Abstract
Cysts of Giardia lamblia and Entamoeba histolytica and oocysts of Toxoplasma gondii and Cryptosporidium parvum are the infectious and sometimes diagnostic forms of these parasites. To discover the structural components of cyst and oocyst walls, we have developed strategies based upon a few simple assumptions. Briefly, the most abundant wall proteins are identified by monoclonal antibodies or mass spectrometry. Structural components include a sugar polysaccharide (chitin for Entamoeba, β-1,3-linked glucose for Toxoplasma, and β-1,3-linked GalNAc for Giardia) and/or acid-fast lipids (Toxoplasma and Cryptosporidium). Because Entamoeba cysts and Toxoplasma oocysts are difficult to obtain, studies of walls of nonhuman pathogens (E. invadens and Eimeria, respectively) accelerate discovery. Biochemical methods to dissect fungal walls work well for cyst and oocyst walls, although the results are often unexpected. For example, echinocandins, which inhibit glucan synthases and kill fungi, arrest the development of oocyst walls and block their release into the intestinal lumen. Candida walls are coated with mannans, while Entamoeba cysts are coated in a dextran-like glucose polymer. Models for cyst and oocyst walls derive from their structural components and organization within the wall. Cyst walls are composed of chitin fibrils and lectins that bind chitin (Entamoeba) or fibrils of the β-1,3-GalNAc polymer and lectins that bind the polymer (Giardia). Oocyst walls of Toxoplasma have two distinct layers that resemble those of fungi (β-1,3-glucan in the inner layer) or mycobacteria (acid-fast lipids in the outer layer). Oocyst walls of Cryptosporidium have a rigid bilayer of acid-fast lipids and inner layer of oocyst wall proteins.
INTRODUCTION
Acid-fast lipids,
Fibrils of beta-glucan,
Dityrosine glows.
—Anonymous, Haiku of the oocyst wall
In this minireview, we discuss strategies that have worked well to discover structural components of protist walls that allow them to travel by the fecal-oral route from one person to the next (1–5). Proteins and sugars are the major components of cyst walls of Entamoeba and Giardia, which cause amebic dysentery and diarrhea, respectively (6–8). In addition to proteins and carbohydrates, lipids are important structural components of oocyst walls of Cryptosporidium and Toxoplasma, which cause diarrhea and disseminated infections, respectively (9–11).
To characterize the structural components of walls of so many different organisms, we have had to focus on what is likely to matter most. Many of these wall-oriented strategies are based upon a set of assumptions that may not be obvious to investigators working on stages of the parasites that are motile and dividing (12). Therefore, we review here the strategies and assumptions most useful to determine the protein, carbohydrate, and lipid compositions of cyst and oocyst walls. The compositions of these cyst and oocyst walls are then used to make simple, if incomplete, models for their structure and assembly.
FIRST STRATEGY: ASSUME THAT AT LEAST SOME OF THE IMPORTANT COMPONENTS OF CYST AND OOCYST WALLS HAVE ALREADY BEEN IDENTIFIED
The life cycle of each parasite reveals important properties of cyst and oocyst walls (Fig. 1) (13–15). Each of Giardia, Entamoeba, and Cryptosporidium has a single walled form, which is infectious and diagnostic. In contrast, Toxoplasma has three walled forms (underlined): oocysts shed in cat feces, within which sporocysts form when oocysts sporulate in the environment and tissue cysts in brains and muscles of warm-blooded animals. Eimeria, which is a pathogen of chickens, makes oocysts and sporocysts that closely resemble those of Toxoplasma (9, 11, 16).
Fig 1.
Cyst and oocyst walls of human pathogens contain a structural sugar polymer (chitin, β-1,3-GalNAc, β-1,3-glucan, or cellulose) and/or acid-fast lipids. Saccharomyces walls contain chitin and β-1,3-glucan. Toxoplasma has three walled forms (oocyst, sporocyst, and tissue cyst). The structural component(s) of tissue cysts of Toxoplasma has not been identified. Bradyzoites are not shown within Toxoplasma tissue cysts. No attempt was made to draw structures to scale.
Giardia cysts and Cryptosporidium oocysts survive for very long periods of time in fresh water, while oocyst walls of Toxoplasma resist treatment with 2% sulfuric acid (17). Chitin, which was first identified biochemically in cyst walls of Entamoeba, is shown with antibodies to Jessie 3, a chitin-binding lectin (sugar-binding protein) (Fig. 2A) (6, 18). Biochemical studies showed that Giardia cyst walls contain fibrils of β-1,3-linked GalNAc, which are detected with antibodies to Giardia CWP1, a lectin that binds the GalNAc homopolymer (Fig. 2B) (7, 19). Acid-fast lipids in oocyst walls of Cryptosporidium stain with carbol fuchsin, which also stains acid-fast lipids in the walls of mycobacteria (Fig. 2C) (20). The autofluorescence of Toxoplasma oocyst and sporocyst walls in UV light indicates the presence of dityrosines, which are formed when Tyr-rich proteins are oxidized (Fig. 2D) (9, 16, 21). Dityrosines are also present in the Saccharomyces spore wall (22).
Fig 2.
Fluorescence and transmission electron micrographs (TEMs) show important structural features of cyst walls of Entamoeba and Giardia and oocyst walls of Cryptosporidium and Toxoplasma. (A) Antibodies (red) show Jessie lectins bound to chitin fibrils of the Entamoeba cyst wall. (B) Antibodies (green) show CWP1 in a shard of the cyst wall of Giardia broken by sonication. (C) Carbol fuchsin (red) stains acid-fast lipids in Cryptosporidium oocyst walls. (D) Dityrosines (blue) in oocyst wall (white arrow) and sporocyst walls (blue arrows) of Toxoplasma are autofluorescent in the UV channel. (E) Three Entamoeba cyst walls isolated by centrifugation have uniform thickness and appearance. (F) Recombinant CWP1 binds to fibrils of β-1,3-GalNAc in cyst walls of Giardia deproteinated with NaOH. (G) The Cryptosporidium oocyst wall has a fibrillar glycocalyx (G), a bilayer (Bil), an inner layer containing oocyst wall proteins (OWPs), and tethers (T) that appear globular after they have broken. (H) Recombinant dectin-1 (red) binds to the oocyst wall (white arrow) but not to sporocyst walls of Toxoplasma (same oocyst as shown in panel D). (I) Recombinant Jessie lectin of Entamoeba self-aggregates and forms a biofilm composed of branched fibrils. (J) Deproteinated cyst walls of Giardia form a hollow sphere of curled fibrils of β-1,3-GalNAc. (K) A sonicated and pronase-treated wall of Cryptosporidium contains a rigid bilayer (Bil) and nothing else. (L) A sonicated Eimeria oocyst wall has an inner layer, which is a porous scaffold of fibrils of β-1,3-glucan. Micrographs of Entamoeba are reprinted from references 6 (A and I) and 24 (E), of Giardia from reference 7 (F), of Cryptosporidium from references 10 (G and K) and 11 (C), and of Toxoplasma from reference 9 (D, H, and L). Panels B and J are original here.
Whether intact, broken by excystation, or disrupted by sonication and/or glass beads, walls of all parasites appear rigid by fluorescence microscopy and transmission electron microscopy (TEM). Sonicated Giardia walls shatter and form fragments with sharp edges not unlike shards of broken pots (Fig. 2B) (7). The rigidity of the Cryptosporidium oocyst wall is shown by the uniform, oval shape of intact oocysts, the cup-like appearance of excysted walls, and the tight scrolls formed by mechanically broken walls (Fig. 2K) (10). Atomic force microscopy shows that the oocyst wall resembles common plastic materials (23).
TEM reveals the relative simplicity of cyst walls of Giardia and Entamoeba, each of which has a single layer of uniform thickness and staining (Fig. 2E) (24). Similarly, the walls of Saccharomyces and the sheath of nematodes are a single layer of uniform thickness and staining. In contrast, oocyst walls of Toxoplasma and Cryptosporidium, as well as walls of nematode eggs, have multiple layers (Fig. 2G) (10, 16). Since different structural components will likely bind different proteins, one can expect that the protein composition of oocyst walls is much more complicated than that of cyst walls.
A monoclonal antibody identified the major protein of cyst walls of Giardia (CWP1), which has an N-terminal signal peptide, a Leu-rich repeat domain, and a C-terminal Cys-rich domain (Fig. 2B) (25, 26). A monoclonal antibody identified the most abundant protein in Cryptosporidium oocyst walls, COWP1, which contains an N-terminal signal peptide and numerous Cys-rich repeat domains and His-rich domains (27). Monoclonal antibodies also identified Ser- and Thr-rich glycoproteins which are tethers that attach to the surface of sporozoites on the interior of the Cryptosporidium oocyst wall (Fig. 2G) (10, 28).
These observations have been essential for preparing reagents for detection of these parasites in stool samples: acid-fast stains of Cryptosporidium and monoclonal antibodies to COWP1 of Cryptosporidium and CWP1 of Giardia (20, 29). Because E. histolytica cysts were not available to raise monoclonal antibodies, diagnostic assays for Entamoeba infections use monoclonal antibodies to the Gal/GalNAc protein that is present on trophozoites and cysts (30). As most infections are transient and mild, Toxoplasma oocysts are not usually diagnosed in cat stools (17). Our goal has been to use these observations, as well as mass spectrometric identification of wall proteins, sugars, and lipids (if any), to make simple models of how cyst and oocyst walls are constructed.
SECOND STRATEGY: THE PREDICTED PROTEINS OF EACH ORGANISM SUGGEST IMPORTANT STRUCTURAL COMPONENTS OF THE CYST AND OOCYST WALLS
Here we assume that fibrils of sugar polymers are major structural components for eukaryotic walls in the same way that peptidoglycans and capsular sugars are major structural components of bacterial walls. Sugar polymers that make fibrils include β-1,4-linked GlcNAc (chitin), β-1,4-linked glucose (cellulose), and β-1,3-glucose (glucan). Cellulose in walls of plants and algae is the most abundant sugar polymer in nature. Chitin in walls of fungi, insects, and nematodes (eggs and sheath) is the second-most-abundant sugar polymer (Fig. 1). Fibrils of β-1,3-glucan are a major structural component of fungal walls, and the glucan synthase is the target of antifungal drugs called echinocandins (22, 31).
Chitin, cellulose, and β-1,3-glucan can be recognized by the enzymes that synthesize and hydrolyze them. Assuming that each of these enzymes evolved once, one can use homology searches of proteins predicted by whole-genome sequences to determine whether or not a given organism is capable of making each of these sugar polymers (32). Alternatively, one can use a key word search in EuPathDB, but beware that automated annotations may be inaccurate (33). Entamoeba is the only protist that makes chitin (6, 18, 34), and Toxoplasma and Eimeria make β-1,3-glucan (9), while Acanthamoeba and Dictyostelium make cellulose (Fig. 1 and Table 1) (35, 36). Cryptosporidium makes none of these sugar polymers (so how does it make a wall?). These homology searches can correct mistakes resulting from the use of plant lectins that are not always specific. For example, wheat germ agglutinin appeared to identify chitin in cyst walls of Giardia and tissue cyst walls of Toxoplasma, even though neither parasite has a chitin synthase and so neither can make chitin (37, 38).
Table 1.
Structural components of fungal, cyst, and oocyst walls
| Molecule(s) | Structural component(s) |
||||
|---|---|---|---|---|---|
| Saccharomyces | Entamoeba | Giardia | Toxoplasma (oocyst) | Cryptosporidium | |
| Sugar polymer(s) | Chitin, β-1,3-glucan | Chitin | β-1,3-GalNAc | β-1,3-Glucan | None |
| Lipids | None | None | None | Acid-fast lipids | Acid-fast lipids |
| Proteins | Glucanase, chitinase, dityrosines (spores), ∼100 proteins with modified GPI anchors | Chitinase, Jacob lectin, Jessie lectin | CWP1 to CWP3 (GalNAc-binding lectins) | Glucanase, Tyr-rich proteins, Cys- and His-rich OWPs, Cys-rich repeat protein | Cys- and His-rich OWPs, POWPs, Ser- and Thr-rich tethers |
| Abundant glycans | Mannans (high-mannose N-glycans), Man-rich O-glycans | Dextran-like O-P-glycans, N-glycans with galactose | Very short N-glycans | GalNAc- and fucose-rich O-glycans | GalNAc- and fucose-rich O-glycans |
If the life cycle of the parasite contains multiple walled forms (e.g., oocysts, sporocysts, and tissue cysts of Toxoplasma), the predicted glucan synthases and glucan hydrolases do not identify which walled stage(s) contains β-1,3-glucan (15). Fortunately, there are multiple glucan-binding reagents (macrophage dectin-1, anti-glucan antibodies, and glucan-binding domains of fungal glucan hydrolases) that may be used to show that oocyst walls of Toxoplasma contain fibrils of β-1,3-glucan whereas walls of sporocysts and tissue cysts do not (Fig. 2D and H) (9, 39).
The enzymes that make and break the unique β-1,3-GalNAc polymer of Giardia cyst walls are not homologous to those that make the other sugar polymers and so cannot be discovered by homology searches (19, 32). Similarly, none of the plant lectins that bind GalNAc bind to the β-1,3-GalNAc fibrils in cyst walls of Giardia. Instead, the most abundant cyst wall protein of Giardia (CWP1) is a lectin that binds to the β-1,3-GalNAc polymer (Fig. 2B and F) (7).
Phylogenetic profiling may be used to search for proteins and/or glycans that are present in coccidian parasites that make oocyst walls and are transmitted by the fecal-oral route (Cryptosporidium, Toxoplasma, and Eimeria) but are absent from related apicomplexan hemoparasites, which are transmitted by arthropods (Plasmodium, Babesia, and Theileria) (Fig. 3 and Table 1). Our assumption is that proteins common to coccidia and hemoparasites are unlikely to be part of oocyst walls, while proteins that are specific to coccidia are more likely to be part of oocyst walls. Further, because the proteins share common ancestry, one may assume, until proven wrong, that shared proteins have the same function in the oocyst walls of all coccidia.
Fig 3.

Venn diagrams show strategies to identify proteins that are common to oocyst walls of all coccidia (Cryptosporidium, Toxoplasma, and Eimeria) (purple) (oocyst wall proteins [OWPs], polyketide synthases [PKSs], O-GalNAc transferases [GalNAcTs], and N-glycans with Man5GlcNac2) but absent in hemoparasites (green). There are also proteins specific to Cryptosoporidium (blue) (possible oocyst wall proteins [POWPs]) or to Toxoplasma and Eimeria (red), which make similar oocysts with two walled forms.
Oocyst wall proteins (OWPs), which are the most abundant proteins in Cryptosporidium walls, are also present in the oocyst walls of Toxoplasma and Eimeria (references 40, 41, and 42 and our unpublished data). Polyketide synthases (PKSs) of Cryptosporidium, Toxoplasma, and Eimeria, which are very large enzymes similar to fatty acid synthases, resemble the PKSs that make acid-fast lipids in mycobacterial walls (43, 44). As the oocyst walls of each parasite are acid-fast (Fig. 2C), it is likely that PKSs are involved in synthesis of lipids in oocyst walls (11, 20).
O-GalNAc transferases (GalNAcT), which resemble mammalian enzymes, add GalNAc to Ser and Thr of secreted and membrane proteins of Cryptosporidium, Toxoplasma, and Eimeria (45, 46). These O-linked glycans, which are not stage specific, can be detected with the plant lectin MPA, which binds avidly to oocyst and sporocyst walls (9, 10). Finally, Asn-linked glycans (N-glycans) of coccidia contain 7 to 10 sugars (Glc0–3Man5GlcNAc2) (47). In contrast, the N-glycans of Plasmodium and Babesia contain two sugars (GlcNAc2), while Theileria makes no N-glycans. Whether N-glycans with mannose are important for oocyst wall formation has not been determined. In contrast, N-glycan length appears to be irrelevant for cyst walls, as Entamoeba makes N-glycans with Man5GlcNAc2, while Giardia makes N-glycans with GlcNAc2 (48).
Phylogenetic profiling may also be used to identify proteins or glycans shared by Toxoplasma and Eimeria that make oocysts with two walls versus Cryptosporidium proteins that make oocysts with one wall (Fig. 1 and 3). These proteins include enzymes that make or hydrolyze β-1,3-glucan, as well as tyrosine-rich proteins that are oxidized to form dityrosines that autofluoresce in UV (Fig. 2D and H) (9, 21, 42). In addition, there is a Cys-rich repeat (CRR) protein in oocysts of Toxoplasma and Eimeria that binds to deproteinated oocyst walls (our unpublished data).
THIRD STRATEGY: STUDIES OF CYST AND OOCYST WALLS OF NONHUMAN PATHOGENS MAY ACCELERATE DISCOVERY
Entamoeba histolytica, a human pathogen, does not encyst in axenic culture (growth without bacteria) or in the mouse model of intestinal infection. It is therefore necessary to travel to places where ameba infections are common such as India, Bangladesh, or Mexico to collect fecal samples that contain E. histolytica cysts. Alternatively, one can study cysts of E. invadens, an important pathogen of reptiles in zoos, which readily form cysts in axenic cultures (49). All of the abundant proteins in E. invadens cyst walls are present in cyst walls of E. histolytica, and the enzymes that make, degrade, and deacetylate chitin are the same (6, 24, 34, 50–55).
The most likely reason that relatively little is known about oocysts and sporocysts of Toxoplasma is the difficulty associated with infecting kittens with the parasite (4, 15). The life cycle of Toxoplasma in the research laboratory usually involves orally infecting mice with sporulated oocysts and then orally infecting cats with mouse brains containing tissue cysts. Genetic crosses are made by feeding cats brains from mice infected with different strains of Toxoplasma (56). The rapid attenuation of Toxoplasma in culture means that gene knockout technologies, which are used for in vitro cultures and for mouse infections, are not presently available for cat stages of the parasite life cycle (57). For example, the current KU80 knockout strains, like their parent strains, cannot complete their sexual cycle in the cat.
Kittens infected with Toxoplasma do not appear sick but shed tens of millions of oocysts in the stool. Within 48 h in the environment, these oocysts sporulate and become highly infectious. Eimeria provides a safe and relatively inexpensive model for cat stages of Toxoplasma, as oocysts and sporocysts of the chicken parasite closely resemble those of Toxoplasma in morphology and biochemistry (9, 11, 16). Discoveries made with Eimeria in chickens which may contribute to our understanding of Toxoplasma in cats include (i) identification of Tyr-rich proteins in oocyst walls and characterization of the peroxidases that form dityrosines (21), (ii) identification of prominent secretory vesicles (wall-forming bodies) during oocyst wall formation (58), and (iii) development of attenuated strains of Eimeria to vaccinate chickens (59).
FOURTH STRATEGY: TOOLS DEVELOPED TO STUDY YEAST WALLS DEMONSTRATE UNIQUE PROPERTIES OF CYST AND OOCYST WALLS
Biochemical methods for studying yeast walls work well for studying cyst and oocyst walls (22). Like fungal walls, parasite walls can be broken with glass beads and/or sonication and then purified on density gradients (10, 24, 51). Proteins may then be released with trypsin and analyzed with mass spectrometry, using whole-genome sequences of each parasite to predict tryptic peptides (10, 51). N-glycans released with peptide:N-glycanaseF (PNGaseF) and O-glycans released by β-elimination may be digested with glycohydrolases and identified by gel filtration or mass spectrometry (48, 51, 60).
The underlying fibrillar structure of the GalNAc homopolymer of Giardia cyst walls is revealed by treatment with NaOH, which deproteinates fungal walls and exposes fibrils of chitin and β-1,3-glucan (Fig. 2J) (7). NaOH-treated walls may be used to pull down proteins from lysed parasites that bind fibrils of the sugar polymer (7). The lectin activities of recombinant cyst or oocyst wall proteins may also be determined using deproteinated walls of parasites or fungi (Fig. 2F) (6–9).
Zymolyase, an extract of Streptomyces that contains proteases and β-glucanases, releases the same glucose disaccharides from fungal walls and oocyst walls of Eimeria (9). Trifluoroacetic acid, which releases glycans in O-phosphodiester-linked bonds to other sugars, also releases Entamoeba sugars linked by O-phosphodiester bonds to Ser and Thr of glycoproteins (51, 61). Nucleotide sugars (e.g., UDP-GalNAc or GDP-fucose) are transported from the cytosol to the lumen of the ER or Golgi apparatus, where sugars are added to secreted and membrane proteins (62). Nucleotide sugar transport assays, first developed with yeast membranes, may be used to identify sugars (e.g., GalNAc or fucose) that each parasite can add to its N-glycans or O-glycans (10).
The function of parasite enzymes may be determined by complementing yeast knockouts (e.g., the Entamoeba chitin synthase 2 complements a Saccharomyces chs1/chs2 double knockout) (34). Finally, organic solvents, which have little effect on fungal and cyst walls, efficiently extract acid-fast lipids from mycobacterial and oocyst walls (11).
While the biochemical methods for investigating fungal and parasite walls are similar, the results with the parasite walls are almost never what one would predict from the fungal wall model (Table 1). For example, the majority of fungal wall proteins are cross-linked to fibrils of chitin and β-1,3-glucan via sugars remaining in glycosylphosphatidylinositol (GPI) anchors after the lipid portion has been removed (22). As far as we know, none of the parasites use modifications of GPI anchors to cross-link proteins into cyst or oocyst walls.
With the exception of a chitinase in Entamoeba and a glucan hydrolase in Toxoplasma, none of the proteins of fungal walls is also present in parasite walls (6, 8, 9, 51). Although the chitin-binding and glucan-binding domains of the parasite chitinases and glucanases share common ancestry with those of fungi, the parasite carbohydrate-binding domains do not share common ancestry with those of fungi (Fig. 4) (9, 50, 53). While the number of internal repeats in flocculation proteins of Saccharomyces determines whether brewer's yeast remains in solution or sediments (causing opaque or clear beer, respectively) (63), there appears to be no particular function for the polymorphic internal repeats in spacer regions between chitin-binding domains of Entamoeba Jacob lectins (although these polymorphisms allow us to distinguish clinical isolates by PCR) (52).
Fig 4.

Lectins present in cyst and oocyst walls (8). (A) Entamoeba and Saccharomyces chitinases have catalytic domains that share common ancestry (purple), but they have chitin-binding domains (ChBD) that do not share common ancestry and are at different ends of the lectins (24, 53). The Entamoeba chitin-binding domain (orange) is also present at the N terminus of the Jessie lectin, which has a self-aggregating or daub domain (khaki) (6, 50). (B) Entamoeba Jacob lectins have multiple chitin-binding domains (gray), which are distinct from those of the amebic chitinase and Jessie lectin (24, 52). The spacer (pink) has Ser- and Thr-rich regions, which contain O-phosphodiester-linked glycans (51). Acanthamoeba proteins with three cellulose-binding domains (CeBD) have a structure similar to that of the Entamoeba Jacob lectins (36). (C) Giardia cyst wall proteins (CWP1 to CWP3) have a Leu-rich repeat (blue-green) that binds fibrils of the β-1,3-GalNAc polymer (7). The Cys-rich region (CRR in pink) is necessary for binding to the wall (70). (D) Toxoplasma and Schizosaccharomyces glucan hydrolases have catalytic domains that share common ancestry (dark blue), but they have glucan-binding domains (GBD) that do not share common ancestry and are at different ends of the lectins (9).
While mass spectrometry identifies ∼100 proteins in the walls of Saccharomyces, similar methods identify just three proteins in cyst walls of Entamoeba, each of which contains one or more chitin-binding domains (Table 1 and Fig. 2A and 4A and B) (6, 24, 50–52). Giardia also has three abundant cyst wall proteins (CWP1 to CWP3), each of which has a Leu-rich repeat that binds the GalNAc homopolymer (Fig. 2B and F and 4C) (7, 25, 26). Cys- and His-rich OWPs are by far the most abundant proteins in oocyst walls of Cryptosporidium, which contain a small number of other possible oocyst wall proteins (POWPs) (10, 27). Remarkably, these OWPs and POWPs are present in just two of ∼200 transcription groups identified in Cryptosporidium synchronously forming oocysts in vitro (64). In contrast, oocyst walls of Toxoplasma and Eimeria oocysts, which contain an outer layer with acid-fast lipids and an inner layer with fibrils of β-1,3-glucan, contain dozens of proteins (Fig. 2H and L) (references 9, 11, and 42 and our unpublished data).
Inhibitors of glucan synthases, called echinocandins, are potent drugs for treating disseminated infections with Candida and Aspergillus (31). Echinocandins kill fungi, while these drugs arrest the development of the oocyst walls of Eimeria and block release of the parasites into the intestinal lumen (9). Fibrils of β-1,3-glucan form a dense mesh on the surface of fungi (22). In contrast, fibrils of the same sugar polymer form a porous scaffold in the inner layer of the oocyst wall of Toxoplasma and Eimeria (Fig. 2L) (9).
The wall of Saccharomyces is covered with mannans, which are glycoproteins containing N-glycans with long mannose chains (Table 1) (22). Cryptococcus is covered by a carbohydrate capsule that is important for pathogenesis (65). While cyst walls of Entamoeba and oocyst walls of Toxoplasma and Cryptosporidium also contain proteins with N-glycans, the mannose chains of these parasites are not nearly as long as those in fungi (47, 48, 60). The most abundant carbohydrate on Entamoeba cysts is a polymer of α-1,6-linked glucose similar to that of bacterial dextran but made by distinct enzymes (51, 61).
In summary, if you know everything about the walls of fungi, you may know a little about the walls of parasites, mainly, the synthesis and hydrolysis of chitin and of β-1,3-glucan.
FIFTH STRATEGY: MAKE MODELS FOR THE STRUCTURE OF CYST AND OOCYST WALLS
Entamoeba.
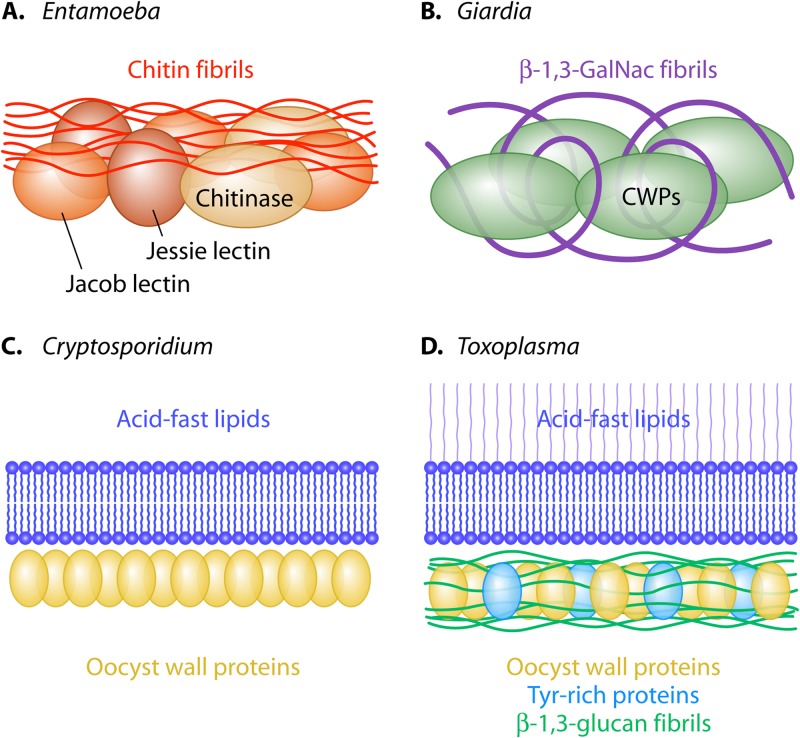
As a starting point, we describe a four-lectin model of the cyst wall of Entamoeba, which we have studied for the longest time and in the most depth (Fig. 1 and 5A) (6, 8, 24, 34, 50–55). Three abundant cyst wall proteins of Entamoeba, each of which was identified by mass spectrometry with excellent coverage, are chitin-binding lectins that cross-link chitin fibrils (Jacob), hydrolyze chitin (chitinase), or self-aggregate (Jessie lectins) and form the daub that holds walls together and makes them impermeable (Fig. 2A and 4A and B and Table 1). The evidence for these conclusions includes (i) demonstration that each cyst wall protein (expressed as a recombinant protein in bacteria or as an epitope-tagged protein in Entamoeba) binds particulate chitin and (ii) use of antibodies to recombinant proteins to confirm their presence in cyst walls (as well as their absence in trophozoites).
Fig 5.
Models for cyst walls of Entamoeba and Giardia and for oocyst walls of Cryptosporidium and Toxoplasma. (A) The Entamoeba cyst wall contains chitin fibrils and three chitin-binding lectins (chitinase, Jessie, and Jacob) (6, 8, 18, 24, 34, 50–54). (B) The Giardia cyst wall contains cyst wall proteins (CWPs), which are lectins that bind to curled fibrils of the β-1,3-GalNAc polymer (7, 19, 25, 29). (C) The Cryptosporidium oocyst wall contains a layer of acid-fast lipids and a layer of oocyst wall proteins (OWPs) (10, 11). (D) The Toxoplasma oocyst wall contains a layer of acid-fast lipids and a layer that contains fibrils of β-1,3-glucan, oocyst wall proteins, and Tyr-rich proteins that form dityrosines (9, 11, 16, 21).
Jacob lectins have two to seven tandemly arranged, Cys-rich, chitin-binding domains, which are separated by low-complexity spacers (Fig. 4B) (50). The spacers contain Ser and Thr residues that are decorated with O-phosphodiester-linked, dextran-like glycans (51). The dextran-like glycans of Entamoeba cysts are the same as those on trophozoites, only the reducing end sugar is rhamnose rather than galactose (61). The Jacob lectin spacers also contain sites for cleavage by parasite Cys proteases, which reduce the number of tandemly arranged chitin-binding domains (51). Very similar lectins with sets of Cys-rich, chitin-binding domains, between which are Ser- and Thr-rich spacers, are present in sheaths of nematodes and in peritrophic membranes formed by insects around the blood meal (66). Dictyostelium and Acanthamoeba have spore wall proteins that have similar designs, only cellulose-binding domains replace chitin-binding domains (35, 36).
The N-terminal chitin-binding domains of chitinase and Jessie lectin are the same but are distinct from the chitin-binding domains of Jacob lectins or those of other organisms (Fig. 4A) (50). Jessie lectins are secreted after Jacob lectins have cross-linked chitin fibrils on the surface of encysting Entamoebae (Fig. 2A) (6). The self-aggregating domain of Jessie lectins was shown by overexpressing the Entamoeba protein in transformed bacteria, which aggregated and made sheets of branching fibrils of the Jessie lectin (Fig. 2I).
Because the Jacob and Jessie lectins and chitinase are secreted proteins with no anchor to the plasma membrane, a fourth lectin (the Gal/GalNAc lectin) is necessary to hold the cyst wall to the parasite (24). This Gal/GalNAc lectin, which is an important virulence factor that binds the parasite to host glycans, is expressed on the plasma membrane of trophozoites and cysts (67). The Gal/GalNAc lectin binds Gal present on N-glycans of cyst wall proteins, so that Entamoeba forms wall-less cysts in the presence of excess galactose.
Two properties of chitin in the Entamoeba cyst wall are of note. First, chitin is made in secretory vesicles that are distinct from those containing Jacob lectins, chitinase, and Jessie lectins (6). In contrast, chitin is made at the plasma membrane of fungi (22). Second, much of the chitin is deacetylated by an Entamoeba chitin deacetylase to form a positively charged species called chitosan (54). Chitosan, which is resistant to hydrolysis by chitinases, is also present in walls of the pathogenic fungus Mucor (68). Finally, the activities of recombinant Entamoeba chitin synthases, chitinases, and chitin deacetylases have all been confirmed by recombinant expression in yeast or bacteria (34, 53, 54).
Giardia.
The β-1,3-linked GalNAc homopolymer present in the cyst wall of Giardia has not been identified in any other organism (Fig. 1 and 5B and Table 1) (19). Fibrils of the GalNAc homopolymer, which are made in vesicles that are distinct from those containing cyst wall proteins, are curled and form a hollow sphere in the absence of protein (Fig. 2J) (7). Deproteinated walls of Giardia were used to show that native CWP1 and CWP2, which are encystation specific, are lectins that bind fibrils of the GalNAc homopolymer (Fig. 4C). Recombinant versions of CWP1 showed that the Leu-rich repeats at the N terminus rather than the Cys-rich region at the C terminus contain the lectin domain (Fig. 2F). This result was a surprise, as chitin-binding lectins of Entamoeba and insect peritrophins, as well as glucan-binding domains of Toxoplasma glucan hydrolases, are Cys rich (6, 9, 50, 66). On the other hand, Leu-rich repeats present in plant receptor kinases and Toll-like receptors of metazoans bind a wide range of ligands (69, 70).
CWP2 has a very basic tail, which is cleaved by a Cys protease and is deposited along with CWP3 onto fibrils of the GalNAc homopolymer on the surface of encysting Giardia after CWP1 and the rest of CWP2 have been deposited (71, 72). When the Cys-rich region of CWP3 is removed, the protein is secreted but is not retained in the Giardia cyst wall (73). A novel transglutaminase activity is required for cyst wall formation, while Cys protease activity is required for excystation of Giardia (74, 75). There is a Cys-rich repeat protein unrelated to the CWP proteins present in cyst walls of Giardia, and there is massive overexpression of a family of glycine-rich repeat proteins in encysting parasites (76, 77).
While the GalNAc polymer synthase has been enriched in membrane extracts of encysting Giardia, it has not been identified (78). In contrast, the cytosolic pathway for synthesis of UDP-GalNAc, the substrate for the GalNAc polymer synthase, has been extensively characterized and shown to be dramatically increased in encysting parasites (79). While many bacteria make glycohydrolases that degrade chitin, cellulose, and/or β-1,3-glucan, no enzymes that degrade the β-1,3-GalNAc have been identified from bacteria or from the host (which has multiple chitinases). Deproteinated walls of Giardia were used to identify a robust cyst-specific glycohydrolase that degrades fibrils of the GalNAc homopolymer (7). This glycohydrolase is not active against intact walls of Giardia, wherein GalNAc fibrils are covered by protein. In contrast, treatment of Giardia cysts with the pancreatic enzyme chymotrypsin exposes GalNAc fibrils and likely contributes to excystation by the parasites in the duodenum.
Wheat germ agglutinin, which mistakenly identifies chitin in Giardia cyst walls, binds to very short N-glycans containing GlcNAc2 (like those present in Plasmodium) (Table 1) (7, 37, 47, 77). These very short N-glycans of Giardia appear to be important for encystation, as morpholinos (small nondegradable DNA homologs) that block N-glycan synthesis cause Giardia to form dysmorphic cysts, often with more than the usual four nuclei (our unpublished data).
In summary, there is a large amount of biochemical detail with regard to cyst wall formation by Giardia, much of which is not readily incorporated into a structural model of the cyst wall.
Cryptosporidium and Toxoplasma.
The Cryptosporidium oocyst wall has a glycocalyx on its surface, a rigid bilayer, and an inner layer rich in Cys- and His-rich OWPs (Fig. 2G and 5C) (10). Attached to the inner layer of the oocyst wall are electron-dense globules, which are remnants of Ser- and Thr-rich glycoproteins that tether sporozoites to the oocyst wall. The lectin MPA, which binds O-linked GalNAc, labels very well the outer and inner surfaces of the Cryptosporidium oocyst wall. In contrast, the fucose-binding lectin UEA1 binds only to the inner surface of oocyst walls. While mass spectrometry suggests that OWPs are by far the most abundant proteins in Cryptosporidium oocyst walls, their (self-aggregating, sugar-binding, or lipid-binding) function is not known (10, 27).
Although Cryptosporidium was shown to be acid-fast 30 years ago (when opportunistic Cryptosporidium infection was used as a diagnostic for AIDS prior to the identification of HIV) (20), the function of acid-fast lipids has begun to be understood only recently (Fig. 2C) (11). Acid-fast lipids in Cryptosporidium oocyst walls are part of a rigid bilayer that remains after pronase treatment of oocyst walls (Fig. 2K and 5C) (10, 11). The rigid bilayer is dissolved by organic solvents, and acid-fastness is lost. Similarly, the rigid bilayer and the outer layer of the oocyst wall of Toxoplasma are removed by organic solvents, while the inner layer containing the scaffold of fibrils of β-1,3-glucan remains, for the most part, intact (Fig. 2L and 5D) (11). In contrast, proteases and glucanases dissolve the inner layer of Toxoplasma oocyst walls and leave the outer layer and rigid bilayer intact (9).
Mass spectrometry of oocyst wall lipids released in organic solvents shows triglycerides with elongated fatty acyl chains (in Cryptosporidium) or polyhydroxy fatty acyl chains like those of lipids in the waxy cuticle of plant leaves (in Toxoplasma) (11). Sodium hydroxide cleaves ester linkages in triglycerides and gives a soap bubble appearance to the outer layer of the oocyst wall of Eimeria (a model for Toxoplasma). Finally, a polyketide synthase similar to the enzyme that makes mycobacterial wall lipids is massively overexpressed in oocysts of Toxoplasma and is present in Cryptosporidium (11, 43, 44). Sporocyst walls of Toxoplasma are also acid-fast, suggesting that triglycerides with polyhydroxy fatty acyl chains are important components of these walls, which superficially resemble Cryptosporidium oocyst walls (Fig. 1).
Toxoplasma oocyst walls have two features (dityrosines and β-1,3-glucan) found in fungal walls but not found in Cryptosporidium walls (Fig. 5D and Table 1) (9, 10, 16, 21). Dityrosines, which are formed in Toxoplasma when Tyr-rich proteins are oxidized, are present in oocyst and sporocyst walls but not in tissue cyst walls of Toxoplasma (Fig. 2D). The porous scaffold of fibrils of β-1,3-glucan, which is detected with dectin-1, is present in the inner layer of the oocyst walls but is absent from sporocyst and tissue cyst walls (Fig. 2H). A Toxoplasma glucan hydrolase, which has a unique Cys-rich, glucan-binding domain at its N terminus, is present in the inner layer of the oocyst wall (Fig. 4D) (9). A Schizosaccharomyces glucan hydrolase has at its C terminus a glucan-binding domain which does not share common ancestry with that of Toxoplasma.
Treatment of Eimeria-infected chickens with echinocandins that inhibit fungal glucan synthases arrests oocysts in a wall-less form that is not released into the lumen of the ceca (9, 31). Whether echinocandins also affect acid-fast lipids has not yet been determined. These results show that the Eimeria glucan synthase is druggable and that glucans are essential for development of the oocyst wall. The absence of β-1,3-glucan from tissue cyst walls suggests that echinocandins cannot be used to treat residual tissue cysts from humans infected with Toxoplasma.
Toxoplasma oocyst walls contain many more proteins than oocyst walls of Cryptosporidium (Table 1) (references 10 and 42 and our unpublished data). Toxoplasma oocyst wall proteins include Cys- and His-rich OWPs similar to those of Cryptosporidium oocyst walls and Tyr-rich proteins similar to those of Eimeria oocyst walls (21, 40–42). A Cys-rich repeat protein which is also present in Eimeria oocyst walls binds to deproteinated oocyst walls, suggesting that it is either a lectin or a lipid-binding protein (our unpublished data). Ser- and Thr-rich proteins in oocyst walls of Toxoplasma resemble Ser- and Thr-rich proteins present in tethers that attach sporozoites of Cryptosporidium to the inner layer of the oocyst walls (reference 10 and our unpublished data). Like oocyst walls of Cryptosporidium, Toxoplasma oocyst walls label with lectins that bind GalNAc (MPA) and fucose (UEA1) (Table 1) (9–11).
Although β-1,3-glucan is present in oocyst walls but absent in sporocyst walls of Toxoplasma, there is likely considerable overlap of proteins in the oocyst and sporocyst walls that look similar to each other and are both acid-fast (Fig. 2H) (9, 11). In contrast, there is likely little commonality between oocyst walls of Toxoplasma and tissue cyst walls, which do not look similar by TEM, do not have similar staining properties, and do not share many proteins (according to gene expression studies at ToxoDB) (15, 16, 33, 40–42). While fluorescence microscopy, expression studies, and knockouts have suggested a number of important properties of Toxoplasma tissue cysts (sugars bound by dolichos lectin, bradyzoite-specific antigens, and rhoptry proteins), there is presently no detailed model of the structure of tissue cyst walls, and no sugar polymers or acid-fast lipids have been identified (Fig. 1) (12, 38, 56, 57, 80–84).
In summary, no sugar polymer has been identified in the Cryptosporidium oocyst wall and the Toxoplasma sporocyst wall, each of which contains acid-fast lipids that appear to be a major structural component. Oocyst walls of Toxoplasma contain Tyr-rich proteins with dityrosine cross-links and fibrils of β-1,3-glucan, which are important structural components of fungal walls, as well as acid-fast lipids that are major structural components of mycobacterial walls.
CONCLUSIONS AND FUTURE STRATEGIES
Important strategies to determine the structure of cyst and oocyst walls derive from a few core ideas. TEM shows whether a parasite wall is relatively simple (Entamoeba and Giardia) or complex (Toxoplasma and Cryptosporidium). Each parasite wall has a major sugar polymer (chitin, cellulose, β-1,3-glucan, or β-1,3-GalNAc) or, in rare instances, acid-fast lipids. Monoclonal antibodies and mass spectrometry identify the most abundant proteins, many of which are lectins that bind the sugar polymers. Advantage may be taken of nonhuman pathogens (Entamoeba invadens and Eimeria) to better understand walls of Entamoeba histolytica and Toxoplasma, respectively. The biochemical methods used to study parasite walls are the same as those used to study fungal walls, although the results are usually different. We look forward to the time when a sixth strategy (targeted knockouts and forward genetics) can be applied to studies of cyst and oocyst walls. This is already the case for tissue cysts of Toxoplasma and may soon be the case for Entamoeba cysts and Eimeria oocysts (56, 57, 80, 84–86). Then we will be able to test the structural models of the cyst and oocyst walls derived from identification of their major components reviewed here.
Finally, there are reasons to be cautiously optimistic that studies of the cyst and oocyst walls may lead to new diagnostics and/or therapeutics for some of these parasites. Already, diagnostic antibodies target proteins in the cyst wall of Giardia and the oocyst wall of Cryptosporidium (25, 27, 29, 30). Jacob and Jessie lectins, which are abundant proteins in the amebic cyst wall, are excellent targets for new diagnostic antibodies for Entamoeba (6, 24, 51, 52, 55). Oral glucan synthase inhibitors might be developed to reduce transmission of Eimeria among commercial chickens (9). Alternatively, it might be possible to reduce transmission of Eimeria in chickens and Cryptosporidium in cattle using drugs that target synthesis of acid-fast lipids (11). Advantage might be taken of the new culture techniques for Eimeria and Cryptosporidium, which make it possible to screen drugs for their effects on oocyst wall development in vitro rather than in the whole animal (87).
ACKNOWLEDGMENTS
We appreciate the many contributions of graduate students and post-docs in the Samuelson and Robbins laboratories, as well as those of collaborators.
This work was supported in part by NIH grants AI48082 and AI44070 (to J.S.), AI07642 (T32 to G.G.B.), and GM31318 (to P.W.R.) and by a grant from the Mizutani Foundation for Glycoscience.
Footnotes
Published ahead of print 4 October 2013
REFERENCES
- 1.Baldursson S, Karanis P. 2011. Waterborne transmission of protozoan parasites: review of worldwide outbreaks—an update 2004–2010. Water Res. 45:6603–6614 [DOI] [PubMed] [Google Scholar]
- 2.Esch KJ, Petersen CA. 2013. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin. Microbiol. Rev. 26:58–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ximénez C, Morán P, Rojas L, Valadez A, Gómez A. 2009. Reassessment of the epidemiology of amebiasis: state of the art. Infect. Genet. Evol. 9:1023–1032 [DOI] [PubMed] [Google Scholar]
- 4.Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP. 2010. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 26:190–196 [DOI] [PubMed] [Google Scholar]
- 5.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222 [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee A, Ghosh SK, Jang K, Bullitt E, Moore LL, Robbins PW, Samuelson J. 2009. Evidence for a “wattle and daub” model of the cyst wall of Entamoeba. PLoS Pathog. 5:e1000498. 10.1371/journal.ppat.1000498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee A, Carpentieri A, Ratner DM, Bullitt E, Costello CE, Robbins PW, Samuelson J. 2010. Giardia cyst wall protein 1 is a lectin that binds curled fibrils of the GalNAc homopolymer. PLoS Pathog. 6:e1001059. 10.1371/journal.ppat.1001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuelson J, Robbins P. 2011. A simple fibril and lectin model for cyst walls of Entamoeba and perhaps Giardia. Trends Parasitol. 27:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bushkin GG, Motari E, Magnelli P, Gubbels MJ, Dubey JP, Miska KB, Bullitt E, Costello CE, Robbins PW, Samuelson J. 2012. β-1,3-Glucan, which can be targeted by drugs, forms a trabecular scaffold in the oocyst walls of Toxoplasma and Eimeria. mBio 3:e00258–12. 10.1128/mBio.00258-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee A, Banerjee S, Steffen M, Moore LL, O'Connor RM, Ward HD, Robbins PW, Samuelson J. 2010. Evidence for mucin-like glycoproteins that tether sporozoites of Cryptosporidium parvum to the inner surface of the oocyst wall. Eukaryot. Cell 9:84–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bushkin GG, Motari E, Carpentieri A, Dubey JP, Costello CE, Robbins PW, Samuelson J. 2013. Evidence for a structural role for acid-fast lipids in oocyst walls of Cryptosporidium, Toxoplasma, and Eimeria. mBio 4:e00387–13. 10.1128/mBio.00387-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boothroyd JC. 2009. Toxoplasma gondii: 25 years and 25 major advances for the field. Int. J. Parasitol. 39:935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson RC, Monis P. 2012. Giardia—from genome to proteome. Adv. Parasitol. 78:57–95 [DOI] [PubMed] [Google Scholar]
- 14.Tzipori S, Widmer G. 2008. A hundred-year retrospective on cryptosporidiosis. Trends Parasitol. 24:184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubey JP. 2009. History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol. 39:877–882 [DOI] [PubMed] [Google Scholar]
- 16.Belli SI, Smith NC, Ferguson DJP. 2006. The coccidian oocyst: a tough nut to crack! Trends Parasitol. 22:416–423 [DOI] [PubMed] [Google Scholar]
- 17.Dubey JP. 2010. Toxoplasmosis of animals and humans, 2nd ed. CRC Press, New York, NY [Google Scholar]
- 18.Arroyo-Begovich A, Cárabez-Trejo A, Ruíz-Herrera J. 1980. Identification of the structural component in the cyst wall of Entamoeba invadens. J. Parasitol. 66:735–741 [PubMed] [Google Scholar]
- 19.Gerwig GJ, van Kuik JA, Leeflang BR, Kamerling JP, Vliegenthart JF, Karr CD, Jarroll EL. 2002. The Giardia intestinalis filamentous cyst wall contains a novel beta (1–3)-N-acetyl-D-galactosamine polymer: a structural and conformational study. Glycobiology 12:499–505 [DOI] [PubMed] [Google Scholar]
- 20.Garcia LS, Bruckner DA, Brewer TC, Shimizu RY. 1983. Techniques for the recovery and identification of Cryptosporidium oocysts from stool specimens. J. Clin. Microbiol. 18:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mai K, Smith NC, Feng ZP, Katrib M, Slapeta J, Slapetova I, Wallach MG, Luxford C, Davies MJ, Zhang X, Norton RS, Belli SI. 2011. Peroxidase catalysed cross-linking of an intrinsically unstructured protein via dityrosine bonds in the oocyst wall of the apicomplexan parasite, Eimeria maxima. Int. J. Parasitol. 41:1157–1164 [DOI] [PubMed] [Google Scholar]
- 22.Lesage G, Bussey H. 2006. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70:317–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumètre A, Dubey JP, Ferguson DJ, Bongrand P, Azas N, Puech PH. 2013. Mechanics of the Toxoplasma gondii oocyst wall. Proc. Natl. Acad. Sci. U. S. A. 110:11535–11540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frisardi M, Ghosh SK, Field J, Van Dellen K, Rogers R, Robbins P, Samuelson J. 2000. The most abundant glycoprotein of cyst walls (Jacob) is a lectin with five Cys-rich, chitin-binding domains. Infect. Immun. 68:4217–4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luján HD, Mowatt MR, Conrad JT, Bowers B, Nash TE. 1995. Identification of a novel Giardia lamblia cyst wall protein with leucine-rich repeats. Implications for secretory granule formation and protein assembly into the cyst wall. J. Biol. Chem. 270:29307–29313 [DOI] [PubMed] [Google Scholar]
- 26.Lauwaet T, Davids BJ, Reiner DS, Gillin FD. 2007. Encystation of Giardia lamblia: a model for other parasites. Curr. Opin. Microbiol. 10:554–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spano F, Puri C, Ranucci L, Putignani L, Crisanti A. 1997. Cloning of the entire COWP gene of Cryptosporidium parvum and ultrastructural localization of the protein during sexual parasite development. Parasitology 114:427–437 [DOI] [PubMed] [Google Scholar]
- 28.Cevallos AM, Bhat N, Verdon R, Hamer DH, Stein B, Tzipori S, Pereira ME, Keusch GT, Ward HD. 2000. Mediation of Cryptosporidium parvum infection in vitro by mucin-like glycoproteins defined by a neutralizing monoclonal antibody. Infect. Immun. 68:5167–5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boone JH, Wilkins TD, Nash TE, Brandon JE, Macias EA, Jerris RC, Lyerly DM. 1999. TechLab and alexon Giardia enzyme-linked immunosorbent assay kits detect cyst wall protein 1. J. Clin. Microbiol. 37:611–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christy NC, Hencke JD, Escueta-De Cadiz A, Nazib F, von Thien H, Yagita K, Ligaba S, Haque R, Nozaki T, Tannich E, Herbein JF, Petri WA., Jr 2012. Multisite performance evaluation of an enzyme-linked immunosorbent assay for detection of Giardia, Cryptosporidium, and Entamoeba histolytica antigens in human stool. J. Clin. Microbiol. 50:1762–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eschenauer G, Depestel DD, Carver PL. 2007. Comparison of echinocandin antifungals. Ther. Clin. Risk Manag. 3:71–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zhang N, Zheng C, Bryant SH. 2011. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 39:D225–D229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aurrecoechea C, Heiges M, Wang H, Wang Z, Fischer S, Rhodes P, Miller J, Kraemer E, Stoeckert CJ, Jr, Roos DS, Kissinger JC. 2010. EuPathDB: a portal to eukaryotic pathogen databases. Nucleic Acids Res. 38:D415–D419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dellen KL, Bulik DA, Specht CA, Robbins PW, Samuelson JC. 2006. Heterologous expression of an Entamoeba histolytica chitin synthase in Saccharomyces cerevisiae. Eukaryot. Cell 5:203–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West CM. 2003. Comparative analysis of spore coat formation, structure, and function in Dictyostelium. Int. Rev. Cytol. 222:237–293 [DOI] [PubMed] [Google Scholar]
- 36.Clarke M, Lohan AJ, Liu B, Lagkouvardos I, Roy S, Zafar N, Bertelli C, Schilde C, Kianianmomeni A, Bürglin TR, Frech C, Turcotte B, Kopec KO, Synnott JM, Choo C, Paponov I, Finkler A, Heng Tan CS, Hutchins AP, Weinmeier T, Rattei T, Chu JS, Gimenez G, Irimia M, Rigden DJ, Fitzpatrick DA, Lorenzo-Morales J, Bateman A, Chiu CH, Tang P, Hegemann P, Fromm H, Raoult D, Greub G, Miranda-Saavedra D, Chen N, Nash P, Ginger ML, Horn M, Schaap P, Caler L, Loftus BJ. 2013. Genome of Acanthamoeba castellanii highlights extensive lateral gene transfer and early evolution of tyrosine kinase signaling. Genome Biol. 14:R11. 10.1186/gb-2013-14-2-r11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward HD, Alroy J, Lev BI, Keusch GT, Pereira ME. 1985. Identification of chitin as a structural component of Giardia cysts. Infect. Immun. 49:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boothroyd JC, Black M, Bonnefoy S, Hehl A, Knoll LJ, Manger ID, Ortega-Barria E, Tomavo S. 1997. Genetic and biochemical analysis of development in Toxoplasma gondii. Philos. Trans. R. Soc. Lond. B Biol. Sci. 352:1347–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drummond RA, Brown GD. 2011. The role of Dectin-1 in the host defence against fungal infections. Curr. Opin. Microbiol. 14:392–399 [DOI] [PubMed] [Google Scholar]
- 40.Templeton TJ, Lancto CA, Vigdorovich V, Liu C, London NR, Hadsall KZ, Abrahamsen MS. 2004. The Cryptosporidium oocyst wall protein is a member of a multigene family and has a homolog in Toxoplasma. Infect. Immun. 72:980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Possenti A, Cherchi S, Bertuccini L, Pozio E, Dubey JP, Spano F. 2010. Molecular characterization of a novel family of cysteine-rich proteins of Toxoplasma gondii and ultrastructural evidence of oocyst wall localization. Int. J. Parasitol. 40:1639–1649 [DOI] [PubMed] [Google Scholar]
- 42.Fritz HM, Bowyer PW, Bogyo M, Conrad PA, Boothroyd JC. 2012. Proteomic analysis of fractionated Toxoplasma oocysts reveals clues to their environmental resistance. PLoS One 7:e29955. 10.1371/journal.pone.0029955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu G, LaGier MJ, Stejskal F, Millership JJ, Cai X, Keithly JS. 2002. Cryptosporidium parvum: the first protist known to encode a putative polyketide synthase. Gene 298:79–89 [DOI] [PubMed] [Google Scholar]
- 44.Portevin D, De Sousa-D'Auria C, Houssin C, Grimaldi C, Chami M, Daffé M, Guilhot C. 2004. A polyketide synthase catalyzes the last condensation step of mycolic acid biosynthesis in mycobacteria and related organisms. Proc. Natl. Acad. Sci. U. S. A. 101:314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stwora-Wojczyk MM, Kissinger JC, Spitalnik SL, Wojczyk BS. 2004. O-glycosylation in Toxoplasma gondii: identification and analysis of a family of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Int. J. Parasitol. 34:309–322 [DOI] [PubMed] [Google Scholar]
- 46.Bhat N, Wojcyk BS, DeCicco M, Castrodad C, Spitalnik SL, Ward HD. 2013. Identification of a family of four UDP-polypeptide N-acetylgalactosaminyl transferases in Cryptosporidium species. Mol. Biochem. Parasitol. 191:24–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bushkin GG, Ratner DM, Cui J, Banerjee S, Duraisingh MT, Jennings CV, Dvorin JD, Gubbels MJ, Robertson SD, Steffen M, O'Keefe BR, Robbins PW, Samuelson J. 2010. Suggestive evidence for Darwinian selection against asparagine-linked glycans of Plasmodium and Toxoplasma. Eukaryot. Cell 9:228–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuelson J, Banerjee S, Magnelli P, Cui J, Kelleher DJ, Gilmore R, Robbins PW. 2005. The diversity of dolichol-linked precursors to Asn-linked glycans likely results from secondary loss of sets of glycosyltransferases. Proc. Natl. Acad. Sci. U. S. A. 102:1548–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eichinger D. 2001. Encystation in parasitic protozoa. Curr. Opin. Microbiol. 4:421–426 [DOI] [PubMed] [Google Scholar]
- 50.Van Dellen K, Ghosh SK, Robbins PW, Loftus B, Samuelson J. 2002. Entamoeba histolytica lectins contain unique 6-Cys or 8-Cys chitin-binding domains. Infect. Immun. 70:3259–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Dellen KL, Chatterjee A, Ratner DM, Magnelli PE, Cipollo J, Steffen M, Robbins PW, Samuelson J. 2006. Unique posttranslational modifications of chitin-binding lectins of Entamoeba invadens cyst walls. Eukaryot. Cell 5:836–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghosh SK, Van Dellen KL, Chatterjee A, Dey T, Haque R, Robbins PW, Samuelson J. 2010. The Jacob2 lectin of the Entamoeba histolytica cyst wall binds chitin and is polymorphic. PLoS Negl. Trop. Dis. 4:e750. 10.1371/journal.pntd.0000750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de la Vega H, Specht CA, Semino CE, Robbins PW, Eichinger D, Caplivski D, Ghosh S, Samuelson J. 1997. Cloning and expression of chitinases of Entamoebae. Mol. Biochem. Parasitol. 85:139–147 [DOI] [PubMed] [Google Scholar]
- 54.Das S, Van Dellen K, Bulik D, Magnelli P, Cui J, Head J, Robbins PW, Samuelson J. 2006. The cyst wall of Entamoeba invadens contains chitosan (deacetylated chitin). Mol. Biochem. Parasitol. 148:86–92 [DOI] [PubMed] [Google Scholar]
- 55.Ali IK, Haque R, Siddique A, Kabir M, Sherman NE, Gray SA, Cangelosi GA, Petri WA., Jr 2012. Proteomic analysis of the cyst stage of Entamoeba histolytica. PLoS Negl. Trop. Dis. 6:e1643. 10.1371/journal.pntd.0001643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sibley LD. 2009. Development of forward genetics in Toxoplasma gondii. Int. J. Parasitol. 39:915–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fox BA, Falla A, Rommereim LM, Tomita T, Gigley JP, Mercier C, Cesbron-Delauw MF, Weiss LM, Bzik DJ. 2011. Type II Toxoplasma gondii KU80 knockout strains enable functional analysis of genes required for cyst development and latent infection. Eukaryot. Cell 10:1193–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferguson DJ, Belli SI, Smith NC, Wallach MG. 2003. The development of the macrogamete and oocyst wall in Eimeria maxima: immuno-light and electron microscopy. Int. J. Parasitol. 33:1329–1340 [DOI] [PubMed] [Google Scholar]
- 59.Sharman PA, Smith NC, Wallach MG, Katrib M. 2010. Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunol. 32:590–598 [DOI] [PubMed] [Google Scholar]
- 60.Magnelli P, Cipollo JF, Ratner DM, Cui J, Kelleher D, Gilmore R, Costello CE, Robbins PW, Samuelson J. 2008. Unique Asn-linked oligosaccharides of the human pathogen Entamoeba histolytica. J. Biol. Chem. 283:18355–18364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moody-Haupt S, Patterson JH, Mirelman D, McConville MJ. 2000. The major surface antigens of Entamoeba histolytica trophozoites are GPI-anchored proteophosphoglycans. J. Mol. Biol. 297:409–420 [DOI] [PubMed] [Google Scholar]
- 62.Caffaro CE, Hirschberg CB. 2006. Nucleotide sugar transporters of the Golgi apparatus: from basic science to diseases. Acc. Chem. Res. 39:805–812 [DOI] [PubMed] [Google Scholar]
- 63.Verstrepen KJ, Jansen A, Lewitter F, Fink GR. 2005. Intragenic tandem repeats generate functional variability. Nat. Genet. 37:986–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oberstaller J, Joseph SJ, Kissinger JC. 2013. Genome-wide upstream motif analysis of Cryptosporidium parvum genes clustered by expression profile. BMC Genomics 14:516. 10.1186/1471-2164-14-516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doering TL. 2009. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu. Rev. Microbiol. 63:223–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen Z, Jacobs-Lorena M. 1998. A type I peritrophic matrix protein from the malaria vector Anopheles gambiae binds to chitin. Cloning, expression, and characterization. J. Biol. Chem. 273:17665–17670 [DOI] [PubMed] [Google Scholar]
- 67.Ralston KS, Petri WA., Jr 2011. Tissue destruction and invasion by Entamoeba histolytica. Trends Parasitol. 27:254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kafetzopoulos D, Martinou A, Bouriotis V. 1993. Bioconversion of chitin to chitosan: purification and characterization of chitin deacetylase from Mucor rouxii. Proc. Natl. Acad. Sci. U. S. A. 90:2564–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Torii KU. 2004. Leucine-rich repeat receptor kinases in plants: structure, function, and signal transduction pathways. Int. Rev. Cytol. 234:1–46 [DOI] [PubMed] [Google Scholar]
- 70.Kang JY, Lee JO. 2011. Structural biology of the Toll-like receptor family. Annu. Rev. Biochem. 80:917–941 [DOI] [PubMed] [Google Scholar]
- 71.Touz MC, Nores MJ, Slavin I, Carmona C, Conrad JT, Mowatt MR, Nash TE, Coronel CE, Luján HD. 2002. The activity of a developmentally regulated cysteine proteinase is required for cyst wall formation in the primitive eukaryote Giardia lamblia. J. Biol. Chem. 277:8474–8481 [DOI] [PubMed] [Google Scholar]
- 72.Konrad C, Spycher C, Hehl AB. 2010. Selective condensation drives partitioning and sequential secretion of cyst wall proteins in differentiating Giardia lamblia. PLoS Pathog. 6:e1000835. 10.1371/journal.ppat.1000835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun CH, McCaffery JM, Reiner DS, Gillin FD. 2003. Mining the Giardia lamblia genome for new cyst wall proteins. J. Biol. Chem. 278:21701–21708 [DOI] [PubMed] [Google Scholar]
- 74.Davids BJ, Mehta K, Fesus L, McCaffery JM, Gillin FD. 2004. Dependence of Giardia lamblia encystation on novel transglutaminase activity. Mol. Biochem. Parasitol. 136:173–180 [DOI] [PubMed] [Google Scholar]
- 75.Ward W, Alvarado L, Rawlings ND, Engel JC, Franklin C, McKerrow JH. 1997. A primitive enzyme for a primitive cell: the protease required for excystation of Giardia. Cell 89:437–444 [DOI] [PubMed] [Google Scholar]
- 76.Chiu PW, Huang YC, Pan YJ, Wang CH, Sun CH. 2010. A novel family of cyst proteins with epidermal growth factor repeats in Giardia lamblia. PLoS Negl. Trop. Dis. 4:e677. 10.1371/journal.pntd.0000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ratner DM, Cui J, Steffen M, Moore LL, Robbins PW, Samuelson J. 2008. Changes in the N-glycome, glycoproteins with Asn-linked glycans, of Giardia lamblia with differentiation from trophozoites to cysts. Eukaryot. Cell 7:1930–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karr CD, Jarroll EL. 2004. Cyst wall synthase: N-acetylgalactosaminyltransferase activity is induced to form the novel N-acetylgalactosamine polysaccharide in the Giardia cyst wall. Microbiology 150:1237–1243 [DOI] [PubMed] [Google Scholar]
- 79.Lopez AB, Sener K, Jarroll EL, van Keulen H. 2003. Transcription regulation is demonstrated for five key enzymes in Giardia intestinalis cyst wall polysaccharide biosynthesis. Mol. Biochem. Parasitol. 128:51–57 [DOI] [PubMed] [Google Scholar]
- 80.Striepen B, Soldati D. 2007. Genetic manipulation of Toxoplasma gondii, p 391–418 In Weiss LM, Kim K. (ed), Toxoplasma gondii, the model apicomplexan: perspectives and methods. Academic Press, London, United Kingdom [Google Scholar]
- 81.Caffaro CE, Koshy AA, Liu L, Zeiner GM, Hirschberg CB, Boothroyd JC. 2013. A nucleotide sugar transporter involved in glycosylation of the toxoplasma tissue cyst wall is required for efficient persistence of bradyzoites. PLoS Pathog. 9:e1003331. 10.1371/journal.ppat.1003331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boothroyd JC. 2013. Have it your way: how polymorphic, injected kinases and pseudokinases enable toxoplasma to subvert host defenses. PLoS Pathog. 9:e1003296. 10.1371/journal.ppat.1003296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Craver MP, Rooney PJ, Knoll LJ. 2010. Isolation of Toxoplasma gondii development mutants identifies a potential proteophosphogylcan that enhances cyst wall formation. Mol. Biochem. Parasitol. 169:120–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gubbels MJ, Lehmann M, Muthalagi M, Jerome ME, Brooks CF, Szatanek T, Flynn J, Parrot B, Radke J, Striepen B, White MW. 2008. Forward genetic analysis of the apicomplexan cell division cycle in Toxoplasma gondii. PLoS Pathog. 4:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morf L, Singh U. 2012. Entamoeba histolytica: a snapshot of current research and methods for genetic analysis. Curr. Opin. Microbiol. 15:469–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanig S, Entzeroth R, Kurth M. 2012. Chimeric fluorescent reporter as a tool for generation of transgenic Eimeria (Apicomplexa, Coccidia) strains with stage specific reporter gene expression. Parasitol. Int. 61:391–398 [DOI] [PubMed] [Google Scholar]
- 87.Müller J, Hemphill A. 2013. In vitro culture systems for the study of apicomplexan parasites in farm animals. Int. J. Parasitol. 43:115–124 [DOI] [PubMed] [Google Scholar]