) were compared for MAP2698c, MAP1272c, MAP2487c, and MAP3567. All four proteins were able to differentiate between the infected and uninfected groups of sheep in both expression systems. The OD values obtained from an infected group of sheep using His-tagged recombinant proteins were significantly higher (P < 0.05) than those obtained using the corresponding MBP fusion proteins (Table 6).
Table 6.
Comparison of antigenicity of MBP fusion, His-tagged, and factor Xa-cleaved proteinsa
| Protein | Data by protein type |
|||||
|---|---|---|---|---|---|---|
| MBP fusion |
His tagged |
Factor Xa cleaved |
||||
| P | AUCROC | P | AUCROC | P | AUCROC | |
| MAP1272c | 0.002 | 0.728 | <0.0001 | 0.90 | NEb | NE |
| MAP2487c | 0.331 | NE | 0.004 | 0.697 | NE | NE |
| MAP2698c | 0.150 | NE | 0.003 | 0.685 | 0.007 | 0.70 |
| MAP3567 | 0.005 | 0.703 | 0.009 | 0.751 | NE | NE |
P and AUCROC values are for comparisons between infected and uninfected groups of sheep. Mean OD values for the exposed group are significantly higher than those for the unexposed group (P < 0.05).
NE, not examined.
The ELISA results obtained from the infected sheep using MAP2698c protein as a MBP fusion, factor Xa-cleaved, or His-tagged protein were analyzed by one-way ANOVA with a Bonferroni correction for multiple comparisons. The OD values obtained using factor Xa-cleaved M. avium subsp. paratuberculosis proteins and His-tagged M. avium subsp. paratuberculosis proteins were significantly higher than those obtained with MBP fusion proteins (P < 0.001). The OD values obtained with factor Xa-cleaved M. avium subsp. paratuberculosis proteins and His-tagged M. avium subsp. paratuberculosis proteins were similar (P > 0.05).
DISCUSSION
This study investigated the antigenicity of M. avium subsp. paratuberculosis proteins in either MBP-fused, factor Xa-cleaved, or His-tagged forms, using sera that were known to have M. avium subsp. paratuberculosis-specific antibodies, as detected by the commercial Institut Pourquier ELISA (32). The proportion of antigenic proteins increased from 58% to 62% after factor Xa cleavage. Sufficient amounts of MBP fusion proteins were available for 22 proteins to be cleaved using factor Xa. Ten of 22 proteins were successfully purified and evaluated in both MBP fusion and factor Xa-cleaved forms. Four proteins were evaluated in the His-tagged form to compare the expression systems.
Factor Xa cleaves proteins after the arginine residue in its preferred cleavage site Ile-(Glu or Asp)-Gly-Arg in the fusion link between MBP and the target protein. However, reports suggest that it quite often cleaves at secondary sites, depending on the conformation of the protein substrate (37, 38). The most common secondary site is Gly-Arg, usually in a partially unfolded protein (39). The probable cause of the appearance of multiple bands on SDS-PAGE gels in this study may be proteolysis or cleavage of target proteins at such secondary sites. Furthermore, on screening of the amino acid sequences of proteins that showed multiple bands on SDS-PAGE gels, several such sites were identified in proteins MAP0435c, MAP1846, and MAP1017c. MAP0435c had two Gly-Arg sites (amino acid positions 24-25 and 142-143), MAP1846 had three Gly-Arg sites (amino acid positions 68-69, 99-100, and 159-160), and MAP1017c had one Gly-Arg site (amino acid positions 168-169).
The protein cleavage experiment showed that 1 unit of factor Xa was sufficient to cleave 100 μg of MBP fusion protein after as little as 18 h of incubation at RT (22 to 23°C), and this finding was consistent with the findings from another study (40). However, factor Xa cleavage was not complete even after 40 h of incubation for proteins MBP-MAP0068c, MBP-MAP0593c, MBP-MAP1297, MBP-MAP1846c, and MBP-MAP3701c. The probable causes of incomplete cleavage may be an inaccessible factor Xa recognition site or alteration of the factor Xa recognition site during cloning (41). This process of protein cleavage, and particularly the need for such long cleavage times, is not well understood, and further investigation may be useful.
Factor Xa is a heterodimer protease composed of two disulfide-linked polypeptide chains with apparent molecular masses of 17 and 42 kDa (42). In this study, no obvious bands of these sizes were observed, which may be due to too low a concentration to be detected in SDS-PAGE analysis. Thus, removal of factor Xa using p-aminobenzamidine resin was not necessary. ELISA results for MBP fusion proteins were analyzed using OD values obtained for MBP fusion proteins without subtracting the OD values obtained for MBP-LacZ to allow for a comparative analysis between the MBP fusion proteins and the factor Xa-cleaved forms. This was justified, as levels of seroreactivity to MBP-LacZ were not significantly different between infected and uninfected samples.
The MBP fusion proteins evaluated in this study were previously reported to be differentially regulated under different in vitro stress conditions (8–10). More than one-half of the 50 MBP fusion proteins evaluated were found to be antigenic, suggesting that the majority of these proteins are expressed by M. avium subsp. paratuberculosis in vivo and are recognized by the host immune response. A recent study on the antigenicity of some of these proteins expressed as His-tagged recombinant proteins showed that 66% of the proteins (18/27 proteins) were detected by sera from M. avium subsp. paratuberculosis-infected sheep (13). Of those 27 proteins, 22 were included in this study. The proteins that were found to be antigenic in both studies are MAP0516c, MAP0834c, MAP1846c, MAP2450c, MAP3200, and MAP3555. The proteins that were found to be nonantigenic in both studies are MAP1339, MAP1885c, MAP1889c, MAP2705c, MAP3701c, MAP3577, and MAP4125. The proteins MAP0435c, MAP0593c, MAP1027c, MAP2281c, and MAP2411 were found to be antigenic by Kawaji et al. (13) but were not antigenic in this study. Similarly, proteins MAP0068, MAP2007, MAP2864c, and MAP3864 were found to be antigenic in this study but not by Kawaji et al. (13). Disagreement in the results for the remaining proteins may be attributed to factors such as different types of antigen (His-tagged or MBP fusion proteins) and the animals tested.
The antigenicity evaluation results for MBP fusion proteins MAP2698c and MAP3567 were in agreement with the findings from a previous study on the evaluation of their His-tagged recombinant forms (12). Another recent study reported protein MAP1272c NlpC/P60 to be strongly antigenic in cattle infected with M. avium subsp. paratuberculosis (29). Evaluation of this protein in sheep infected with M. avium subsp. paratuberculosis in this study also showed strong immunoreactivity, suggesting that this protein may be of potential use in the diagnosis of JD in both sheep and cattle. This is the first antigenicity evaluation of the remaining 28 MBP fusion proteins.
Twelve of the 50 MBP fusion proteins included in this study were previously identified to have a high number of conformational B cell epitopes, and so they may be useful for detection of M. avium subsp. paratuberculosis-specific serum antibodies in infected hosts (43). Antigenicity evaluation of these proteins in this study revealed 75% (9/12 proteins) to be antigenic.
The proteins found to be antigenic were from clusters such as amino acid metabolism (four proteins), antioxidant enzymes (four proteins), fatty acid metabolism (six proteins), hypothetical proteins (three proteins), proteolysis (two proteins), two-component response regulators (two proteins), and cellular processes (three proteins), as well as cell wall synthesis, cell division, ATP biosynthesis, signal recognition, and a putative virulence factor (one protein each). These clusters of M. avium subsp. paratuberculosis proteins recognized by the host immune system may be involved in the ability of M. avium subsp. paratuberculosis to evade host defense mechanisms, which may be augmented by in vivo expression. Antioxidant enzymes are important for protecting M. avium subsp. paratuberculosis from the oxidative stress response mounted by the host (10, 44, 45); similarly, proteins involved in fatty acid metabolism play important roles in intracellular survival, growth of mycobacteria, and pathogenicity in mycobacterial infections (46). Proteins and lipoproteins associated with cell wall synthesis and lipid membranes are critical for protecting mycobacteria from damage (47).
In conclusion, this study has identified several stress-regulated M. avium subsp. paratuberculosis proteins as being antigenic in infected sheep. Proteins that were found to be able to detect paucibacillary infections and Institut Pourquier ELISA low reactors may be of potential use in early diagnosis. The proteins that were identified as antigenic in this study (MBP fusion or cleaved) and by Kawaji et al. (His-tagged) (13) may be of potential use in the diagnosis and control of JD. The antigenicity results for cleaved or His-tagged M. avium subsp. paratuberculosis proteins were marginally superior to those for MBP fusion forms. Further evaluation of these proteins using a larger panel of serum samples without the bias of prior positive ELISA results is required to select potentially useful proteins for diagnosis.
ACKNOWLEDGMENTS
This work was supported by Meat and Livestock Australia and by the Cattle Council of Australia, the Sheepmeat Council of Australia, and WoolProducers Australia through Animal Health Australia.
Footnotes
Published ahead of print 16 October 2013
REFERENCES
- 1.Morris CA, Hickey SM, Henderson HV. 2006. The effect of Johne's disease on production traits in Romney, Merino and Merino × Romney-cross ewes. N. Z. Vet. J. 54:204–209 [DOI] [PubMed] [Google Scholar]
- 2.Ott SL, Wells SJ, Wagner BA. 1999. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev. Vet. Med. 40:179–192 [DOI] [PubMed] [Google Scholar]
- 3.Hope AF, Kluver PF, Jones SL, Condron RJ. 2000. Sensitivity and specificity of two serological tests for the detection of ovine paratuberculosis. Aust. Vet. J. 78:850–856 [DOI] [PubMed] [Google Scholar]
- 4.Nielsen SS, Toft N. 2008. Ante mortem diagnosis of paratuberculosis: a review of accuracies of ELISA, interferon-γ assay and faecal culture techniques. Vet. Microbiol. 129:217–235 [DOI] [PubMed] [Google Scholar]
- 5.Yokomizo Y, Merkal RS, Lyle PA. 1983. Enzyme-linked immunosorbent assay for detection of bovine immunoglobulin G1 antibody to a protoplasmic antigen of Mycobacterium paratuberculosis. Am. J. Vet. Res. 44:2205–2207 [PubMed] [Google Scholar]
- 6.Yokomizo Y, Yugi H, Merkal RS. 1985. A method for avoiding false-positive reactions in an enzyme-linked immunosorbent assay (ELISA) for the diagnosis of bovine paratuberculosis. Nihon Juigaku Zasshi 47:111–119 [DOI] [PubMed] [Google Scholar]
- 7.Whittington RJ, Marshall DJ, Nicholls PJ, Marsh IB, Reddacliff LA. 2004. Survival and dormancy of Mycobacterium avium subsp. paratuberculosis in the environment. Appl. Environ. Microbiol. 70:2989–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gumber S, Taylor DL, Marsh IB, Whittington RJ. 2009. Growth pattern and partial proteome of Mycobacterium avium subsp. paratuberculosis during the stress response to hypoxia and nutrient starvation. Vet. Microbiol. 133:344–357 [DOI] [PubMed] [Google Scholar]
- 9.Gumber S, Whittington RJ. 2009. Analysis of the growth pattern, survival and proteome of Mycobacterium avium subsp. paratuberculosis following exposure to heat. Vet. Microbiol. 136:82–90 [DOI] [PubMed] [Google Scholar]
- 10.Kawaji S, Zhong L, Whittington RJ. 2010. Partial proteome of Mycobacterium avium subsp. paratuberculosis under oxidative and nitrosative stress. Vet. Microbiol. 145:252–264 [DOI] [PubMed] [Google Scholar]
- 11.Gumber S, Taylor DL, Whittington RJ. 2009. Evaluation of the immunogenicity of recombinant stress-associated proteins during Mycobacterium avium subsp. paratuberculosis infection: implications for pathogenesis and diagnosis. Vet. Microbiol. 137:290–296 [DOI] [PubMed] [Google Scholar]
- 12.Gurung RB, Purdie AC, Begg DJ, Whittington RJ. 2012. In silico screened Mycobacterium avium subsp. paratuberculosis (MAP) recombinant proteins upregulated under stress conditions are immunogenic in sheep. Vet. Immunol. Immunopathol. 149:186–196 [DOI] [PubMed] [Google Scholar]
- 13.Kawaji S, Gumber S, Whittington RJ. 2012. Evaluation of the immunogenicity of Mycobacterium avium subsp. paratuberculosis (MAP) stress-associated recombinant proteins. Vet. Microbiol. 155:298–309 [DOI] [PubMed] [Google Scholar]
- 14.Banasure KD, Basagoudanavar SH, Chaudhury P, Tiwari V, Parihar NS, Goswami PP. 2001. Identification and characterization of a gene encoding a 35-kDa protein from Mycobacterium avium subspecies paratuberculosis. FEMS Microbiol. Lett. 196:195–199 [DOI] [PubMed] [Google Scholar]
- 15.Bannantine JP, Paulson AL, Chacon O, Fenton RJ, Zinniel DK, McVey DS, Smith DR, Czuprynski CJ, Barletta RG. 2011. Immunogenicity and reactivity of novel Mycobacterium avium subsp. paratuberculosis PPE MAP1152 and conserved MAP1156 proteins with sera from experimentally and naturally infected animals. Clin. Vaccine Immunol. 18:105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bannantine JP, Paustian ML. 2006. Identification of diagnostic proteins in Mycobacterium avium subspecies paratuberculosis by a whole genome analysis approach. Methods Mol. Biol. 345:185–196 [DOI] [PubMed] [Google Scholar]
- 17.Cho D, Sung N, Collins MT. 2006. Identification of proteins of potential diagnostic value for bovine paratuberculosis. Proteomics 6:5785–5794 [DOI] [PubMed] [Google Scholar]
- 18.Hughes V, Bannantine JP, Denham S, Smith S, Garcia-Sanchez A, Sales J, Paustian ML, McLean K, Stevenson K. 2008. Immunogenicity of proteome-determined Mycobacterium avium subsp. paratuberculosis-specific proteins in sheep with paratuberculosis. Clin. Vaccine Immunol. 15:1824–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koets AP, Rutten V, Hoek A, Bakker D, van Zijderveld F, Muller KE, van Eden W. 1999. Heat-shock protein-specific T-cell responses in various stages of bovine paratuberculosis. Vet. Immunol. Immunopathol. 70:105–115 [DOI] [PubMed] [Google Scholar]
- 20.Leroy B, Viart S, Trinchero N, Roupie V, Govaerts M, Letesson JJ, Huygen K, Wattiez R. 2009. Use of Mycobacterium avium subsp. paratuberculosis specific coding sequences for serodiagnosis of bovine paratuberculosis. Vet. Microbiol. 135:313–319 [DOI] [PubMed] [Google Scholar]
- 21.Mullerad J, Michal I, Fishman Y, Hovav AH, Barletta RG, Bercovier H. 2002. The immunogenicity of Mycobacterium paratuberculosis 85B antigen. Med. Microbiol. Immunol. 190:179–187 [DOI] [PubMed] [Google Scholar]
- 22.Olsen I, Boysen P, Kulberg S, Hope JC, Jungersen G, Storset AK. 2005. Bovine NK cells can produce gamma interferon in response to the secreted mycobacterial proteins ESAT-6 and MPP14 but not in response to MPB70. Infect. Immun. 73:5628–5635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bannantine JP, Hansen JK, Paustian ML, Amonsin A, Li LL, Stabel JR, Kapur V. 2004. Expression and immunogenicity of proteins encoded by sequences specific to Mycobacterium avium subsp. paratuberculosis. J. Clin. Microbiol. 42:106–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachdev D, Chirgwin JM. 2000. Fusions to maltose-binding protein: control of folding and solubility in protein purification. Methods Enzymol. 326:312–321 [DOI] [PubMed] [Google Scholar]
- 25.Bannantine JP, Rosu V, Zanetti S, Rocca S, Ahmed N, Sechi LA. 2008. Antigenic profiles of recombinant proteins from Mycobacterium avium subsp. paratuberculosis in sheep with Johne's disease. Vet. Immunol. Immunopathol. 122:116–125 [DOI] [PubMed] [Google Scholar]
- 26.Pediaditakis P, Monga SPS, Mars WM, Michalopoulos GK. 2002. Differential mitogenic effects of single chain hepatocyte growth factor (HGF)/scatter factor and HGF/NK1 following cleavage by factor Xa. J. Biol. Chem. 277:14109–14115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu D, Zou L, Li W, Wang L, Wu Y. 2009. High-level expression and large-scale preparation of soluble HBx antigen from Escherichia coli. Biotechnol. Appl. Biochem. 54:141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasek M, Boeggeman E, Ramakrishnan B, Qasba PK. 2010. Galectin-1 as a fusion partner for the production of soluble and folded human β-1,4-galactosyltransferase-T7 in E. coli. Biochem. Biophys. Res. Commun. 394:679–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bannantine JP, Lingle CK, Stabel JR, Ramyar KX, Garcia BL, Raeber AJ, Schacher P, Kapur V, Geisbrecht BV. 2012. MAP1272c encodes an NlpC/P60 protein, an antigen detected in cattle with Johne's disease. Clin. Vaccine Immunol. 19:1083–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bannantine JP, Stabel JR, Bayles DO, Geisbrecht BV. 2010. Characteristics of an extensive Mycobacterium avium subspecies paratuberculosis recombinant protein set. Protein Expr. Purif. 72:223–233 [DOI] [PubMed] [Google Scholar]
- 31.Ellis TM, Norris RT, Martin P, Casey RH, Hawkins CD. 1998. Evidence for freedom from Johne's disease in cattle and goats in Western Australia. Aust. Vet. J. 76:630–633 [DOI] [PubMed] [Google Scholar]
- 32.Gumber S, Eamens G, Whittington RJ. 2006. Evaluation of a Pourquier ELISA kit in relation to agar gel immunodiffusion (AGID) test for assessment of the humoral immune response in sheep and goats with and without Mycobacterium paratuberculosis infection. Vet. Microbiol. 115:91–101 [DOI] [PubMed] [Google Scholar]
- 33.Perez V, Marin JFG, Badiola JJ. 1996. Description and classification of different types of lesion associated with natural paratuberculosis infection in sheep. J. Comp. Pathol. 114:107–122 [DOI] [PubMed] [Google Scholar]
- 34.Begg DJ, de Silva K, Di Fiore L, Taylor DL, Bower K, Zhong L, Kawaji S, Emery D, Whittington RJ. 2010. Experimental infection model for Johne's disease using a lyophilised, pure culture, seedstock of Mycobacterium avium subspecies paratuberculosis. Vet. Microbiol. 141:301–311 [DOI] [PubMed] [Google Scholar]
- 35.Burton AB, Wagner B, Erb HN, Ainsworth DM. 2009. Serum interleukin-6 (IL-6) and IL-10 concentrations in normal and septic neonatal foals. Vet. Immunol. Immunopathol. 132:122–128 [DOI] [PubMed] [Google Scholar]
- 36.Zweig MH, Campbell G. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39:561–577 [PubMed] [Google Scholar]
- 37.Eaton D, Rodriguez H, Vehar GA. 1986. Proteolytic processing of human factor VIII: correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry 25:505–512 [DOI] [PubMed] [Google Scholar]
- 38.Nagai K, Perutz MF, Poyart C. 1985. Oxygen binding properties of human mutant hemoglobins synthesized by Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 82:7252–7255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang X, Crute BE, Sun C, Tang YY, Kelley JJ, III, Lewis AF, Hartman KI, Laue TM, Speck NA, Bushweller JH. 1998. Overexpression, purification, and biophysical characterization of the heterodimerization domain of the core-binding factor β subunit. J. Biol. Chem. 273:2480–2487 [DOI] [PubMed] [Google Scholar]
- 40.Ludeman JP, Pike RN, Bromfield KM, Duggan PJ, Cianci J, Le Bonniec B, Whisstock JC, Bottomley SP. 2003. Determination of the P1′, P2′ and P3′ subsite-specificity of factor Xa. Int. J. Biochem. Cell Biol. 35:221–225 [DOI] [PubMed] [Google Scholar]
- 41.He M, Jin L, Austen B. 1993. Specificity of factor Xa in the cleavage of fusion proteins. J. Protein Chem. 12:1–5 [DOI] [PubMed] [Google Scholar]
- 42.Himmelspach M, Pfleiderer M, Fischer BE, Plaimauer B, Antoine G, Falkner FG, Dorner F, Schlokat U. 2000. Recombinant human factor X: high yield expression and the role of furin in proteolytic maturation in vivo and in vitro. Thromb. Res. 97:51–67 [DOI] [PubMed] [Google Scholar]
- 43.Gurung RB, Purdie AC, Begg DJ, Whittington RJ. 2012. In silico identification of epitopes in Mycobacterium avium subsp. paratuberculosis proteins that were upregulated under stress conditions. Clin. Vaccine Immunol. 19:855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zahrt TC, Deretic V. 2002. Reactive nitrogen and oxygen intermediates and bacterial defenses: unusual adaptations in Mycobacterium tuberculosis. Antioxid. Redox Signal. 4:141–159 [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Tu ZJ, Coussens PM, Kapur V, Janagama H, Naser S, Sreevatsan S. 2008. Transcriptional analysis of diverse strains Mycobacterium avium subspecies paratuberculosis in primary bovine monocyte derived macrophages. Microbes Infect. 10:1274–1282 [DOI] [PubMed] [Google Scholar]
- 46.Dyer DH, Lyle KS, Rayment I, Fox BG. 2005. X-ray structure of putative acyl-ACP desaturase DesA2 from Mycobacterium tuberculosis H37Rv. Protein Sci. 14:1508–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He Z, De Buck J. 2010. Localization of proteins in the cell wall of Mycobacterium avium subsp. paratuberculosis K10 by proteomic analysis. Proteome Sci. 8:21. 10.1186/1477-5956-8-21 [DOI] [PMC free article] [PubMed] [Google Scholar]