Abstract
Human TRIT1 is a tRNA isopentenyltransferase (IPTase) homologue of Escherichia coli MiaA, Saccharomyces cerevisiae Mod5, Schizosaccharomyces pombe Tit1, and Caenorhabditis elegans GRO-1 that adds isopentenyl groups to adenosine 37 (i6A37) of substrate tRNAs. Prior studies indicate that i6A37 increases translation fidelity and efficiency in codon-specific ways. TRIT1 is a tumor suppressor whose mutant alleles are associated with cancer progression. We report the systematic identification of i6A37-containing tRNAs in a higher eukaryote, performed using small interfering RNA knockdown and other methods to examine TRIT1 activity in HeLa cells. Although several potential substrates contained the IPTase recognition sequence A36A37A38 in the anticodon loop, only tRNASerAGA, tRNASerCGA, tRNASerUGA, and selenocysteine tRNA with UCA (tRNA[Ser]SecUCA) contained i6A37. This subset is a significantly more restricted than that for two distant yeasts (S. cerevisiae and S. pombe), the only other organisms comprehensively examined. Unlike the fully i6A37-modified tRNAs for Ser, tRNA[Ser]SecUCA is partially (∼40%) modified. Exogenous selenium and other treatments that decreased the i6A37 content of tRNA[Ser]SecUCA led to increased levels of the tRNA[Ser]SecUCA. Of the human mitochondrion (mt)-encoded tRNAs with A36A37A38, only mt tRNAs tRNASerUGA and tRNATrpUCA contained detectable i6A37. Moreover, while tRNASer levels were unaffected by TRIT1 knockdown, the tRNA[Ser]SecUCA level was increased and the mt tRNASerUGA level was decreased, suggesting that TRIT1 may control the levels of some tRNAs as well as their specific activity.
INTRODUCTION
Translation of mRNA should be faithful in order to produce functional proteins, as codon misreading can lead to misfolding and aggregation, yet quality control must be balanced with speed and efficiency in fast-growing cells (1–3). Modifications of the tRNA anticodon loop at positions 34 and 37 contribute to efficiency and fidelity in a codon-specific manner. In addition, these modifications can extend or restrict the potential for wobble base recognition of cognate and noncognate codons and thus contribute to deciphering the genetic code (4, 5). A deficiency of different types of tRNA wobble base U34 modification can have different mRNA-specific effects, with consequent effects on phenotypes, as different subsets of mRNAs are overenriched in the cognate codons (6–8). A link between tRNA modification and specific mRNA subsets was recently extended to the A37 position in the anticodon loop (9).
Three types of complex modifications can exist at position 37: N6-isopentenyladenosine (i6A37); N6-threonylcarbamoyladenosine (t6A37) or its cyclized derivative, ct6A, in archaea and bacteria; and wybutosine (yW37). These are present in distinct subsets of tRNAs in all three domains of life (10–12). The importance of the A37 modification in mammals is illustrated by tRNALysUUU, which contains a methyl-sulfur derivative of t6A37, ms2t6A37. Cdkal1 was identified as an enzyme of unknown function that was associated with diabetes risk. It was later found to be the enzyme that adds the methylsulfur group to t6A37 of tRNALysUUU and that its deletion from pancreatic β cells causes type 2 diabetes in mice (13, 14).
Evidence from the fission yeast Schizosaccharomyces pombe indicates that i6A37 increases the specific activity of its tRNAs, increasing fidelity at cognate codons, decreasing fidelity at noncognate codons, and promoting the translation efficiency of abundant mRNAs that bear an overabundance of cognate codons (9). Although i6A37 or its derivatives are found on subsets of tRNAs in Bacteria, Archaea, and Eucarya, the subsets differ significantly, in some cases even among related species. In bacteria, ms2i6A37 or ms2i(o)-i6A37 versions of i6A37 are found on tRNAs for UNN codons, Cys, Leu, Phe, Ser, Trp, and Tyr (15, 16), as this modification helps stabilize the weak U·A codon-anticodon pair in all three tRNA binding sites of the bacterial ribosome (17). I6A37 appears to be more restricted in eukaryotes and is found on Ser, Tyr, and sometimes Cys or Trp tRNAs. tRNATrpCCA has i6A37 in S. pombe but not in Saccharomyces cerevisiae, whereas tRNACysGCA has i6A37 in S. cerevisiae but not in S. pombe (18). This species specificity can be accounted for by two variables: (i) whether or not the tRNA bears the isopentenyltransferase (IPTase) recognition sequence A36A37A38 in the anticodon loop and (ii) differences among the fine specificities of the IPTases (18). An example of the first is that S. pombe tRNACysGCA contains A36G37A38 and is not a substrate, whereas S. cerevisiae tRNACysGCA has A36A37A38 and is a substrate (the boldface G37 and A37 indicate the different sequences between S. pombe and S. cerevisiae). An example of the second variable is that while tRNATrpCCA has A36A37A38, it is not modified by Mod5 but is modified by Tit1 (18). Thus, while A36A37A38 appears to be sufficient for modification by Tit1, it is not sufficient for modification by Mod5. Substitution of a single nucleotide at position 34 of tRNATrpCCA upstream of A36A37A38 converted it to an efficient substrate of Mod5 (18), consistent with a cocrystal structure that shows that Mod5 contacts the position 34 nucleotide in the anticodon loop of its substrate (19). Thus, although potential IPTase substrates may be identified by database searches for A36A37A38, whether or not they are modified by their IPTase must be experimentally determined.
TRIT1 appears to be a tumor suppressor (20). In lung tumors examined, TRIT1 mRNA levels were 6- to 14-fold lower than those in healthy lung (20). The tumorigenic potential of A549 cells in nude mice was reduced by ectopic expression of TRIT1 (20), and genetic linkage disequilibrium refined a region responsible for cancer progression on chromosome 1p34 that includes a TRIT1 rare mutation allele associated with poor survival in some ethnic groups (21). Human TRIT1 was confirmed to be an IPTase by complementation of a suppressor tRNASerUCA-mediated phenotype in mod5-deficient S. cerevisiae (20) and by a direct IPTase activity assay of recombinant TRIT1 protein (18).
Selenocysteine tRNA (tRNA[Ser]Sec) is the only tRNA known to contain i6A37 in mammals (22, 23). Human TRIT1 has not been examined for its activity with native tRNA[Ser]Sec and other potential human tRNA substrates. We used small interfering RNA (siRNA)-mediated knockdown of HeLa cell TRIT1 as part of a systematic search for potential substrates. Compared to S. cerevisiae and S. pombe, the only other organisms that have been systematically examined for i6A37, human has the most limited subset of cytosolic i6A37-containing tRNAs, comprised of the three tRNAs (tRNASerUGA, -AGA, and -CGA) and tRNA[Ser]Sec, and tRNA[Ser]Sec is relatively hypomodified (∼40%). Further, we found that the levels of some tRNAs are differentially increased or decreased in TRIT1-deficient cells.
MATERIALS AND METHODS
siRNA-mediated TRIT1 knockdown.
One day before transfection, 1 × 106 HeLa cells were seeded in 10-cm2 culture plates. Three siRNA duplexes against TRIT1 (Trifecta kit; IDT Technologies) were mixed equally, and 24 pmol was transfected per plate using the Oligofectamine reagent (Invitrogen) according to the manufacturer's instructions. Mock transfections with Oligofectamine but no siRNA and with NC1 nontargeting siRNA (IDT Technologies) were used as controls. At 72 h posttransfection, the cells were harvested, RNA was isolated using the TRIzol reagent (Invitrogen), and protein was isolated separately to confirm TRIT1 knockdown by immunoblot analysis with anti-TRIT1 (GTX120508; Genetex).
The siRNA sequences against TRIT1 (TRIT1 siRNA; Trifecta kit) were as follows: NC1 (control siRNA), 5′-CGUUAAUCGCGUAUAAUACGCGUAT-3′ (accession number NM_017646); duplex 1 (in the 3′ untranslated region [UTR]), 5′-AGCUGUGACAUAGGACUUGAAGACC-3′ (accession number NM_017646); duplex 2 (in the 3′ UTR), 5′-CCAAACUAGUUCUCAGAAUUCUACA-3′ (accession number NM_017646), and duplex 3 (in the protein coding DNA sequence), 5′-GACCAUAACAAAGAACCUAAAGAGA-3′.
Preparation of recombinant TRIT1 protein.
The cDNA open reading frame clone of human TRIT1 was obtained from OriGene (catalog number RC210476), PCR amplified, and cloned into the pET15b vector with a His tag at the N terminus. Escherichia coli BL21 Rosetta 2(DE3)/pLysS was transformed with pET15b-TRIT1, and the cells were cultured in LB medium with ampicillin at 37°C. When the culture reached an A600 of 0.5, 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added for 5 h. His-tagged TRIT1 was purified using Talon metal affinity resin. Fractions containing His-TRIT1 were collected and dialyzed in 50 mM Tris-Cl, pH 8.0, 200 mM NaCl, 2 mM dithiothreitol. The dialyzed protein was mixed with an equal volume of glycerol and stored at −20°C.
An in vitro isopentenylation assay was carried out using recombinant His-TRIT1, [14C]dimethylallyl pyrophosphate ([14C]DMAPP), and total RNA from HeLa cells or synthetic RNA from Integrated DNA Technologies (IDT), as described previously (18). For the two-part reaction for tRNA[Ser]Sec, 100 nmol unlabeled DMAPP was used.
PHA6 assay.
In the (positive hybridization in the absence of i6A (PHA6) assay, anticodon loop (ACL) and body probes were designed to hybridize to the anticodon stem-loop (ASL) and TψC region of tRNAs, respectively. Denaturing, high-resolution polyacrylamide-urea gels were stained with ethidium bromide, photographed, and then blotted onto GeneScreen plus, cross-linked with UV light (Stratalizer), and vacuum baked. Blots were preincubated with 15 ml of hybridization buffer (2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.2% SDS, 1× Denhardt's solution [catalog number 750018; Life Technologies], 100 μg/ml yeast RNA [catalog number AM7118; Ambion]) at the oligonucleotide DNA probe hybridization temperature for 1 to 2 h. 32P-end-labeled antisense DNA oligonucleotide at 1.6 × 1010 cpm/μg DNA was added to the hybridization buffer (final concentration, 105 cpm/ml) and left overnight (12 to 16 h) for hybridization at the melting temperature (Tm) minus 15°C, where Tm is equal to 16.6 log[M] + 0.41[Pgc] + 81.5 − Pm − (B/L) − 0.65, where M is the molar salt concentration, Pgc is the percent G+C content in the oligonucleotide DNA probe, Pm is the percentage of mismatched bases, if any, B is 675, and L is the oligonucleotide DNA probe length (24). The blot was washed with 15 ml of 2× SSC, 0.2% SDS four times at room temperature (10 min each time). A final wash of 20 ml was performed at the hybridization temperature for 20 min. Before the next hybridization, the blot was stripped with 0.1× SSC, 0.1% SDS at 95°C, and the nearly complete removal (≥90%) of 32P was confirmed by phosphorimager analysis.
RNase H-mediated elimination of specific tRNA species.
For most tRNAs, 25-mer antisense oligonucleotide DNAs complementary to positions 47 to 23 of the tRNA were used with RNase H as described previously (18). For tRNA[Ser]Sec elimination, the antisense oligonucleotides were directed to the region from positions 86 to 69 at the 3′ end of the tRNA. The oligonucleotides used in this study are described in Table 1.
Table 1.
Oligonucleotides used
| Purpose and DNA oligonucleotide | Sequence |
|---|---|
| Probes | |
| Ser AGA ACL | CCAATGGATTTCTAGTCCATCGC |
| Ser AGA body | GCAGGATTCGAACCTGCGCGG |
| Ser CGA ACL | CCCATTGGATTTCGAGTCCAACGCCT |
| Ser CGA body | AGCAGGATTCGACCTGCGCGGGG |
| Ser UGA ACL | CCCATTGGATTTCAAGTCCAACGC |
| Ser UGA body | GCAGGATTCGAACCTGCGCGGG |
| Leu CAA ACL | CCATTCAGATTTTGAGACTGATGCG |
| Leu CAA body | CCTCTGAGGCTTGAACTCAG |
| Leu UAA-1 ACL | GATTATAAAATTTTAAGTTTTATGC |
| Leu UAA-1 body | GTTAAGAAGAGGAGTTGAACC |
| Leu UAA-2 ACL | TTATAAAGTTTTAAGTCTTATG |
| Leu UAA-2 body | GTTAATGTGAGGAGTTGTACC |
| Sec ACL | GCTACAGGTTTGAAGCCTGCACC |
| Sec body | CAAAGGTGGAATTGAACCACTC |
| Sec-3 body | CACAAAGGGACTCAAACC |
| mt Ser UGA ACL | CAAAGCTGGTTTCAAGCCAACCCC |
| mt Ser UGA body | AAGGAAGGAATCGAACCCCCC |
| mt Trp UCA ACL | CTGAGGGCTTTGAAGGCTCTTGG |
| mt Trp UCA body | GAAATTAAGTATTGCAACTTAC |
| mt Phe GAA ACL | GTCTAAACATTTTCAGTGTATTGC |
| mt Phe GAA body | GTGTTTATGGGGTGATGTGATGTGAGCC |
| mt Leu UUA ACL | GACTGTAAAGTTTTAAGTTTTATGC |
| mt Leu UUA body | GTTAAGAAGAGGAATTGAACCTC |
| mt Tyr GUA ACL | TCTTTAGATTTACAGTCCAATGC |
| mt Tyr GUA body | GGTAAAAAGAGGCCTAACCCCTG |
| mt Cys GCA ACL | TCTTCGAATTTGCAATTCAATATG |
| mt Cys GCA body | AGCCCCGGCAGGTTTGAAGCT |
| Antisense to eliminate tRNAs with RNase H | |
| mt Ser RNase H | GGCAAAAAAGGAAGGAATCGAACCCCCCAAAG |
| mt Trp RNase H | GGCAGAAATTAAGTATTGCAACTTACTG |
| mt Phe RNase H | GGTGTTTATGGGGTGATGTGAGCCCGTCTA |
| mt Leu RNase H | GGTGTTAAGAAGAGGAATTGAACCTCTGACTG |
| mt Tyr RNase H | GGTGGTAAAAAGAGGCCTAACCCCTGTCT |
| mt Cys RNase H | GGAAGCCCCGGCAGGTTTGAAGCTGCTCT |
| cyto-Leu CAA RNase H | CCCCTCTGAGGCTTGAACTCAGGACCATTC |
| cyto-Leu UAA-1 RNase H | AGAAGAGGAGTTGAACCTCTGATTATAAA |
| cyto-Leu UAA-2 RNase H | TGATTATAAAGTTTTAAGTCTTATGCAA |
| cyto-Cys RNase H | GGGGGCACCTGGATTTGAATCAGGGACCTC |
| Sec-1 AAS/RNase H | GCCCGAAAGGTGGAATTG |
| Sec-2 AAS/RNase H | CCTACAAAGGTGGAATTG |
| Subcloning | |
| TRIT1 clone NdeI forward primer | AATCGATA CAT ATG GCG TCC GTG GCG GCT GC |
| TRIT1 clone XhoI reverse primer | TACGTACG CTC GAG TTA AAC GCT GCA TTT CAG C |
| In vitro modification oligonucleotides | |
| Ser AGA RNA | rG rArUrG rGrArC rUrArG rArArA rUrCrC rArUrC |
| Ser CGA RNA | rG rUrUrG rGrArC rUrCrG rArArA rUrCrC rArA rC |
| Ser UGA RNA | rG rArUrG rGrArC rUrUrG rArArA rUrCrC rArUrC |
| Leu CAA RNA | rG rUrCrArG rUrCrU rCrArA rArArU rCrUrGrA rC |
| Leu UAA-1 RNA | rG rUrArArArA rCrUrU rArArArA rUrUrUrUrArC |
| Leu UAA-2 RNA | rG rUArA rGrArC rUrUrA rArArA rCrUrU rUrArC |
| Cys GCA RNA | rG rUrUrU rGrArC rUrGrC rArArA rUrCrA rArGrC |
| Sec UCA RNA | rG rUrGrC rArGrG rCrUrU rCrArA rArCrC rUrGrU rArC |
Effects of selenium (Se) on tRNA[Ser]Sec levels.
Sodium selenite (catalog number 214485; Sigma) solution was freshly prepared and filter sterilized prior to addition to the growth medium. Cells were harvested after 48 h, and RNA was isolated and analyzed.
RESULTS
Human tRNAome analysis identifies a limited set of tRNAs with i6A37.
The total tRNA gene composition of a genome is known as the tRNAome and can be highly variable even among related species (25–27). In all bacteria, archaea, and eukaryotes examined, the only tRNAs that have been found to contain i6A37 are those that decode UNN codons and therefore contain anticodon base A36; these are for the codons for Phe, Leu, Ser, Tyr, Cys, and Trp. tRNAs with U36 also form weak A·U base pairs with their codons, but these are modified with threonylcarbamoyl-A37 (t6A37), e.g., human tRNALysUUU, to stabilize this interaction (also see references 12 and 28). All genes encoding tRNAs that have been found to contain i6A37 bear AAA at positions 36 to 38, and this motif has been shown to be a common specificity determinant for the IPTases from different species (29, 30).
We examined the gene sequences for all human tRNAs for the potential for i6A37 modification. The 506 annotated human tRNA genes (26) collectively produce 48 tRNA anticodon families that decode all of the 61 codons for the standard 20 amino acids. Thirteen codons have no tRNA that recognizes them by direct Watson-Crick base pairing and must rely on wobble decoding. Excluding these, the human tRNAome contains large inequalities in the gene copy numbers, from 1 to 32, a range not too dissimilar from that for other mammals (26). Table 2 reports that all of the human tRNA genes for Phe, Tyr, and Trp encode G at position 37, and therefore, none of these can be a substrate for i6A37 modification. As will be shown below, all of the genes for Ser tRNAs that decode UNN codons do indeed contain i6A37. Table 2 also shows that only 1 of the 7 genes for tRNALeuCAA and 2 of the 7 genes for tRNALeuUAA bear A37. Similarly, only 1 of the 30 genes for tRNACysGCA bears A37.
Table 2.
Human genes in human genome 19 build encoding cytosolic tRNAs with A in position 36 and their base identities at positions 37 and 38
| Anticodon family | Nucleotide at position: |
No. of genes |
Candidate for i6A37 | |||
|---|---|---|---|---|---|---|
| 36 | 37 | 38 | Total | With AAA | ||
| tRNASerAGA | A | A | A | 10 | 10 | Yes |
| tRNASerAGA | A | G | A | 1 | 0 | No |
| tRNASerUGA | A | A | A | 5 | 5 | Yes |
| tRNASerCGA | A | A | A | 4 | 4 | Yes |
| tRNASecUCA | A | A | A | 2 | 2 | Yes |
| tRNALeuUAA | A | A | A | 2 | 2 | Yesa |
| tRNALeuUAA | A | G | A | 5 | 0 | No |
| tRNALeuCAA | A | A | A | 1 | 1 | Yesb |
| tRNALeuCAA | A | G | U | 6 | 0 | No |
| tRNACysGCA | A | A | A | 1 | 1 | Maybec |
| tRNACysGCA | A | G | A | 29 | 0 | No |
| tRNAPheGAA | A | G | A | 12 | 0 | No |
| tRNATyrGUA | A | G | A | 14 | 0 | No |
| tRNATyrAUA | A | G | G | 1 | 0 | No |
| tRNATrpCCA | A | G | A | 9 | 0 | No |
While one of these, tRNALeuUAA-1, was found to be in the i6A37-unmodified form, the other, tRNALeuUAA-2, was undetectable in any form in HeLa cells.
While this is a potential candidate, it was found to be in the i6A37-unmodified form.
Whether this is modified or not was not determined.
We previously reported that while S. pombe Tit1 was inactive on synthetic substrates containing AAY, it was active for AAG but that no tRNAs that contain A36A37G38 exist among the 186 S. pombe tRNA sequences (18). In light of this specificity and the limited set of AAA-containing substrates that we found for TRIT1, we considered the (unlikely) possibility that human tRNAs other than ones with A at position 38 are substrates for i6A37. We report here the same for TRIT1, i.e., inactivity on synthetic substrates containing AAY and activity on synthetic substrates containing AAG, but that no tRNAs that contain A36A37G38 exist among the 506 human tRNA sequences. Further, we found that there are no genes for tRNAs with AAG, AAC, or AAU at positions 36 to 38 in the human genome. Thus, tRNA sequences with A36 and A37 always have A at position 38. This is also true for E. coli tRNA gene sequences (not shown) and may reflect negative selection.
siRNA-mediated knockdown of TRIT1 leads to tRNAs lacking i6A37.
Compared to mock- and control siRNA-treated HeLa cells, there was an efficient decrease in TRIT1 protein levels in cells treated with siRNA against TRIT1 (Fig. 1A). tRNAs that are hypomodified due to a deficiency of IPTase activity in vivo can be modified in vitro using recombinant IPTase and [14C]DMAPP (18). When this assay was carried out on total RNA isolated from wild-type S. pombe or S. cerevisiae, there was no detectable transfer of the [14C]dimethylallyl (isopentenyl) pyrophosphate group to any RNA because the tRNAs were saturated with i6A37 (18). When the assay was performed on total RNA from S. pombe or S. cerevisiae with deletion of their IPTase, multiple tRNAs of different sizes were labeled with [14C]i6A37 (18). The in vitro 14C isopentenylation assay on total RNA from control and TRIT1 knockdown cells produced two unexpected results (Fig. 1B and C). First, two [14C]RNA bands were present in mock- and control siRNA-treated cells, a fast-migrating band, designated a, of about ∼70 nucleotides (nt) and a slow-migrating band, designated b, in the 18S-28S rRNA region (Fig. 1C, lanes 1 to 3). The reaction in which TRIT1 was omitted produced no bands, as expected (Fig. 1C, lane 4). The ability to accept the transfer of 14C indicates that these two unidentified RNAs are unmodified in vivo but upon purification become TRIT1 substrates in vitro. The second unexpected feature of Fig. 1C was that, unlike S. cerevisiae and S. pombe RNAs, which produced multiple bands corresponding to different tRNA substrates (18), only one tRNA-sized band was specific to the TRIT1 knockdown cells (lane 3). The size of this substrate at ∼90 nt suggests that it is a class II tRNA with a large variable arm, most likely tRNA(s)Ser. A conclusion is that siRNA-mediated knockdown of TRIT1 led to hypomodification of its tRNA substrate(s).
Fig 1.
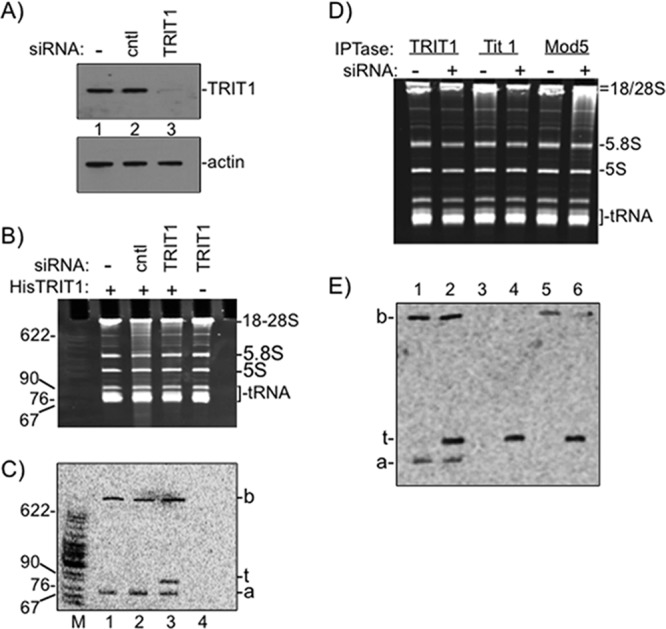
siRNA-mediated TRIT1 knockdown identifies its human tRNA substrates. (A) TRIT1 knockdown in HeLa cells was confirmed by immunoblotting. (Top) anti-TRIT1; (bottom) antiactin. cntl, control. (B and C) In vitro i6A37 modification by recombinant His-TRIT1 and [14C]DMAPP. (B) Ethidium bromide-stained total RNA after in vitro modification; RNA species are indicated to the right. Lane M, size markers, with sizes (in nucleotides) indicated to the left. (C) Autoradiogram of the gel in panel B after drying and detection by phosphorimaging. The a, b, and t bands are indicated to the right (see the text). (D and E) Three IPTases modify the t RNA band. RNAs from HeLa control (lanes −) or TRIT1 siRNA-treated (lanes +) cells were subjected to in vitro modification by the recombinant IPTases indicated above the lanes together with [14C]DMAPP. (D) Ethidium bromide-stained gel; (E) autoradiogram; the a, b, and t bands are indicated.
As noted in the introduction, IPTases from different yeast species can exhibit different substrate specificities for tRNAs with the A36A37A38 motif (18). To further characterize the substrates derived from HeLa RNA, we subjected them to parallel modification reactions that differed only in the source of the IPTase (Fig. 1D and E). All three IPTases modified the genuine tRNA candidate band, designated t, as a major substrate (Fig. 1E, lanes 2, 4, and 6) but differed in recognition of the other bands. While TRIT1 modified the a- and b-band in vitro substrates, Tit1 did not modify either one, and Mod5 modified the b band, albeit less efficiently than TRIT1, but not the a band (Fig. 1E). Although we noted less b band in lane 6 than lane 5, suggesting that it may not compete well against the t band for Mod5, the enzymological basis of the differences in these IPTases has not been determined.
We emphasize that the a and b bands appear to be artifacts of the in vitro assay, as the data indicate that these are not TRIT1 substrates in vivo. Nonetheless, we used a previously described assay with antisense oligonucleotide DNA plus RNase H to attempt to identify the a band as a tRNA (18). However, targeting several potential cytosolic and mitochondrial (mt) tRNAs indicated that none was the a band, while a positive-control tRNA was shown to be eliminated by RNase H plus a specific oligonucleotide (see Fig. S1 in the supplemental material). We did not further pursue identification of the a- or b-band RNAs that were modified only in vitro.
Three tRNASers are modified by TRIT1 in HeLa cells.
The majority of the potential TRIT1 substrates (Table 2) are comprised of three tRNASerNGA tRNAs and selenocysteine tRNA (tRNA[Ser]Sec); of the 22 genes for these, 21 have the A36A37A38 motif. Four other genes have A36A37A38: 2 of 7 tRNALeuUAA genes, 1 of 7 tRNALeuCAA genes, and 1 of 30 tRNACysGCA genes (Table 2). We performed analysis using the positive hybridization in the absence of i6A37 (PHA6) Northern blot assay, which can verify if the tRNAs carry i6A37 in vivo. In the PHA6 assay, the signal intensity with an ACL probe increases as the modification decreases (18) because the large hydrophobic isopentenyl group on N6 of A37 interferes with base pairing to the ACL probe (9, 18). A body probe complementary to the pseudouridine stem-loop region of the same tRNA serves as an internal control for loading. The ethidium bromide-stained gel is shown in Fig. 2A, in which each sample was loaded with 1× (5 μg) and 2× (10 μg) amounts of total RNA. This gel was blotted and sequentially probed, stripped, and reprobed for the tRNAs indicated in Fig. 2B to K.
Fig 2.
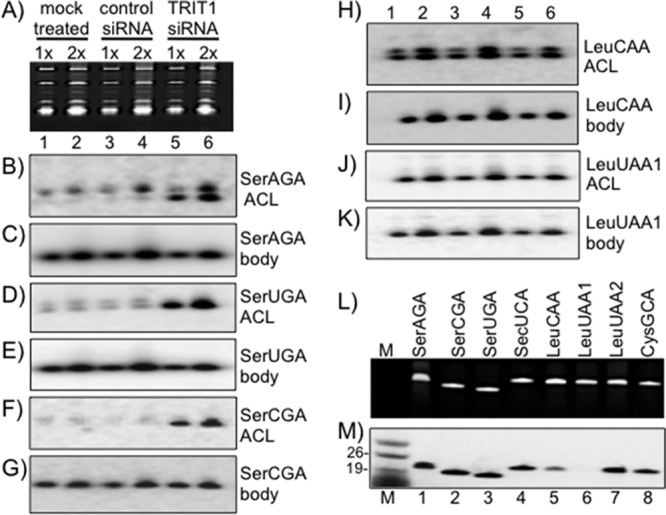
In vivo measurement of i6A modification of different tRNAs by the PHA6 assay. (A) Ethidium bromide-stained gel loaded with two different concentrations of total RNA. 1× and 2×, 5 μg and 10 μg of total RNA per lane, respectively. (B to G) Hybridization with ACL and body (TψC) probes for three different tRNASer tRNAs. The ACL probe hybridizes to the anticodon stem-loop region of tRNA. The body probe, also called the TψC probe, was used as a loading control probe and covers the entire TψC loop of tRNA. (H to K) Measurement of i6A modification of tRNALeuCAA and tRNALeuUAA in vivo by the PHA6 assay. The same blot was probed, stripped, and reprobed sequentially with the probes indicated to the right. (L and M) In vitro isopentylation assay using purified recombinant human TRIT1, [14C]DMAPP, and synthetic minihelices representing the anticodon stem-loops (ASLs) of the tRNAs indicated above the lanes. (L) Ethidium bromide-stained gel; (M) autoradiograph. Lane M, size markers, with sizes (in nucleotides) indicated to the left.
tRNASerAGA is encoded by 11 genes, of which 10 have A36A37A38; the other gene has G37. Eight of these genes have the same ACL sequence, to which we designed a probe. The tRNASerAGA ACL probe hybridization was less intense for mock-treated and control siRNA samples than samples treated with siRNA against TRIT1 (indicating the paucity of i6A37 in the latter) (Fig. 2B; the upper band of the doublet would appear to reflect a precursor tRNASerAGA that is hypomodified relative to the lower mature tRNASerAGA species). The tRNASerAGA body probe showed almost equal levels of tRNAs in all three types of samples (Fig. 2C).
All five tRNASerUGA genes have identical sequences in the ACL region. Figures 2D and E indicate that tRNASerUGA contains i6A37. All four tRNASerCGA genes have identical sequences in their ACL region. Figures 2F and G indicate that tRNASerCGA contains i6A37.
Minor fractions of tRNALeuUAA and -CAA have A36A37A38 but not i6A37.
Only 1 of 7 genes encoding tRNALeuCAA has A36A37A38 (Table 2). The similar hybridization patterns for the ACL and body probes in the mock, control, and TRIT1 siRNA samples indicate that tRNALeuCAA accumulates in HeLa cells but does not contain i6A37 (Fig. 2H and I). Only 2 of 7 genes encoding tRNALeuUAA have the A36A37A38 motif (Table 2). The sequences of the ACL regions of these two isodecoders (tRNALeuUAA-1 on chromosome 4 [Chr4] and tRNALeuUAA-2 on ChrX) are different and therefore require different ACL probes in the PHA6 assay. The PHA6 assay indicated that although tRNALeuUAA-1 accumulates in HeLa cells, it lacks i6A37 (Fig. 2J and K). tRNALeuUAA-2 was not detectable by use of either the ACL or body probe (not shown).
Only 1 of 30 genes for tRNACysGCA (Chr7, tRNA18) has A36A37A38 (Table 2), while the others have G37 but otherwise identical or nearly identical ACLs and surrounding sequences. We did not attempt to verify whether i6A37 is on tRNACysGCA because the PHA6 assay is not expected to discern this for 1 of 30 nearly identical ACLs. Although we could not confidently evaluate a single tRNACysGCA for i6A37 in HeLa cells, we determined that it is a poor substrate of Mod5 in S. cerevisiae cells (not shown). In any case, the significance of 1 potential tRNACysGCA substrate among 30 nonsubstrates is unknown.
A synthetic minihelix analog of tRNALeuUAA-1 is a poor substrate of TRIT1.
We and others have used 19-nt synthetic oligonucleotide RNAs as minihelix analogs of tRNA anticodon stem-loops (ASLs) to assess IPTase substrate specificity (18, 30, 31). We examined the substrate activities of eight synthetic ASLs shown in the ethidium bromide-stained gel in Fig. 2L. Note that while each ASL is 19 nt in length, subtle sequence differences can affect gel mobility (18). The three Ser ASLs, used here as positive controls, were modified in vitro by recombinant TRIT1 (Fig. 2M, lanes 1 to 3). The tRNALeuCAA ASL was less modified and was present at trace levels (lane 5), whereas tRNALeuUAA-1 was largely unmodified (lane 6). Although ASLs are not natural substrates and are expected to be more sensitive to perturbations than a tRNA, these results suggest (even though the assay is not strictly quantitative) that the lack of i6A37 on these tRNAs in vivo may be because they are relatively poor substrates for TRIT1. We also note that although the tRNALeuUAA2 ASL was modified in vitro (lane 7), as noted above, the corresponding tRNA was not detected in vivo, leaving open the possibility that it is expressed in other cell types, tissues, or metabolic states.
tRNA[Ser]SecUCA is only partially modified in HeLa cells.
tRNA[Ser]SecUCA carries selenocysteine to the translating ribosome for insertion at certain UGA codons in mRNAs that encode selenoproteins (32). We examined the tRNA[Ser]SecUCA i6A37 status in vivo. The human genome contains sequences for two isodecoders of tRNA[Ser]SecUCA (Sec-1 and Sec-2) that differ mostly in the acceptor stem of tRNA (Fig. 3A). A third candidate, annotated as tRNASec in the genomic tRNA database, is on Chr17, but this lacks a large variable arm found on all mammalian tRNASec tRNAs, has very low sequence homology to other mammalian tRNA[Ser]SecUCA tRNAs, has a critical mismatch in the B-box promoter and no transcription terminator within 250 bp of the 3′ end of the tRNA sequence (not shown), and is probably a pseudogene. Due to an imperfect acceptor stem, we suspected that Sec-2 would be nonfunctional and/or not accumulate (Fig. 3A). To examine the latter possibility, each isoform was targeted for elimination by a complementary oligonucleotide DNA specific to that isoform, followed by RNase H digestion (18), both in control cells and in TRIT1 siRNA-treated cells. The oligonucleotide complementary to Sec-1 eliminated all of the signal, while the oligonucleotide complementary to Sec-2 had no effect (Fig. 3B). Hybridization with probes complementary to the 3′ strand of the aminoacyl acceptor stem (AAS) of either isoform confirmed that tRNA[Ser]SecUCA Sec-1 accumulates and that tRNA[Ser]SecUCA Sec-2 does not (Fig. 3B to D). We also noted that TRIT1 siRNA-treated cells reproducibly contained significantly higher levels of tRNA[Ser]Sec than the control cells (compare lanes 1 to 4 of Fig. 3B with those of Fig. 3D). This suggests an inverse relationship between i6A37 hypomodification and the steady-state levels of tRNA[Ser]SecUCA, which was also reproducibly observed, as described below (see Fig. 4).
Fig 3.

tRNA[Ser]Sec is partially modified with i6A37. (A) Schematic depictions of the aminoacyl stems of the two tRNA[Ser]Sec tRNAs predicted from the human genome. (B to D) Targeted RNase H-mediated identification of cellular tRNA[Ser]Sec isodecoder 2. Oligonucleotide DNAs complementary to the 3′ end of the acceptor stem of either potential isodecoder 1 (Sec-1) or isodecoder 2 (Sec-2) were incubated with RNase H, as indicated above the lanes, and their presence and/or elimination was monitored by Northern blotting using either the Sec-1 aminoacyl acceptor stem (AAS) probe or the Sec-2 AAS probe, as indicated. Odd- and even-numbered lanes contain RNA from control (c) and TRIT1 siRNA (si)-treated cells, respectively, as indicated above the lanes. (D) As a control, hybridization with the tRNASerCGA body probe was carried out. (E and F) The same blot used in Fig. 2 was probed for tRNA[Ser]Sec using ACL and body probes. Bands were quantified from three independent experiments and applied to the following formula: percent modification = {1 − [(amount of ACL control/amount of BP control)/(amount of ACL TRIT1 siRNA/amount of BP TRIT1 siRNA)]} × 100, where BP is body probe. The results are reported in panel L. (G to K) In vitro modification of tRNA[Ser]Sec was measured using unlabeled DMAPP and recombinant His-TRIT1 and assessed by a two-part assay. First, control or TRIT1 siRNA-treated cellular RNA was subjected to in vitro modification, followed by the PHA6 assay. (G) Ethidium bromide-stained gel, including control RNA or TRIT1 siRNA with or without the addition of DMAPP to the TRIT1 reaction mixture, as indicated above the lanes. (H and I) Sec ACL and Sec body probings. (J and K) As a control, the blot was hybridized with tRNASerUGA ACL and body probes. The bands were quantified, and the results are presented in panel L. (L) Comparison of i6A37 levels from in vivo and in vitro assays. The absence of detection of hybridization with the ACL probe in the control samples was considered 100% i6A37 modification. The error bars were determined from four independent experiments for the in vivo assays and two independent experiments for the in vitro assays. The data for tRNA[Ser]Sec were derived from experiments whose results are shown in panels E and F and panels H and I; the data for tRNASerUGA were derived from experiments whose results are shown in Fig. 2D and E and panels J and K. (M) The ratios of the steady-state levels of tRNA[Ser]Sec and tRNASerCGA in control and TRIT1 siRNA-treated samples from three independent experiments, including the probings whose results are shown in panels B and D, were compared. The ratio obtained for the control samples was set to 100%, and the percent change due to TRIT1 depletion was compared to this.
Fig 4.
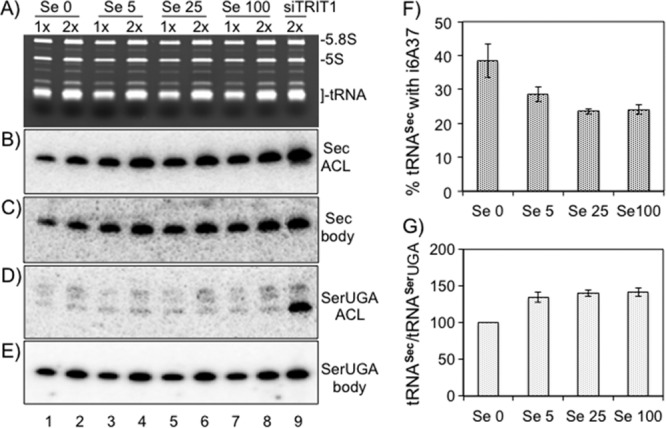
Selenium alters the i6A37 content and steady-state levels of tRNA[Ser]Sec. (A) HeLa cells were grown in medium containing additional Se at 0, 5, 25, and 100 ng/ml, as indicated above the lanes, and total RNA was purified and examined at 1× (5 μg) and 2× (10 μg) concentrations by the PHA6 assay. (B and C) Hybridization with the tRNA[Ser]Sec ACL probe and body probe, respectively. (D and E) Hybridization with tRNASerUGA ACL and body probes, respectively. (F) The percentage of tRNAs with the i6A37 modification was measured by comparing the signal intensities from the gels in panels B and C using the formula noted in the text; error bars indicate differences in two independent experiments. (G) The ratio of steady-state levels of tRNA[Ser]Sec and tRNASerUGA in the presence of different concentrations of Se was derived from quantification of the bands in panels C and E. The ratio obtained with no added selenium (Se 0) was considered 100%, and the percent change with higher Se concentrations was compared to this. The standard errors (bars) were determined from two independent experiments.
To examine the i6A37 status of tRNA[Ser]SecUCA, we used the same HeLa cell RNA blot for Fig. 3E and F that was used in Fig. 2B to K. Surprisingly, tRNA[Ser]SecUCA was found to be only partially modified (Fig. 3E and F). Unlike the results observed for tRNASer ACL probes, each of which showed a large difference in signal intensity in the TRIT1 siRNA sample relative to that in the mock-treated and control samples (Fig. 2B, D, and F), there was much less difference for tRNA[Ser]SecUCA between the TRIT1 siRNA and control cells (Fig. 3E and F; compare lanes 1 to 4 with lanes 5 and 6). This indicates hypomodification in the control and mock-treated cells that is more extensive for the tRNA[Ser]SecUCA tRNAs than for the tRNASer tRNAs.
We next examined the i6A37 status of tRNA[Ser]SecUCA using a two-part approach (Fig. 3G to K). If the N6 position of A37 is unmodified in cellular tRNA[Ser]SecUCA, it should be a substrate for i6A modification in vitro by TRIT1 and (unlabeled) DMAPP. The products were then assessed by the PHA6 assay for tRNA[Ser]SecUCA from control and TRIT1 siRNA-treated cells (Fig. 3G to K). The small decrease in the ratio of the Sec ACL probe to the Sec body probe after in vitro modification with TRIT1 in the control sample indicated that only a fraction of tRNA[Ser]SecUCA was a substrate for in vitro modification (Fig. 3H and I, lanes 1 and 2). A larger decrease in the ratio of the Sec ACL probe to Sec body probe was observed in the TRIT1 siRNA-treated cells (Fig. 3H and I, lanes 3 and 4). These data indicate that TRIT1 knockdown led to hypomodification and that the hypomodified fraction could then be modified by TRIT1 in vitro. However, in both the control and the TRIT1 siRNA-treated cells, only a fraction of tRNA[Ser]SecUCA was available for in vitro modification by TRIT1 in vitro. In contrast, a large fraction of tRNASerUGA was a substrate for in vitro modification in the TRIT1 siRNA sample (Fig. 3H and I, lanes 3 and 4). We can quantify the percent i6A37 modification by a method that includes internal normalization to the control sample (9): percent modification = {1 − [(amount of ACL control/amount of BP control)/(amount of ACL TRIT1 siRNA/amount of BP TRIT1 siRNA)]} × 100, where BP is the body probe. Quantification of the data from three independent experiments revealed that only about 40% of the tRNA[Ser]SecUCA in HeLa control cells was modified with i6A37, whereas the same methods applied to tRNASer tRNAs revealed about 90 to 95% modification (Fig. 3L, in vivo).
We performed quantification of triplicate data for TRIT1 knockdown and control samples and plotted the results in Fig. 3M. The data indicate that the increase in overall tRNA[Ser]SecUCA levels that occurs upon TRIT1 knockdown is specific relative to the levels of tRNASerCGA (and tRNASerUGA), as the levels of the latter remained unchanged (Fig. 3B, D, and M and data not shown).
Attempts to increase the fraction of tRNA[Ser]SecUCA that could be modified in vitro by increasing the concentrations of TRIT1 and DMAPP led to no more than a 55% modification (not shown), suggesting that its A36A37A38 recognition sequence is altered or inaccessible or that the N6 of A37 is blocked. Furthermore, significant overexpression of TRIT1 in HeLa cells did not increase the fraction of i6A37-containing tRNA[Ser]SecUCA (see Fig. S2 in the supplemental material). The collective data support the conclusion that a significant fraction of tRNA[Ser]SecUCA is modified with i6A37 in vivo and that a significant fraction does not contain i6A and is recalcitrant to modification (see Discussion).
The tRNA[Ser]Sec i6A37 content is decreased as the tRNA[Ser]Sec content is increased by selenium.
Partial modification suggests the potential for i6A37 to regulate selenoprotein production. Addition of selenium (Se) to the diet of rats or the growth medium of human cells led to increased tRNA[Ser]Sec levels with a shift in the ratio of two chromatographically distinct isoforms that appeared to differ in ribose 2′-O-methylation of mcmU34 (33, 34). We examined whether addition of Se affects the i6A37 content of tRNA[Ser]Sec in HeLa cells. We examined Se by adding it to the medium at four final concentrations (0, 5, 25, and 100 ng/ml), examined the tRNA[Ser]Sec levels and the percentage of cells with i6A37 (Fig. 4A to E), and quantified the results from 3 independent experiments (Fig. 4F and G). To quantify the fraction of i6A37, we used the formula percent modification = {1 − [(ACL Se0/BP Se0)/(ACL SeX/BP SeX)]} × 100, where SeX is the selenium concentration of the test condition, Se0 is the selenium concentration of the no-addition control, and BP is body probe. This revealed significant decreases in the i6A37 content (in percent) of tRNA[Ser]Sec (Fig. 4F), from 38% modified with no addition to 24% modified in cultures with ≥23.5 ng/ml Se. There was no detectable decrease in the i6A37 content of tRNASerUGA with increasing Se (Fig. 4D and E).
Examination of the Sec body probe data revealed increased amounts of tRNA[Ser]Sec in the samples treated with excess Se that were not observed for tRNASerUGA (Fig. 4C and E). Quantification of the results of triplicate experiments revealed a significant increase (∼40%) in tRNA[Ser]Sec levels relative to tRNASerUGA levels in response to supplemental Se (Fig. 4G).
HeLa cell mt tRNASerUGA and tRNATrpUCA contain i6A37.
Mod5 and Tit1 modify mitochondrial (mt) tRNAs (9, 35). TRIT1 has a targetP signal and was reported to be localized in part in mitochondria (36) (see the human MitoCarta database). A systematic examination of human mt tRNAs for i6A37 has not been reported. In a study of Tit1, the i6A37 mt tRNAs were only partially modified (∼30%), whereas cytosolic i6A37 tRNAs were more completely modified (9). We searched through the human mt tRNAs and found that those for Ser, Trp, Phe, Leu, Cys, and Tyr carry the A36A37A38 ASL motif. We applied the PHA6 assay to control and TRIT1 siRNA-treated cells (Fig. 5A to N). Quantitative analysis of the ACL probe data from triplicate experiments for control and TRIT1 siRNA-treated samples, each normalized to the amount of their corresponding body probe, was performed (Fig. 5O). This provided convincing evidence that mt tRNASerUGA and mt tRNATrpUCA contain i6A37. Interestingly, the other three candidate mt tRNAs showed much less of a difference in ACL probe hybridization between control and TRIT1 siRNA-treated samples (Fig. 5O). It should be noted that in each case in which siRNA against TRIT1 was used, it was very effective, showing results similar to those shown in Fig. 1A.
Fig 5.
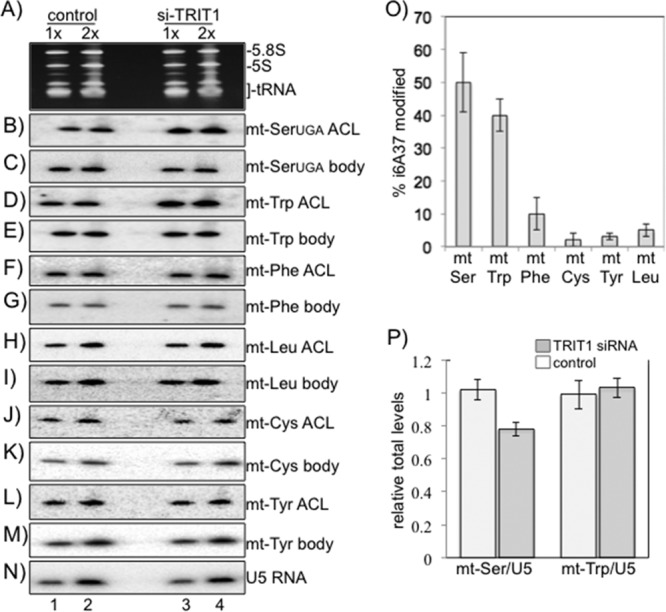
Mitochondrial tRNASerUGA and tRNATrpCCA contain i6A37, and the steady-state level of mt tRNASerUGA decreases in its absence. (A to N) PHA6 analysis of the six mt tRNAs that contain the A36A37A38 motif, using ACL or body probes, as indicated to the right of each panel. (O) Quantification of the PHA6 assay results in panels B to N using the formula noted in the text. The standard errors (bars) were determined from three independent experiments. (P) The ratio of mt tRNASerUGA or mt tRNATrpCCA to U5 is shown.
Loss of i6A37 is accompanied by a decrease in mt tRNASerUGA levels.
We examined the effect of TRIT1 knockdown on the overall levels of mt tRNASerUGA and mt tRNATrpUCA by quantifying the data for their body probes relative to the amount of the loading control, U5 RNA, in triplicate experiments (Fig. 5N) and display the results in Fig. 5P. This revealed that mt tRNASerUGA was significantly decreased, by 20%, in TRIT1 siRNA-treated cells, while the mt tRNATrpUCA was not affected by TRIT1 knockdown (Fig. 5P).
DISCUSSION
In this study, we identified human TRIT1 tRNA substrates. The data support multiple conclusions, including the fact that the presence of an A36A37A38 motif in a tRNA ASL domain is not necessarily sufficient for modification by a eukaryotic IPTase. This was first observed for S. cerevisiae tRNATrpCCA, which has the A36A37A38 motif but does not contain i6A37 and is not a substrate for Mod5, while it is a substrate for Tit1 (18). In that case, the tRNATrpCCA isolated from S. cerevisiae was not blocked from i6A37 modification because S. pombe Tit1 could readily modify it in vitro (18). Therefore, tRNATrpCCA i6A hypomodification appeared to be due to a restrictive substrate specificity of Mod5 (18). We are less confident as to the reason why human tRNALeuUAA and tRNALeuCAA, which bear A36A37A38, were not found to contain i6A37 and also why tRNA[Ser]Sec was found to be only partially modified. In the latter case, the fraction of hypomodified tRNA[Ser]Sec was recalcitrant to modification even in cells in which active TRIT1 was highly overexpressed (see Fig. S2 in the supplemental material), suggesting that it may somehow be chemically or otherwise blocked, perhaps by the presence of another modification (see below). The cumulative data that argue that tRNALeuUAA-1 appears to be unmodified, even though it contains the A36A37A38 motif, suggest that TRIT1 may have a restrictive substrate specificity, similar to Mod5. Thus, the reason(s) why human tRNA potential substrates are unmodified is unclear and will require significant additional work.
Another conclusion is that the subset of human tRNAs with i6A37 is significantly more limited than it is in S. pombe and S. cerevisiae, in which the levels of the subset of tRNAs with i6A37 also differ from each other. This is also the case for S. pombe and S. cerevisiae mt tRNAs, as well as their cytosolic tRNAs. Knowing which tRNAs carry i6A37 is important toward understanding how disturbances of TRIT1 activity may affect translation in general and of specific mRNAs enriched in cognate codons (9). With regard to physiological effects in fission yeast, the loss of i6A37 appeared to have both direct effects on the translation of certain highly expressed genes and indirect effects mediated in part through the TOR pathway (9).
Biochemical and functional characteristics of the two classes of IPTase substrates.
Another insight that emerges from this work comes from the deciphering of the significance of subclasses of IPTase substrates. Prior analyses suggested that S. pombe contains two classes of i6A37-containing tRNAs on the basis of biochemical and functional features. The two types of IPTase targets are comprised of tRNA targets that contain large variable arms and tRNA targets that have short or absent variable arms as well as anticodon base identity features that affect substrate activity (18). More recent evidence suggests that these two classes of IPTase substrates are also functionally distinct, as their cognate codons are distributed differently among the mRNAs (9). The most abundant S. pombe Tit1 substrate is tRNASerAGA, which decodes one of the most abundant codons, UCU, which is found to be heavily overenriched in the most abundant mRNAs, those that encode ribosome subunits, translation factors, and energy metabolic enzymes (9). Three other serine codons (UCA/G/C) decoded by i6A37 tRNAs are also enriched in this set of mRNAs, although the codons of each of those tRNAs are not as abundant as the tRNASerAGA and/or tRNASerUCU codon. Thus, i6A37 tRNASer tRNAs selectively contribute to decoding the highly abundant mRNAs, as these are overenriched in the cognate codons. This fits with the idea that i6A37 on a substrate tRNA promotes translation efficiency during growth and proliferation. The other serine codons, AGC and AGU, are decoded by S. pombe tRNASerGCU, which does not carry i6A37, and these codons are underrepresented in the abundant mRNAs (9).
The second functional class of Tit1 substrates in S. pombe comprises tRNATyrGUA and tRNATrpCCA, as their cognate codons are not enriched in the abundant mRNAs like the i6A37-sensitive serine codons are but are instead found distributed throughout all mRNAs (see the supplemental material; mRNA abundances are given in reference 9). Intriguingly, the data presented here show that HeLa cells use i6A37 only on one of the two classes found in the yeasts, the tRNASer tRNAs that decode the four UNN serine codons.
We found that tRNA[Ser]Sec was only partially modified in the control HeLa cells. We also observed partial modification of the two i6A37-containing mt tRNAs. Although the biochemical basis of this partial modification is unknown (see below), the data raise the possibility that TRIT1 and/or i6A37 levels on some tRNAs are regulated dynamically, as reported for some other tRNA modifications in S. cerevisiae (37).
TRIT1 has substrate-specific effects on the accumulation of some of its target tRNAs.
We found that two mt tRNAs, mt tRNASerUGA and mt tRNATrp, contain i6A37 in vivo. These were only partially modified and in this way were similar to tRNA[Ser]Sec. Interestingly, the steady-state levels of mt tRNASerUGA were decreased in the absence of i6A37, while mt tRNATrp levels were unaffected. However, contrary to the levels of mt tRNASerUGA, which decreased in the absence of i6A37, the tRNA[Ser]Sec levels increased as the i6A37 content decreased, both upon knockdown of TRIT1 and in response to exogenous selenium. Thus, unlike for the yeast i6A37 tRNAs, whose levels remained unchanged after IPTase depletion, the levels of some of the human i6A37 tRNAs were significantly affected by TRIT1 deficiency.
I6A37 and tRNA[Ser]Sec metabolism.
The prior characterization of murine TRIT1 showed its isopentenyltransferase activity on in vitro-transcribed tRNA[Ser]Sec (22, 23). The advances made by tRNA[Ser]Sec modification reported here are 3-fold. First, we were able to show using cell-derived tRNA[Ser]Sec in a quantifiable assay that it is only partially modified in human cells. Second, we could show that native hypomodified tRNA[Ser]Sec is refractory to further i6A37 modification in vitro and in vivo, suggesting that a fraction is specifically excluded from modification. Third, our data support an inverse relationship between the steady-state levels of tRNA[Ser]Sec and i6A37 content (below).
Modulation of selenium concentrations can affect both the levels of tRNA[Ser]Sec and its wobble U34 nucleotide modification (33, 34). The partial modification of tRNA[Ser]Sec is in agreement with findings described in previous reports (38, 39). A previous study focused on the effects of TRIT1 knockdown on tRNA[Ser]Sec hypomodification and selenoprotein expression but did not examine other potential substrates, such as the tRNASer tRNAs identified here (22, 23).
Upon TRIT1 knockdown we observed a significant increase in the steady-state levels of tRNA[Ser]Sec that was accompanied by a decrease in its i6A37 content, while tRNASer tRNAs were also hypomodified, but their levels remained unchanged. As noted in Results, overexpression of TRIT1 in HeLa and HEK cells did not increase the i6A37-containing fraction of tRNA[Ser]Sec (see Fig. S2 in the supplemental material), suggesting among other possibilities that its isopentylation site was altered or otherwise blocked.
Supplemental selenium led to increased tRNA[Ser]Sec levels with a shift in the ratio of two isoforms that differ in ribose 2′-O-methylation of mcm5U34, i.e., the ratio of mcm5Um to mcm5U (33, 34, 40). More specifically, the i6A content of tRNA[Ser]Sec decreased as the ratio of mcm5Um to mcm5U increased (38, 41). On the basis of these prior observations and our data, it is tempting to speculate that mcm5Um-containing tRNA[Ser]Sec may be resistant to i6A37 modification. This seems plausible for future investigation because Mod5 protein contacts tRNA position 34 (19) and a substitution at this position of S. cerevisiae tRNATrpCCA greatly affects its substrate activity (18), consistent with the idea that TRIT1 and other IPTases may be sensitive to the chemical composition of U34. Surely, the increase in tRNA[Ser]Sec levels is comprised of the unmodified form after TRIT1 knockdown. A possibility is that i6A37 tRNASec has a shorter half-life than the unmodified form.
Supplementary Material
ACKNOWLEDGMENTS
We thank James Iben, members of the R. J. Maraia lab, and The Friday Seminar for discussion.
This work was supported by the Intramural Research Program of the NICHD, NIH.
Footnotes
Published ahead of print 14 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01041-13.
REFERENCES
- 1. Gingold H, Pilpel Y. 2011. Determinants of translation efficiency and accuracy. Mol. Syst. Biol. 7:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drummond DA, Wilke CO. 2008. Mistranslation-induced protein misfolding as a dominant constraint on coding-sequence evolution. Cell 134:341–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johansson M, Zhang J, Ehrenberg M. 2012. Genetic code translation displays a linear trade-off between efficiency and accuracy of tRNA selection. Proc. Natl. Acad. Sci. U. S. A. 109:131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gustilo EM, Vendeix FA, Agris PF. 2008. tRNA's modifications bring order to gene expression. Curr. Opin. Microbiol. 11:134–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yokoyama S, Watanabe T, Murao K, Ishikura H, Yamaizumi Z, Nishimura S, Miyazawa T. 1985. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl. Acad. Sci. U. S. A. 82:4905–4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Begley U, Dyavaiah M, Patil A, Rooney JP, Direnzo D, Young CM, Conklin DS, Zitomer RS, Begley TJ. 2007. Trm9-catalyzed tRNA modifications link translation to the DNA damage response. Mol. Cell 28:860–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bauer F, Matsuyama A, Candiracci J, Dieu M, Scheliga J, Wolf DA, Yoshida M, Hermand D. 2012. Translational control of cell division by elongator. Cell Rep. 1:424–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandez-Vasquez J, Vargas-Perez I, Sanso M, Buhne K, Carmona M, Paulo E, Hermand D, Rodriguez-Gabriel M, Ayte J, Leidel S, Hidalgo E. 2013. Modification of tRNA(Lys)UUU by Elongator is essential for efficient translation of stress mRNAs. PLoS Genet. 9:e1003647. 10.1371/journal.pgen.1003647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lamichhane TN, Blewett NH, Cherkasova VA, Crawford AK, Iben JR, Farabaugh PJ, Begley TJ, Maraia RJ. 2013. Lack of tRNA modification isopentenyl-A37 alters mRNA decoding and causes metabolic deficiencies in fission yeast. Mol. Cell. Biol. 33:2918–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bjork GR. 1995. Biosynthesis and function of modified nucleosides, p 165–205 In Söll D, RajBhandary UL. (ed), tRNA: structure, biosynthesis, and function. ASM Press, Washington, DC [Google Scholar]
- 11. Phizicky EM, Hopper AK. 2010. tRNA biology charges to the front. Genes Dev. 24:1832–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. El Yacoubi B, Bailly M, de Crecy-Lagard V. 2012. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet. 46:69–95 [DOI] [PubMed] [Google Scholar]
- 13. Wei F, Suzuki T, Watanabe S, Kimura S, Kaitsuka T, Fujimura A, Matsui H, Atta M, Michiue H, Fontecave M, Yamagata K, Suzuki T, Tomizawa K. 2011. Deficit of tRNALys modification by Cdkal1 causes the development of type 2 diabetes in mice J. Clin. Invest. 121:3598–3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wei FY, Tomizawa K. 2011. Functional loss of Cdkal1, a novel tRNA modification enzyme, causes the development of type 2 diabetes. Endocr. J. 58:819–825 [DOI] [PubMed] [Google Scholar]
- 15. Bjork G. 1994. Biosynthesis and function of modified nucleosides in tRNA, p 165–206 In Söll D, RajBhandary UL. (ed), tRNA: structure, biosynthesis, and function. ASM Press, Washington, DC [Google Scholar]
- 16. Persson BC, Esberg B, Olafsson O, Bjork GR. 1994. Synthesis and function of isopentenyl adenosine derivatives in tRNA. Biochimie 76:1152–1160 [DOI] [PubMed] [Google Scholar]
- 17. Jenner LB, Demeshkina N, Yusupova G, Yusupov M. 2010. Structural aspects of messenger RNA reading frame maintenance by the ribosome. Nat. Struct. Mol. Biol. 17:555–560 [DOI] [PubMed] [Google Scholar]
- 18. Lamichhane TN, Blewett NH, Maraia RJ. 2011. Plasticity and diversity of tRNA anticodon determinants of substrate recognition by eukaryotic A37 isopentenyltransferases. RNA 17:1846–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou C, Huang RH. 2008. Crystallographic snapshots of eukaryotic dimethylallyltransferase acting on tRNA: insight into tRNA recognition and reaction mechanism. Proc. Natl. Acad. Sci. U. S. A. 105:16142–16147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spinola M, Galvan A, Pignatiello C, Conti B, Pastorino U, Nicander B, Paroni R, Dragani TA. 2005. Identification and functional characterization of the candidate tumor suppressor gene TRIT1 in human lung cancer. Oncogene 24:5502–5509 [DOI] [PubMed] [Google Scholar]
- 21. Spinola M, Falvella FS, Galvan A, Pignatiello C, Leoni VP, Pastorino U, Paroni R, Chen S, Skaug V, Haugen A, Dragani TA. 2007. Ethnic differences in frequencies of gene polymorphisms in the MYCL1 region and modulation of lung cancer patients' survival. Lung Cancer 55:271–277 [DOI] [PubMed] [Google Scholar]
- 22. Fradejas N, Carlson BA, Rijntjes E, Becker NP, Tobe R, Schweizer U. 2013. Mammalian Trit1 is a tRNA([Ser]Sec)-isopentenyl transferase required for full selenoprotein expression. Biochem. J. 450:427–432 [DOI] [PubMed] [Google Scholar]
- 23. Warner GJ, Berry MJ, Moustafa ME, Carlson BA, Hatfield DL, Faust JR. 2000. Inhibition of selenoprotein synthesis by selenocysteine tRNA[Ser]Sec lacking isopentenyladenosine. J. Biol. Chem. 275:28110–28119 [DOI] [PubMed] [Google Scholar]
- 24. Davis LG, Dibner MD, Battey JF. 1986. Basic methods in molecular biology. Elsevier, New York, NY [Google Scholar]
- 25. Iben JR, Maraia RJ. 2012. Yeast tRNAomics: tRNA gene copy number variation and codon use provide bioinformatics evidence of a new wobble pair in a eukaryote. RNA 18:1358–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan PP, Lowe TM. 2009. GtRNAdb: a database of transfer RNA genes detected in genomic sequence. Nucleic Acids Res. 37:D93–D97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lowe TM. 2013. A genomic tRNA database. University of California at Santa Cruz, Santa Cruz, CA: http://gtrnadb.ucsc.edu/ [Google Scholar]
- 28. Yarian C, Marszalek M, Sochacka E, Malkiewicz A, Guenther R, Miskiewicz A, Agris PF. 2000. Modified nucleoside dependent Watson-Crick and wobble codon binding by tRNALysUUU species. Biochemistry 39:13390–13395 [DOI] [PubMed] [Google Scholar]
- 29. Motorin Y, Bec G, Tewari R, Grosjean H. 1997. Transfer RNA recognition by the Escherichia coli delta2-isopentenyl-pyrophosphate:tRNA delta2-isopentenyl transferase: dependence on the anticodon arm structure. RNA 3:721–733 [PMC free article] [PubMed] [Google Scholar]
- 30. Soderberg T, Poulter CD. 2000. Escherichia coli dimethylallyl diphosphate:tRNA dimethylallyltransferase: essential elements for recognition of tRNA substrates within the anticodon stem-loop. Biochemistry 39:6546–6553 [DOI] [PubMed] [Google Scholar]
- 31. Soderberg T, Poulter CD. 2001. Escherichia coli dimethylallyl diphosphate:tRNA dimethylallyltransferase: site-directed mutagenesis of highly conserved residues. Biochemistry 40:1734–1740 [DOI] [PubMed] [Google Scholar]
- 32. Kasaikina MV, Hatfield DL, Gladyshev VN. 2012. Understanding selenoprotein function and regulation through the use of rodent models. Biochim. Biophys. Acta 1823:1633–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diamond AM, Choi IS, Crain PF, Hashizume T, Pomerantz SC, Cruz R, Steer CJ, Hill KE, Burk RF, McCloskey JA, Hatfield DL. 1993. Dietary selenium affects methylation of the wobble nucleoside in the anticodon of selenocysteine tRNA([Ser]Sec). J. Biol. Chem. 268:14215–14223 [PubMed] [Google Scholar]
- 34. Hatfield D, Lee BJ, Hampton L, Diamond AM. 1991. Selenium induces changes in the selenocysteine tRNA[Ser]Sec population in mammalian cells. Nucleic Acids Res. 19:939–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dihanich ME, Najarian D, Clark R, Gillman EC, Martin NC, Hopper AK. 1987. Isolation and characterization of MOD5, a gene required for isopentenylation of cytoplasmic and mitochondrial tRNAs of Saccharomyces cerevisiae. Mol. Cell. Biol. 7:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. 2008. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134:112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan CT, Dyavaiah M, DeMott MS, Taghizadeh K, Dedon PC, Begley TJ. 2010. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet. 6:e1001247. 10.1371/journal.pgen.1001247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim LK, Matsufuji T, Matsufuji S, Carlson BA, Kim SS, Hatfield DL, Lee BJ. 2000. Methylation of the ribosyl moiety at position 34 of selenocysteine tRNA[Ser]Sec is governed by both primary and tertiary structure. RNA 6:1306–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim JY, Carlson BA, Xu XM, Zeng Y, Chen S, Gladyshev VN, Lee BJ, Hatfield DL. 2011. Inhibition of selenocysteine tRNA[Ser]Sec aminoacylation provides evidence that aminoacylation is required for regulatory methylation of this tRNA. Biochem. Biophys. Res. Commun. 409:814–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carlson BA, Yoo MH, Tsuji PA, Gladyshev VN, Hatfield DL. 2009. Mouse models targeting selenocysteine tRNA expression for elucidating the role of selenoproteins in health and development. Molecules 14:3509–3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choi IS, Diamond AM, Crain PF, Kolker JD, McCloskey JA, Hatfield DL. 1994. Reconstitution of the biosynthetic pathway of selenocysteine tRNAs in Xenopus oocytes. Biochemistry 33:601–605 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


