Abstract
Small GTPases of the Ras superfamily are highly conserved proteins that are involved in various cellular processes, in particular morphogenesis, differentiation, and polar growth. Here we report on the analysis of RAS1 and RAC homologues from the gray mold fungus Botrytis cinerea. We show that these small GTPases are individually necessary for polar growth, reproduction, and pathogenicity, required for cell cycle progression through mitosis (BcRAC), and may lie upstream of the stress-related mitogen-activated protein kinase (MAPK) signaling pathway. bcras1 and bcrac deletion strains had reduced growth rates, and their hyphae were hyperbranched and deformed. In addition, both strains were vegetatively sterile and nonpathogenic. A strain expressing a constitutively active (CA) allele of the BcRAC protein had partially similar but milder phenotypes. Similar to the deletion strains, the CA-BcRAC strain did not produce any conidia and had swollen hyphae. In contrast to the two deletion strains, however, the growth rate of the CA-BcRAC strain was normal, and it caused delayed but well-developed disease symptoms. Microscopic examination revealed an increased number of nuclei and disturbance of actin localization in the CA-BcRAC strain. Further work with cell cycle- and RAC-specific inhibitory compounds associated the BcRAC protein with progression of the cell cycle through mitosis, possibly via an effect on microtubules. Together, these results show that the multinucleate phenotype of the CA-BcRAC strain could result from at least two defects: disruption of polar growth through disturbed actin localization and uncontrolled nuclear division due to constitutive activity of BcRAC.
INTRODUCTION
The gray mold fungus Botrytis cinerea is a ubiquitous necrotrophic plant pathogen capable of causing disease in more than 200 plant species. B. cinerea is able to infect different tissues, in which it survives for long periods, thereby causing damage in both fresh and postharvest crops (1, 2). In recent years, many signaling factors have been identified that are involved in disease development (3), including mitogen-activated protein kinase (MAPK) cascades (4–6), calcium-mediated signaling (7–9), cyclic AMP (cAMP)-related signaling pathways (10, 11), heterotrimeric GTPases (1, 4), and protein kinase A (12).
An important group of proteins, known collectively as small GTPases, is involved in many of these signaling pathways. Proteins in this group can facilitate specific activities downstream of a signaling pathway as well as relay signals within and between signal cascades. Small GTPases are divided into superfamilies, which are further divided into smaller families of proteins. Among these groups, members of the RAS and RHO families are the best characterized. Proteins in this group are involved in various cellular mechanisms, such as growth, differentiation, cell cycle, and cell polarity. All of the protein members in this group bind GTP and hydrolyze it to GDP. The transition between GTP- and GDP-bound states correlates with active (GTP) and inactive (GDP) states of the protein (13).
The first characterization of small GTPases in fungi was performed in Saccharomyces cerevisiae (14, 15). Today, numerous fungal GTPases of the RAS and RHO families have been characterized (16). In B. cinerea, RAS signaling is connected to the cAMP pathway, which controls conidial germination (11), whereas in Aspergillus nidulans, RAS is independent of the cAMP signaling pathway (17). Homologues of the RHO-type GTPases RAC and CDC42 have been characterized in a number of other fungi. For example, in A. nidulans, these small GTPases have been associated with control of hyphal morphogenesis and polarity establishment (18). In Colletotrichum gloeosporioides, CgRAC1 is associated with asymmetric development and with the regulation of morphogenesis, pathogenesis, and nuclear division (19). In Ustilago maydis, RAC and CDC42 regulate polarized growth, cytokinesis, and pathogenicity (20).
The relationships between homologues of RAS- and RHO-type GTPases have been investigated in the human pathogen Penicillium marneffei. In P. marneffei, RASA acts upstream of CflA (a CDC42 homologue), which shares functions with CflB (a RAC homologue), but both CflA and CflB have unique roles as well (21). In U. maydis, the effect of RAS2 on morphogenesis and pathogenicity is mediated by the MAPK signaling cascade (22). In Colletotrichum trifolii, expression of a constitutively active (CA) form of RAS (CA-RAS) resulted in accumulation of elevated levels of reactive oxygen species (ROS). This phenotype was restored by the expression of a dominant negative form of RAC, demonstrating the linkage between RAS- and RHO-type GTPases in this fungus (23). A connection between RAS and ROS was also reported for Neurospora crassa, where RAS-1- and ROS-dependent signal transduction modulates circadian- and light-regulated gene expression (24).
In the current study, we analyzed two small GTPases of B. cinerea, BcRAS1 and BcRAC, and determined their involvement in development, cell cycle, pathogenicity, and ROS-dependent signaling. We show that both proteins operate in the same pathway, and although their roles do not completely overlap, both influence the phosphorylation level of the stress-activated MAPK BcSAK1 and share common target genes with it. In addition, we show that BcRAC affects nuclear division, probably through modification of microtubules at mitosis.
MATERIALS AND METHODS
Fungi, media, and plant infection assays.
B. cinerea Pers. [teleomorph Botryotinia fuckeliana (de Bary) Whetzel] strain B05.10 was routinely cultured on potato dextrose agar (PDA). For specific purposes, fungi were cultured on various other solid media, including complete medium (CM) (25), PDA with 10% (wt/vol) mashed bean leaves, and Gamborg's B5 medium with 2% glucose (Duchefa, The Netherlands). Transgenic strains were produced on the background of strain B05.10. Transformants were selected on CM agar containing 70 μg ml−1 nourseothricin or hygromycin. A list of all strains used in this study is provided in Table S1 in the supplemental material.
For DNA isolation, mycelium was produced in potato dextrose broth. For RNA and protein isolation, mycelia were produced in liquid malt medium (5 g liter−1 glucose, 15 g liter−1 malt extract, 1 g liter−1 casein peptone, 1 g liter−1 Casamino Acids, 1 g liter−1 yeast extract, 0.2 g liter−1 RNAs), the mycelia were harvested by filtration and transferred into 100 ml liquid Czapek-Dox medium, and the cultures were incubated for additional times according to the experimental conditions.
Infection assays were performed with Phaseolus vulgaris L. genotype N90598. Primary leaves were inoculated by placing agar plugs with mycelium on both sides of the central leaf vein. Disease was estimated by measuring the numbers and sizes of the lesions at 24 to 96 h postinoculation (p.i.).
Molecular techniques.
Fungal DNA was isolated as previously described (26). Plasmid DNA was isolated using a GeneJet plasmid miniprep kit (Fermentas, Germany). Reverse transcription-PCR (RT-PCR) was carried out with a SuperScript II reverse transcriptase kit (Invitrogen) according to the manufacturer's instructions. Sequences of oligonucleotides that were used in PCRs are listed in Table S2 in the supplemental material. Southern and Northern blot analyses were performed according to the methods in earlier reports (1, 12).
Western blot analysis.
Lyophilized mycelium was ground in liquid nitrogen, and the powder was transferred to Eppendorf tubes containing 1 ml lysis buffer (20 mM Tris-HCl, pH 8.0, 0.05% Triton X-100, 150 mM NaCl, 10 μl ml−1 [each] of phosphatase and protease inhibitors). The samples were incubated for 15 min at 4°C on a rotary shaker, the tubes were centrifuged, and then the supernatant was decanted into fresh tubes and the protein concentration was determined using Bradford reagent. Gel electrophoresis and Western blot analysis were performed as described previously (6, 27).
Vector construction and production of transgenic strains. (i) bcras1 deletion cassette.
A 750-bp fragment upstream of bcras1 (5′ flank) was obtained by digesting genomic DNA with SalI and HindIII restriction enzymes. A 568-bp fragment downstream of bcras1 (3′ flank) was amplified using primers 5 and 6, which introduced SacII and SacI restriction sites at the 5′ and 3′ ends of the fragment, respectively. The fragments were cloned into the vector pNR1 (28), which carries the nourseothricin resistance cassette, resulting in the replacement vector pΔbcras1. Transformation of strain B05.10 was performed with the deletion cassette (3,280 kb) as described previously (1). Nourseothricin-resistant colonies were analyzed by PCR with the primer pairs 7-8 and 9-10, which are diagnostic for homologous integration, and 11-12, which amplifies the wild-type (WT) copy (all the primers used in this study are listed in Table S2 in the supplemental material). Further analysis of the integration pattern was performed by Southern blotting. DNA was digested with ClaI, separated in an agarose gel, blotted, and hybridized with a radioactively labeled DNA fragment from the 3′-flanking part of the replacement vector.
(ii) bcrac deletion cassette.
A 750-bp fragment upstream of bcrac (5′ flank) was amplified using primers 13 and 14, which introduced SacII and SacI restriction sites in the 5′ and 3′ ends of the fragment, respectively. A 340-bp fragment downstream of bcras1 (3′ flank) was amplified using primers 15 and 16, which introduced ClaI and XhoI restriction sites in the 5′ and 3′ ends of the fragment, respectively. The fragments were cloned into the vector pNR1, resulting in the replacement vector pΔbcrac. Transformation of strain B05.10 was performed with the deletion cassette (3,477 bp), and nourseothricin-resistant colonies were analyzed by PCR with the primer pairs 17-9 and 18-8, which are diagnostic for homologous integration, and 18-19, which amplifies the WT copy. Further analysis of the integration pattern was performed by Southern blotting. DNA was digested with EcoRI, separated in an agarose gel, blotted, and hybridized with a radioactively labeled DNA fragment from the 3′-flanking part of the replacement vector.
(iii) Constitutively active BcRAC expression cassette.
For expression of CA-BcRAC, the bcrac cDNA (600 bp) was amplified with primers 20 and 21 and cloned into the PTZ57 vector (Fermentas), resulting in pTZBcRAC. A hemagglutinin (HA) tag was added at the 3′ end by ligation of an oligonucleotide duplex (22-23) into pTZBcRAC at the KpnI and PacI sites, destroying the original PacI site and introducing a new PacI site at the beginning of the tag, resulting in pTZHABcRAC. The CA allele of BcRAC (G17V) was generated by site-directed mutagenesis of pTZHABcRAC, using primers 24 and 25, resulting in pTZHA-CA-BcRAC. The HA-tagged CA-bcrac fragment was excised from pTZHA-CA-BcRAC with PacI and AscI and introduced under the control of the B. cinerea actin promoter into the vector KSHANG (29), which also carries the hygromycin resistance cassette, to form the pKSH-HA-CA-BcRAC plasmid. Diagnostic PCR of the hygromycin-resistant transformants was carried out with primers 22 (tag specific) and 21 to verify expression of the constitutively active protein.
Tissue staining. (i) Trypan blue staining of infected leaf tissue.
Staining of infected tissue was performed according to the method of Keogh et al. (30), with minor changes. Leaf tissue was incubated in lactophenol-trypan blue (1 ml lactic acid, 1 ml glycerol, 10 ml phenol, and 10 mg trypan blue, dissolved in 10 ml distilled water). Following overnight incubation, the samples were boiled for 1 min and then destained by two successive incubations, each for 60 min, in chloral hydrate (2.5 g chloral hydrate dissolved in 1 ml distilled water). Stained samples were mounted in 50% (vol/vol) glycerin and inspected by use of a light microscope.
(ii) Hoechst 33342 staining of nuclei.
A stock solution of 1.5 mg ml−1 Hoechst 33342 dye was prepared in water. Before use, the stock solution was diluted 1:100 in water or growth medium, and aliquots were applied to mycelium samples on microscope slides. The slides were incubated in the dark for 1 to 2 h in a humid chamber at room temperature before microscopic visualization. For staining of septa, the mycelium samples were further incubated for 15 min with 0.2 mM calcofluor white. Nuclei and septa were visualized with a fluorescence microscope, using the 4′,6-diamidino-2-phenylindole (DAPI) channel.
(iii) Live imaging of nuclei and actin patches.
Nuclei were visualized in living cells of transgenic B05.10 strains expressing the histone-GFP (H1-GFP) fusion protein. Actin was visualized in strain B05.10 and CA-BcRAC-transgenic strains expressing a codon-optimized Lifeact-GFP fusion protein (31) (see Table S1 in the supplemental material for details). Nuclei and actin were localized under a fluorescence microscope using a green fluorescent protein (GFP)-specific filter set.
Microscopy.
Fluorescence microscopy was carried out with a Zeiss AxioImager M1 fluorescence microscope. Scanning electron microscopy (SEM) was performed with a Hitachi S-3000N scanning electron microscope. Sample preparation for SEM analyses was performed as previously described (32).
RAC and cell cycle inhibitors.
NSC23766 (Tocris, United Kingdom), a RAC-specific inhibitor, was prepared as a 100 mM stock solution in water. Hydroxyurea (HU), an inhibitor of DNA replication (S phase), was prepared as a 3 M stock solution in water. Benomyl (BEN), an inhibitor of chromatid separation (mitosis), was prepared as a 1-mg ml−1 stock solution in dimethyl sulfoxide (DMSO). All of the compounds were diluted in water or culture medium to their final concentrations prior to use. Studies on the effects of inhibitors of conidial germination, nuclear division, and hyphal growth were conducted using the B05.10, CA-BcRAC, Δbcrac, Δbcras1, and H1-GFP strains. For germination experiments, conidia were suspended in Gamborg's B5 medium containing the inhibitor compounds at specified concentrations, and droplets of the suspension were mounted on microscope slides and incubated in a humid chamber at 22°C. In the case of strains that do not produce conidia, fungi were cultured on cellophane-covered PDA medium, and hyphal plugs (2 mm2) were used instead of conidia. After 24 h, germination rates (for conidia), hyphal growth, and numbers of nuclei were determined using light and fluorescence microscopy.
RESULTS
Isolation of bcras1 and bcrac and generation of transgenic strains.
bcras1 was originally identified as an expressed sequence tag (EST) clone derived from a cDNA library of B. cinerea cultivated under nitrogen starvation conditions (www.genoscope.cns.fr/spip/Botrytis-cinerea-cDNA-library.html). The predicted BcRAS1 protein has 212 amino acids, and the corresponding open reading frame (ORF) consists of 911 bp and includes three introns, of 74, 68, and 133 bp. This protein is annotated with the accession number BC1G_10176.1 in the B. cinerea genome database at the Broad Institute (http://www.broad.mit.edu/). bcrac was identified in the B. cinerea genome sequence based on similarity to RAC proteins from other fungi. The predicted protein has 199 amino acids, and the corresponding ORF consists of 904 bp and includes five introns, of 81, 58, 48, 66, and 54 bp. The gene is annotated B0510_7705 in the Broad Institute database.
The BcRAS1 protein is 99% identical to Sclerotinia sclerotiorum SsRAS1 (accession no. AAT75139.1) and 85% identical to C. trifolii CtRAS1 (accession no. AAC03781.1). BcRAC is 87% identical to C. trifolii CtRAC1 (accession no. AAP89013.1) and 86% identical to Aspergillus fumigatus AfRAC (accession no. XP_754976.1). Both the BcRAS1 and BcRAC proteins have the typical consensus sequences found in all RAS and RAC proteins, respectively. In addition, all of the motifs typical of members of the RAS superfamily of proteins (33) are found in the B. cinerea RAS and RAC proteins (see Fig. S1 in the supplemental material).
The bcras1 or bcrac gene was deleted on the background of WT strain B05.10 by using a deletion construct with a nourseothricin resistance cassette (see Fig. S2A and B in the supplemental material). Strains homokaryotic for the deletion were obtained by single-spore isolation and verified by PCR (data not shown) and Southern blot analyses (see Fig. S2C and D). Growth and morphogenetic phenotypes were verified in several (at least four) independent transgenic colonies for each genotype.
Both the Δbcras1 and Δbcrac strains had severe growth defects, which precluded further analyses of development-related phenotypes. As a complementary approach, a strain expressing a constitutively active (CA) form of BcRAC was produced by replacement of glycine 17 with valine in the GTP binding and hydrolysis site (G1 box) of the bcrac ORF. The clone was transformed into WT strain B05.10, and expression of the modified gene was verified by RT-PCR (data not shown). Growth and morphogenetic phenotypes of colonies expressing CA-BcRAC were verified in six independent isolates. A detailed study was performed using a single isolate of each of the Δbcras1, Δbcrac, and CA-BcRAC strains.
Colony and hyphal morphology of the bcras1 and bcrac mutants.
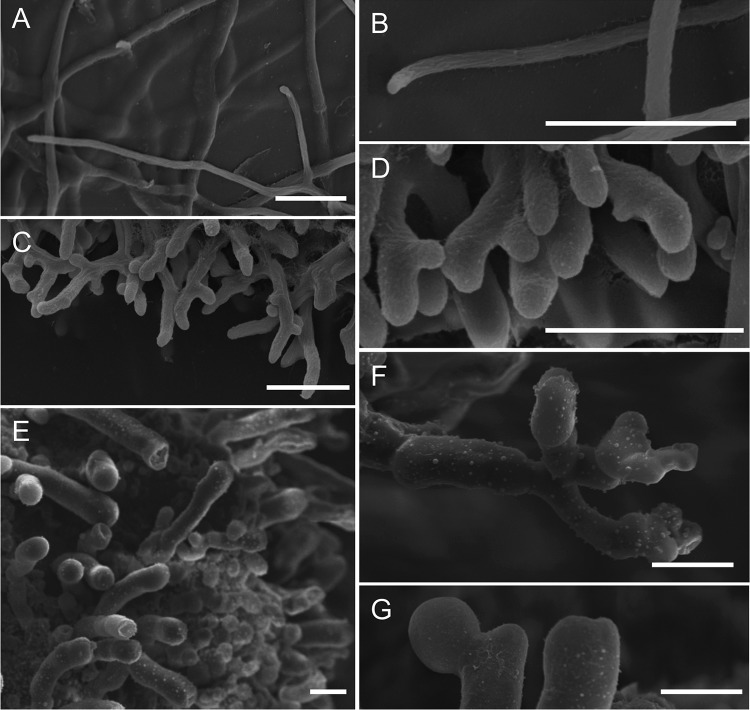
The Δbcras1 and Δbcrac strains were characterized by slow growth and formation of a compact mycelium on solid medium (Fig. 1A). On the Δbcras1 culture plates, we noticed the occasional appearance of sections resembling the WT. Molecular analyses showed that the bcras1-knockout situation was stable in these sections, suggesting the possibility of a suppression mutation. The CA-BcRAC strain had a WT-like growth rate but produced fluffy aerial hyphae. All three strains were vegetatively sterile and did not produce any conidia. In addition, the CA-BcRAC strain also failed to produce sclerotia (Fig. 1B). Microscopic analyses revealed changes in hyphal morphology that were especially pronounced in the two deletion strains. Hyphae were thickened and highly branched, and cell polarity was disturbed (Fig. 2). This hyphal architecture can explain the compact colonies and slow growth of the Δbcras1 and Δbcrac strains. Hyphae of the CA-BcRAC strain also had polarity defects (see Fig. S3 in the supplemental material), and despite the normal growth rate, the septa were more frequent and closer to each other than in WT hyphae (Fig. 3). The CA-BcRAC strain did not produce conidiophores, which is consistent with the lack of conidia (not shown).
Fig 1.
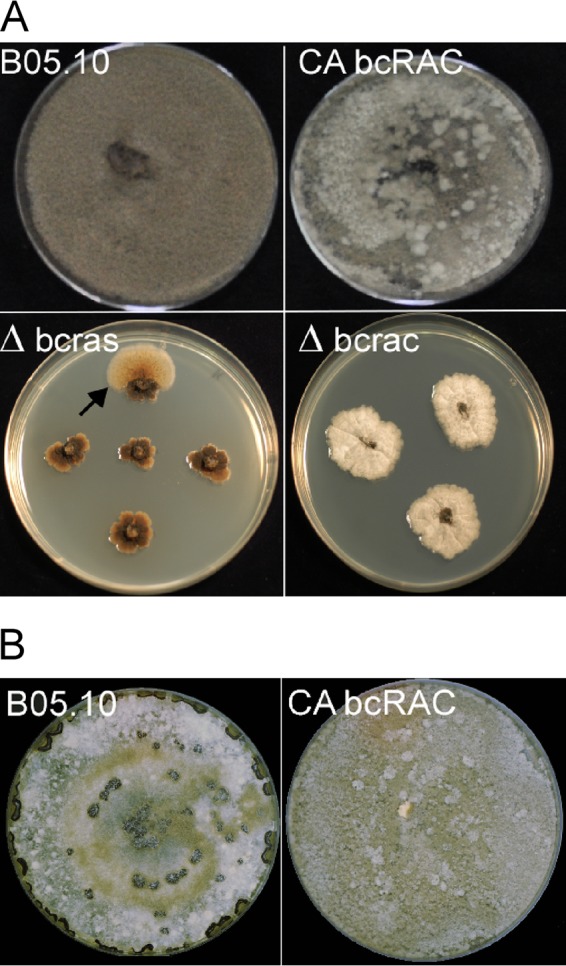
Morphology of bcras1 and bcrac mutants. (A) Colony morphology. The Δbcras1 and Δbcrac strains show slow growth and produce compact colonies; the CA-BcRAC strain has a normal growth rate and produces fluffy aerial hyphae. None of these strains produce conidia. Δbcras1 colonies form sectors of outgrowing brighter mycelia (arrow). (B) The CA-BcRAC strain does not produce sclerotia and has similar colony morphologies when grown in the dark or light.
Fig 2.
Hyphal morphologies of bcras1 and bcrac deletion strains. Fungi were cultivated on CM agar-soaked filter paper, and images were obtained by SEM after appropriate processing of the samples. (A and B) WT; (C and D) Δbcrac strain; (E to G) Δbcras1 strain. Bars = 20 μm.
Fig 3.
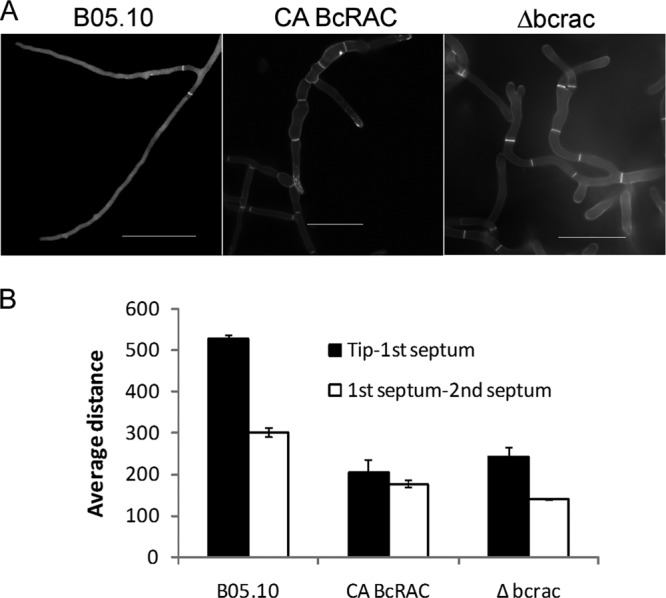
Septum localization and frequency in hyphae of bcrac mutants. Mycelia of WT, Δbcrac, and CA-BcRAC strains were produced in Gamborg's B5 liquid medium. Hyphae of the Δbcras1 mutant were not included in this analysis due to their diminutive and complex hyphal structure. (A) Hyphal septa can be seen by bright fluorescence following staining with calcofluor white. (B) Average distances (in pixels) between the hyphal tip and the first septum and between the first and second septa. In each case, 20 segments were measured. To avoid bias due to location within the hypha, only segments without branching were counted. Numbers are the means ± standard errors (SE) for three independent experiments (P < 0.001). Bars = 50 μm.
Pathogenic development is impaired in the bcras1 and bcrac mutant strains.
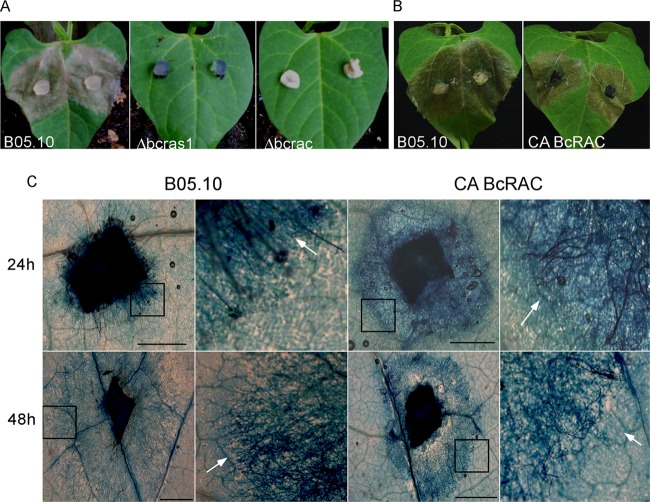
Due to the lack of conidial production in all of the transgenic strains, we used mycelia for infection assays. The Δbcras1 and Δbcrac strains were completely nonpathogenic and failed to produce disease symptoms on intact or wounded leaves (Fig. 4A) or on various types of B. cinerea-sensitive fruit (data not shown). These results demonstrated that both genes are essential for pathogenicity. However, since both strains were also severely defective in growth, it was impossible to conclude whether the lack of symptoms was due to specific defects in pathogenic development or was an indirect result of the growth defects.
Fig 4.
Pathogenicity tests on bean leaves. Due to a lack of conidial production by the mutants, infection assays were performed with mycelial plugs. (A) Leaves inoculated with WT, Δbcras1, and Δbcrac strains, shown at 72 h p.i. Inoculations with the deletion mutants did not develop any further on the following days (not shown). (B) Leaves inoculated with WT and CA-BcRAC mycelia, shown at 72 h p.i. Infection by the CA-BcRAC strain was slightly delayed but approached WT levels at 6 days p.i. (not shown). (C) Microscope images of lactophenol blue-stained leaves inoculated with WT and CA-BcRAC mycelia, shown at 24 and 48 h p.i. Hyphae and dead plant cells stain dark blue (arrows). Faster lesion and mycelium spread is evident in CA-BcRAC-infected leaves at 24 h p.i., and then the order is inverted at 48 h p.i. Insets show regions of higher magnification (right). Bars = 2 mm.
Unlike the complete loss of pathogenicity in the deletion strains, the CA-BcRAC strain caused normal-looking symptoms. There was a slight delay in symptom development during the first 72 h p.i. (Fig. 4B), but eventually, at 6 days p.i., disease levels were comparable to those on WT-infected leaves (data not shown). However, more detailed microscopic analysis of disease progression in this strain showed that at 24 h p.i., necrotic lesions and hyphal growth were both more advanced in CA-BcRAC-inoculated than in WT-inoculated leaves (Fig. 4C). At 48 h p.i., hyphal and lesion spread was slowed down considerably in the CA-BcRAC-infected tissue and increased in the WT-infected tissue (Fig. 4C). As a result, at 72 h p.i., lesion size in mutant-infected leaves was smaller than that in the WT-infected leaves (Fig. 4B). After 72 h, lesion expansion in the CA-BcRAC-infected leaves continued, eventually killing the plant as with the WT strain, at 6 days p.i. (data not shown). These results indicated that the CA-BcRAC strain is altered in early stages of pathogenic development. The initial acceleration of lesion formation could be due to enhanced secretion of necrotizing agents, whereas the pause in development at 48 h is suggestive of defects in the transition from early to late infection phases (34).
Actin localization is disrupted in hyphae of the CA-BcRAC strain.
The morphological abnormalities observed in the bcras1 and bcrac mutants, and in particular the loss of polarity, indicated a possible disturbance of the actin cytoskeleton. This possibility was further supported by studies in other systems showing that fungal RAC proteins modulate polarisome activity, thereby affecting assembly of actin cables (35–37). We used the yeast actin-binding protein Abp140 fused to GFP (Lifeact) for visualization of actin distribution in hyphae of the WT and CA-BcRAC strains. The deletion mutants could not be analyzed in this way due to the severe impairment of hyphal growth and difficulties in transformation with these strains. Furthermore, lack of conidia in the CA-BcRAC strain precluded analysis of actin in conidia or during germination. In WT hyphae, actin was concentrated at the tips and the branching sites and in spots of newly formed septa, in agreement with the known pattern of actin localization in fungal hyphae (Fig. 5A). The typical pattern of actin localization was disrupted in CA-BcRAC hyphae: the actin was more evenly distributed throughout the hyphae (Fig. 5B). Due to the presence of a WT copy of the bcrac gene and the heterokaryotic nature of this strain, the CA-BcRAC culture always contained a low level of WT nuclei. In these WT nucleus-containing hyphae, actin distribution was similar to that observed in hyphae of the WT strain (data not shown), further supporting the coincidence of polarity loss and disruption of the actin cytoskeleton.
Fig 5.
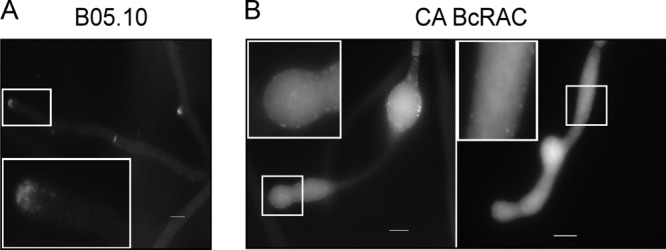
Actin distribution in WT and CA-BcRAC hyphae. The B05.10 (WT) and CA-BcRAC strains were transformed with a Lifeact-GFP expression plasmid. Mycelia were produced in Gamborg's B5 liquid medium, and samples were mounted on microscopic slides and incubated for 24 h to allow hyphal growth. Images were obtained using a fluorescence microscope in the differential interference contrast (DIC) and GFP channels. (A) WT; (B) CA-BcRAC strain. In panel B, two typical hyphal morphologies are illustrated. The one on the left shows a bulbous structure, which is relatively rare, and in which the actin signal is relatively intense. The figure on the right shows a more typically shaped hypha, in which the signal is generally evenly distributed but weaker. Insets show enlarged images. Bars = 10 μm.
Modification of conidial germination by RAC and cell cycle inhibitors.
None of the transgenic strains produced conidia, and therefore the putative roles of BcRAS1 and BcRAC in germination could not be studied in these strains. To address this question, we therefore used a strain that expresses H1-GFP, in which nuclei can be followed by fluorescence microscopy (38). First, we treated conidia of the H1-GFP strain with cell cycle- and RAC-inhibiting compounds and determined the effects on germination and division of nuclei. We then compared the effects of these inhibitory compounds on hyphae of the WT and mutant strains.
Conidia treated with a sublethal concentration (0.5 mM) of the RAC-inhibiting compound NSC23766 (39) produced a short germ tube (Fig. 6A, panel a). At higher concentrations (>0.85 mM), germination was completely prevented, and even the short germ tube did not develop (Fig. 6A, panel d). Treatment of conidia of a Δbccdc42 mutant with NSC23766 (0.5 mM) also resulted in the production of short germ tubes (data not shown). Lack of dependency of NSC23766 on CDC42, which is a small GTPase that is closely related to BcRAC, reinforced the specific effect of NSC23766 on the latter. To determine whether inhibition of BcRAC influences a certain stage in the cell cycle, we compared the effect of NSC23766 with the effects of known cell cycle blockers—HU, which disrupts DNA duplication during the G1/S transition, and BEN, which disrupts microtubule assembly and blocks the G2/M transition. Treatment of conidia with HU had a similar but not identical effect to that of NSC23766: at 0.3 M HU, conidia produced a short germ tube before germination ceased (Fig. 6A, panel b), whereas at a higher concentration (0.5 M HU), germination was completely blocked (Fig. 6A, panel e). However, in NSC23766-treated cells, nuclei continued to divide after treatment with the drug (Fig. 6A, panel a), and cells had the typical swollen morphology of hyphae of the CA-BcRAC or Δbcrac strain. However, in HU-treated cells, nuclear division was completely blocked, and the cells retained WT morphology (Fig. 6A, panel b). Incubation of conidia in medium containing moderate concentrations of both NSC23766 (0.5 mM) and HU (0.3 mM) had an effect similar to that of the treatment with 0.3 mM HU (Fig. 6A, panel g). Collectively, these results showed that the inhibition of BcRAC by NSC23766 affects a process during the cell cycle that is downstream of DNA replication (HU checkpoint), suggesting that RAC activation (by its guanine nucleotide exchange factor [GEF]) influences a later stage during the cell cycle.
Fig 6.

Impairment of spore germination and nuclear division by RAC and cell cycle inhibitors in the WT and mutant strains. Due to the lack of conidial production by the Δbcrac and Δbcras1 mutants, the effects of the inhibitors on germinating conidia were determined in conidia of the H1-GFP strain, which is a B05.10 (WT) strain expressing the H1-GFP cassette for live visualization of nuclei by fluorescence microscopy. (A) Conidia of the H1-GFP strain were germinated on a microscope slide in Gamborg's B5 medium with the specified compounds. Samples were analyzed following incubation for 24 h at 22°C. (a to c) Subinhibitory concentrations of NSC23766 (NSC) (RAC inhibitor; 0.5 mM), hydroxyurea (HU) (G1/S inhibitor; 0.3 M), and benomyl (BEN) (G2/M inhibitor; 250 μg ml−1). (d to f) Inhibitory concentrations of NSC (0.85 mM), HU (0.5 M), and BEN (500 μg ml−1). (g and h) Combinations of subinhibitory concentrations of NSC (0.5 mM) with HU (0.3 M) (g) and BEN (250 μg ml−1) (h). (i) Control. (B) Mycelia of all strains were cultured for 3 days on PDA medium covered with cellophane, and plugs of fresh mycelium were removed and placed on a glass slide in a droplet of Gamborg's B5 medium containing the desired concentration of the inhibitory compounds: 0.5 mM NSC, 0.3 M HU, 250 μg ml−1 BEN, or the combination of NSC with BEN. The slides with mycelia were incubated at 22°C for 24 h and then inspected microscopically. (j to m) WT; (n to q) CA-BcRAC strain; (r to u) Δbcrac strain; (v to y) Δbcras1 strain. White bars on the bottom left of each image represent the average numbers of nuclei per 10 μm of hypha ± SE. Empty squares represent no detectable growth. Bars = 10 μm.
To address this possibility, we treated conidia with BEN. The effect of the BEN treatment was almost identical to that of NSC23766: at 250 μg ml−1, conidia produced a short swollen germ tube (Fig. 6A, panel c), and at 500 μg ml−1, germination and nuclear division were completely blocked (Fig. 6A, panel f). Importantly, the combination of moderate levels of NSC23766 (0.5 mM) and BEN (250 μg ml−1) had an additive effect, namely, germination and nuclear division were completely blocked, similar to treatments with high concentrations of either one of the compounds alone (Fig. 6A, panel h). Together, the results with the RAC and cell cycle blockers showed that BcRAC influences a late stage in the cell cycle, after G1/S and before or at mitosis.
Nuclear division is affected by BcRac.
The results obtained with conidia of the H1-GFP strain suggested that BcRAC is connected with progression of the cell cycle. Moreover, the number of nuclei and their distribution in hyphae of all mutant strains, as opposed to the WT, further confirmed this assumption. In the Δbcras1 and Δbcrac strains, the number of nuclei per compartment was slightly changed (see Table S3 in the supplemental material). However, the nuclei were more densely packed (increased number of nuclei per hyphal length) than in WT hyphae due to the shorter distance between the septa (Fig. 6B, panels j, r, and v; see Table S3). In the CA-BcRAC strain, nuclei were also more densely packed; however, in this strain as well, the number of nuclei in each hyphal compartment was larger than in WT hyphae (Fig. 6B, panel n; see Table S3). These results showed that in addition to morphological changes in hyphal size and shape, which are affected by both GTPases, the BcRAC protein might also affect nuclear division.
Next, we compared the responses of the WT and mutant hyphae to the RAC-inhibitory compound. WT hyphae that were treated with 0.5 mM NSC23766 developed the phenotype of the Δbcrac strain, namely, short, swollen hyphae with compactly packed nuclei (Fig. 6B, panel k; see Table S3 in the supplemental material). Furthermore, the NSC23766 inhibitor had no effect on hyphae of the Δbcrac strain (Fig. 6B, panel s; see Table S3), while slightly enhancing the multinucleated and swollen hyphal phenotype of the CA-BcRAC strain (Fig. 6B, panel o; see Table S3) (probably due to the lack of or reduced competition with the endogenous copy of RAC). These results, individually and collectively, showed the specificity of NSC23766 for BcRAC and indicated BcRAC's association with nuclear division and the cell cycle.
Finally, to better understand the connection of BcRAC with the cell cycle, we compared the effects of cell cycle inhibitors in WT and mutant hyphae. Treatment with 0.3 M HU completely blocked development in the hyphae of all strains (data not shown). BEN, on the other hand, had a differential effect on the hyphae of the WT and mutant strains. BEN-treated WT hyphae continued to grow and had an increased number of nuclei, a phenotype that is highly similar to that of the CA-BcRAC strain (Fig. 6B, panel l; see Table S3 in the supplemental material). In contrast, treatment of Δbcrac and Δbcras1 lines with a similar concentration of BEN (250 μg ml−1) completely blocked hyphal growth in these strains (Fig. 6B, panels t and x; see Table S3). A combination of 250 μg ml−1 BEN with 0.5 mM NSC23766 blocked hyphal growth in the deletion mutants (Fig. 6B, panels u and y; see Table S3) as well as the WT (Fig. 6B, panel m; see Table S3); however, it did not prevent hyphal growth in the CA-BcRAC line, which grew with both treatments (Fig. 6B, panels p and q; see Table S3).
Collectively, the results obtained from the experiments with the RAC and cell cycle inhibitors showed that RAC affects nuclear division and that this effect is probably mediated by microtubules (Fig. 7).
Fig 7.

Model delineating BcRAC's association with growth and nuclear division. Active BcRAC (*RAC) is necessary for normal growth and forces nuclear division. In the complete absence of BcRAC (deletion strain or NSC23766-treated cells), growth is arrested but nuclear division is synchronous with development, resulting in the normal number of nuclei per cell. In the case of hyperactive RAC (CA-BcRAC), the growth rate is normal because the RAC functions are fulfilled, but nuclear division is continuous, resulting in hypernucleated cells. These RAC effects resemble those of BEN and are therefore probably mediated by tubulin.
Connection of BcRAS1 and BcRAC with the BcSAK1 MAPK pathway.
Colonies of the Δbcras1 and Δbcrac strains are sensitive to osmotic and oxidative stress. This sensitivity resembles the phenotype previously observed in deletion mutants of the stress-regulating MAPK BcSAK1 (6), suggesting a possible linkage between BcRAS1/BcRAC and the BcSAK1 MAPK cascade. To test this possibility, we compared development and responses to stress in the Δbcras1 and Δbcrac mutants with those of a Δbcsak1 deletion strain.
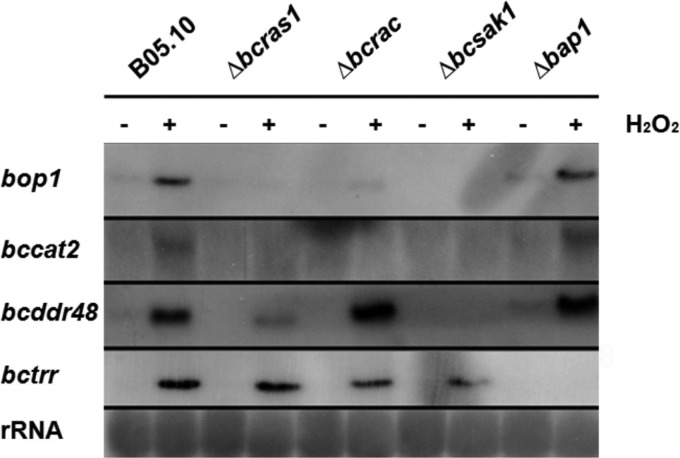
First, we tested sensitivity of the deletion strains to a range of stress regimens. Because of the relatively slow growth of the deletion strains, sensitivity to oxidative or osmotic stress was calculated as the ratio between growth of each strain under stress and normal (Czapek-Dox medium) conditions. In these experiments, all three deletion strains were more sensitive to stress than the WT strain (see Fig. S4 and Table S4 in the supplemental material). Next, we compared the expression levels of stress-regulating genes—bccat2 and bcbop1— two H2O2-inducible genes that are downstream of BcSAK1 (40). Similar to the results reported for the Δbcsak1 strain, expression of these genes was not induced in the Δbcras1 and Δbcrac strains (Fig. 8). This is in contrast to expression of the BcSAK1-dependent bcddr48 gene (41) and the BcSAK1-independent bctrr gene, which was unaffected (upregulated following treatment, as in the wild type) in the Δbcrac and Δbcras1 genetic backgrounds.
Fig 8.
Northern blot analysis of H2O2-induced genes. Cultures were incubated for 30 min in Czapek-Dox liquid medium with or without 10 mM H2O2. Mycelium was collected, total RNA was extracted, and gene expression was evaluated by Northern blot analysis. bcbop1, Opsin-1; bccat2, catalase 2; bctrr, thioredoxin reductase; bcddr48, stress protein. bcbop1, bccat2, and bcddr48 are under the control of BcSAK1 (6, 41), and bctrr is regulated by the transcription factor BcBAP1 (40).
These results suggest a possible link between the two GTPases and the BcSAK1 pathway, possibly with BcRAC and BcRAS1 residing upstream of the MAPK pathway. In this case, the phosphorylation state of the BcSAK1 protein is expected to be altered on the background of the two deletion strains. To test this hypothesis, we determined the phosphorylation state of BcSAK1 in the WT and in the bcrac and bcras1 deletion strains by using anti-p-p38 antibodies (6). Western blot analysis of hyphal protein extracts showed enhanced phosphorylation of BcSAK1 following H2O2 treatment in the WT but not in the two deletion strains, thus supporting a signaling connection between BcRAS1 and BcRAC and the BcSAK1 pathway (Fig. 9).
Fig 9.

Phosphorylation assay of BcSAK1. Western blots were performed with protein samples from mycelia that were produced in liquid medium with or without 10 mM H2O2. The phosphorylated form of BcSAK1 was monitored with anti-p-p38 antibody. The total amount of BcSAK1 protein was detected with an anti-Hog1 antibody.
DISCUSSION
We showed that the B. cinerea small GTPases BcRAS1 and BcRAC regulate growth and morphogenesis, are both necessary for pathogenicity, and might convey stress responses to the BcSAK1 pathway. In addition, BcRAC was found to be necessary for proper progression through the cell cycle, probably during mitosis.
Deletion strains lacking either bcras1 or bcrac produced compact colonies, had hyphae that were hyperbranched and short with obstructed polarity, and were vegetatively sterile. Similar phenotypic alterations in morphogenesis and differentiation in ras and rac mutant strains have been reported for other fungal species. For example, deletion of A. fumigatus rasa resulted in decreased radial growth and shorter hyphae (42). Deletion of a second fungus-specific ras gene in A. fumigatus or in B. cinerea had similar but milder effects (12, 43). In the biotrophic pathogen Claviceps purpurea, deletion of cprac resulted in cauliflower-like three-dimensional growth, a reduced growth rate, and failure to produce conidia (27). Deletion of rac1 in the dimorphic pathogen U. maydis abolished filament formation, while transformation of the fungus with a constitutively active allele was lethal (20), and a deletion strain of ras2 had disrupted cell morphology and was nonpathogenic to maize (22). In N. crassa, CDC42 and RAC localized to the forming septa, whereas Δcdc42 and Δrac strains were hyperseptated (44). Colletotrichum trifolii transgenic strains carrying a dominant active allele of RAS or RAC had abnormal hyphal proliferation and defects in polarized growth, conidium production, and appressorium formation (23, 45), suggesting that they share a signaling cascade.
RAC and RAS proteins are essential for hyphal morphogenesis due to their effect on polarisome assembly and actin localization (35). In Cryptococcus neoformans, RAC acts downstream of RAS, and both proteins are associated with cell division and actin polarization (46, 47). Formin, a component of the polarisome that catalyzes de novo F-actin assembly, is a direct effector of RAC (35, 48). The swollen hyphae of the CA-BcRAC strain resemble polarisome defects, particularly due to disruption of actin function. This possibility is further supported by the disappearance of actin foci in hyphae of CA-BcRAC. The possibility that this phenotype is specifically and directly controlled through interaction of BcRAC with the formin protein warrants further validation.
Similar to the situation in other systems, BcRAS1 and BcRAC seem to share the same signaling cascade. More significantly, however, we showed that defects in the bcras1 and bcrac deletion strains closely resemble those described for a deletion strain of the stress-activated MAPK BcSAK1. These results, together with differences in the phosphorylation state of BcSAK1 and defects in the transcriptional response of BcSAK1-regulated stress-activated genes on the background of the two deletion strains, are indicative of cross talk between the BcSAK1 and BcRAS1/BcRAC signaling cascades.
Pathogenicity of the Δbcras1 and Δbcrac mutants was completely abolished, suggesting a connection between BcRAS1 and BcRAC and pathogenic development. However, the reasons for the lack of pathogenicity in these mutants are unclear, since the two deletion strains are severely defective in vegetative development and growth, which could account for the reduced disease symptoms. Pathogenicity assays with the CA-BcRAC strain, which had a normal growth rate, were, however, more informative. This strain did not lose pathogenicity, and although lesion formation was delayed compared with that in WT infections, leaves infected with the CA-BcRAC strain eventually developed normally sized lesions. This result could be interpreted as a mild and possibly insignificant effect on pathogenicity. However, further examinations of early (24 to 48 h) and late (48 to 96 h) time points p.i. showed specific changes in both fungal and symptom progression. Microscopic analyses revealed that at the early stage of infection, the mutant actually produces faster-growing hyphae and earlier lesion formation than those in the WT strain (Fig. 4). Hyphal development and lesion progression then seem to slow down or even pause for a period of approximately 24 h, after which fungal and disease development continues (at 72 h), resulting in the final phenotype of delayed symptoms observed in CA-BcRAC-infected leaves. While the mechanism behind this phenomenon has yet to be resolved, it points to defects in the coordination of pathogenic development at both early and late infection phases. According to a working model proposed by Shlezinger and coworkers (34), B. cinerea first secretes necrosis-inducing compounds that mediate the early infection stage, and then the fungus produces programmed cell death (PCD)-inducing factors that mediate the spreading lesion. It is possible that regulation of these events is disrupted in the CA-BcRAC strain such that it produces larger amounts of necrosis-inducing factors (e.g., necrosis-inducing proteins or ROS) during the first hours p.i., but the transition between the early and late infection phases and secretion of PCD-inducing factors is delayed. It is also possible that constitutive activation of BcRAC leads to hypersensitivity of the fungus to certain plant defense compounds, such as ROS or phytoalexins (49). With respect to ROS, however, levels of H2O2 (detected by staining with diaminobenzidine) and superoxide (detected by staining with Nitro Blue Tetrazolium [NBT]) were unchanged in BcRAC compared to WT cultures. Furthermore, the two deletion strains also produced nearly WT ROS levels, except for elevated levels of H2O2 in culture filtrates of the Δbcras1 strain (data not shown). It is therefore unlikely that the changes in pathogenic development observed in the CA-BcRAC strain are due to changes in ROS production.
A small number of reports have connected RAC proteins with cell cycle progression (19, 50, 51). We used RAC- and cell cycle-inhibitory compounds to address the possible role of BcRAC in germination and nuclear division. Two sets of experiments were performed, the results of which support a role for BcRAC in regulation of the cell cycle. First, we used the inhibitors to treat conidia of a strain expressing H1-GFP, which enables live visualization of nuclei (38). These experiments showed an additive effect of the microtubule inhibitor BEN and the RAC-inhibitory compound NSC23766. Importantly, there was no such connection between NSC23766 and HU, which blocks DNA replication in the S phase. The higher sensitivity of conidia to NSC23766 is in agreement with the complete lack of conidial production in both the CA-BcRAC and Δbcrac strains, suggesting a specific role for BcRAC in conidial formation and germination.
Experiments with hyphae of the bcrac deletion and CA-BcRAC strains were performed to further address the specificity of NSC23766 for BcRAC and to pinpoint more precisely the stage at which BcRAC affects cells. Results of these experiments confirmed the specific inhibition of BcRAC by NSC23766, showing that treatment of WT hyphae with the compound leads to a phenotype that is almost identical to that of the bcrac deletion mutant: shorter and swollen hyphae, a shorter distance between septa, and a slightly increased number of nuclei/compartments (Fig. 6B, panels k and r; see Table S3 in the supplemental material). Furthermore, the effect of the drug was enhanced in the background of the CA-BcRAC strain, which was expected from inhibition of the BcRAC protein: the CA strain has a WT copy of BcRAC, and the observed phenotype is a net effect of competition between the CA and WT proteins for GTP. Therefore, in the absence of a WT copy (due to inhibition by NSC23766), the phenotype of the CA strain is expected to be enhanced.
Because we unequivocally showed that NSC23766 specifically blocks BcRAC without affecting even the closely related BcRAS1 or BcCDC42 protein, and based on the high similarity of its effects to those of BEN, we can propose that BcRAC affects the cell cycle by interfering with progression through mitosis (Fig. 7). In this case, namely, if activated BcRAC is necessary for the passage through mitosis, it is expected that the CA allele will cause hypernucleation due to promotion of uncontrolled nuclear divisions. Indeed, the larger-than-normal number of nuclei in hyphae of the CA-BcRAC strain is in accordance with this scenario. Thus, collectively, the genetic (mutant strains) and pharmacological data all support an association between BcRAC and cell cycle progression through mitosis. Our findings strengthen a small number of previous reports that hint at an association between RAC and the cell cycle: in the dimorphic fungus U. maydis, CDC42 and RAC affect cytokinesis (20), and in Colletotrichum gloeosporioides, modification of RAC leads to changes in the cell cycle and nuclear division (19). Interestingly, a multinucleated phenotype similar to the one observed in the CA-BcRAC strain (gain of function) was previously reported for B. cinerea BcCDC42 (38). In that case, the deletion (loss of function) mutant had multinucleated hyphae. Thus, two closely related RHO-type small GTPases, BcCDC42 and BcRAC, seem to have opposite effects on nuclear division: BcRAC promotes it, whereas BcCDC42 seems to suppress it. Whether BcRAC and BcCDC42 interact with each other, e.g., such that BcRAC negatively regulates the cell cycle by suppressing BcCDC42 activity, is unknown and warrants further investigation.
Supplementary Material
ACKNOWLEDGMENT
This work was funded by a trilateral research grant of the DFG (Tu50/15).
Footnotes
Published ahead of print 4 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00160-13.
REFERENCES
- 1.Gronover CS, Kasulke D, Tudzynski P, Tudzynski B. 2001. The role of G protein alpha subunits in the infection process of the gray mold fungus Botrytis cinerea. Mol. Plant Microbe Interact. 14:1293–1302 [DOI] [PubMed] [Google Scholar]
- 2.Williamson B, Tudzynski B, Tudzynski P, van Kan JA. 2007. Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol. 8:561–580 [DOI] [PubMed] [Google Scholar]
- 3.Schumacher J, Tudzynski P. 2012. Morphogenesis and infection in Botrytis cinerea. Top. Curr. Genet. 22:225–241 [Google Scholar]
- 4.Doehlemann G, Berndt P, Hahn M. 2006. Different signalling pathways involving a Galpha protein, cAMP and a MAP kinase control germination of Botrytis cinerea conidia. Mol. Microbiol. 59:821–835 [DOI] [PubMed] [Google Scholar]
- 5.Rui O, Hahn M. 2007. The Slt2-type MAP kinase Bmp3 of Botrytis cinerea is required for normal saprotrophic growth, conidiation, plant surface sensing and host tissue colonization. Mol. Plant Pathol. 8:173–184 [DOI] [PubMed] [Google Scholar]
- 6.Segmüller N, Ellendorf U, Tudzynski B, Tudzynski P. 2007. BcSAK1, a stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea. Eukaryot. Cell 6:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viaud M, Brunet-Simon A, Brygoo Y, Pradier JM, Levis C. 2003. Cyclophilin A and calcineurin functions investigated by gene inactivation, cyclosporin A inhibition and cDNA arrays approaches in the phytopathogenic fungus Botrytis cinerea. Mol. Microbiol. 50:1451–1465 [DOI] [PubMed] [Google Scholar]
- 8.Schumacher J, de Larrinoa IF, Tudzynski B. 2008. Calcineurin-responsive zinc finger transcription factor CRZ1 of Botrytis cinerea is required for growth, development, and full virulence on bean plants. Eukaryot. Cell 7:584–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harren K, Schumacher J, Tudzynski B. 2012. The Ca2+/calcineurin-dependent signaling pathway in the gray mold Botrytis cinerea: the role of calcipressin in modulating calcineurin activity. PLoS One 7:e41761. 10.1371/journal.pone.0041761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klimpel A, Gronover CS, Williamson B, Stewart JA, Tudzynski B. 2002. The adenylate cyclase (BAC) in Botrytis cinerea is required for full pathogenicity. Mol. Plant Pathol. 3:439–450 [DOI] [PubMed] [Google Scholar]
- 11.Schumacher J, Kokkelink L, Huesmann C, Jimenez-Teja D, Collado IG, Barakat R, Tudzynski P, Tudzynski B. 2008. The cAMP-dependent signaling pathway and its role in conidial germination, growth, and virulence of the gray mold Botrytis cinerea. Mol. Plant Microbe Interact. 21:1443–1459 [DOI] [PubMed] [Google Scholar]
- 12.Schumacher J, Viaud M, Simon A, Tudzynski B. 2008. The Galpha subunit BCG1, the phospholipase C (BcPLC1) and the calcineurin phosphatase co-ordinately regulate gene expression in the grey mould fungus Botrytis cinerea. Mol. Microbiol. 67:1027–1050 [DOI] [PubMed] [Google Scholar]
- 13.Bustelo XR, Sauzeau V, Berenjeno IM. 2007. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays 29:356–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFeo-Jones D, Scolnick EM, Koller R, Dhar R. 1983. ras-related gene sequences identified and isolated from Saccharomyces cerevisiae. Nature 306:707–709 [DOI] [PubMed] [Google Scholar]
- 15.Powers S, Kataoka T, Fasano O, Goldfarb M, Strathern J, Broach J, Wigler M. 1984. Genes in Saccharomyces cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell 36:607–612 [DOI] [PubMed] [Google Scholar]
- 16.Harris SD. 2011. Cdc42/Rho GTPases in fungi: variations on a common theme. Mol. Microbiol. 79:1123–1127 [DOI] [PubMed] [Google Scholar]
- 17.Fillinger S, Chaveroche MK, Shimizu K, Keller N, d'Enfert C. 2002. cAMP and ras signalling independently control spore germination in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 44:1001–1016 [DOI] [PubMed] [Google Scholar]
- 18.Virag A, Lee MP, Si H, Harris SD. 2007. Regulation of hyphal morphogenesis by cdc42 and rac1 homologues in Aspergillus nidulans. Mol. Microbiol. 66:1579–1596 [DOI] [PubMed] [Google Scholar]
- 19.Nesher I, Minz A, Kokkelink L, Tudzynski P, Sharon A. 2011. Regulation of pathogenic spore germination by CgRac1 in the fungal plant pathogen Colletotrichum gloeosporioides. Eukaryot. Cell 10:1122–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahlert M, Leveleki L, Hlubek A, Sandrock B, Bölker M. 2006. Rac1 and Cdc42 regulate hyphal growth and cytokinesis in the dimorphic fungus Ustilago maydis. Mol. Microbiol. 59:567–578 [DOI] [PubMed] [Google Scholar]
- 21.Boyce KJ, Hynes MJ, Andrianopoulos A. 2005. The Ras and Rho GTPases genetically interact to co-ordinately regulate cell polarity during development in Penicillium marneffei. Mol. Microbiol. 55:1487–1501 [DOI] [PubMed] [Google Scholar]
- 22.Lee N, Kronstad JW. 2002. Ras2 controls morphogenesis, pheromone response, and pathogenicity in the fungal pathogen Ustilago maydis. Eukaryot. Cell 1:954–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Dickman M. 2004. Dominant active Rac and dominant negative Rac revert the dominant active Ras phenotype in Colletotrichum trifolii by distinct signalling pathways. Mol. Microbiol. 51:1493–1507 [DOI] [PubMed] [Google Scholar]
- 24.Belden WJ, Larrondo LF, Froehlich AC, Shi M, Chen CH, Loros JJ, Dunlap JC. 2007. The band mutation in Neurospora crassa is a dominant allele of ras-1 implicating RAS signaling in circadian output. Genes Dev. 21:1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pontecorvo G, Roper JA, Hemmons LM, Macdonald KD, Bufton AW. 1953. The genetics of Aspergillus nidulans. Adv. Genet. 5:141–238 [DOI] [PubMed] [Google Scholar]
- 26.Cenis JL. 1992. Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Res. 20:2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolke Y, Tudzynski P. 2008. The small GTPase Rac and the p21-activated kinase Cla4 in Claviceps purpurea: interaction and impact on polarity, development and pathogenicity. Mol. Microbiol. 68:405–423 [DOI] [PubMed] [Google Scholar]
- 28.Malonek S, Rojas MC, Hedden P, Gaskin P, Hopkins PB. 2004. The NADPH-cytochrome P450 reductase gene from Gibberella fujikuroi is essential for gibberellin biosynthesis. J. Biol. Chem. 279:25075–25084 [DOI] [PubMed] [Google Scholar]
- 29.Finkelshtein A, Shlezinger N, Bunis O, Sharon A. 2011. Botrytis cinerea BcNma is involved in apoptotic cell death but not in stress adaptation. Fungal Genet. Biol. 48:621–630 [DOI] [PubMed] [Google Scholar]
- 30.Keogh RC, Deverall BJ, McLeod S. 1980. Comparison of histological and physiological responses to Phakopsora pachyrhizi in resistant and susceptible soybean. Trans. Br. Mycol. Soc. 74:329–333 [Google Scholar]
- 31.Schumacher J. 2012. Tools for Botrytis cinerea: new expression vectors make the gray mold fungus more accessible to cell biology approaches. Fungal Genet. Biol. 49:483–497 [DOI] [PubMed] [Google Scholar]
- 32.Giesbert S, Lepping HB, Tenberge KB, Tudzynski P. 1998. The xylanolytic system of Claviceps purpurea: cytological evidence for secretion of xylanases in infected rye tissue and molecular characterization of two xylanase genes. Phytopathology 88:1020–1030 [DOI] [PubMed] [Google Scholar]
- 33.Bourne HR, Sanders DA, McCormick F. 1991. The GTPase superfamily: conserved structure and molecular mechanism. Nature 349:117–127 [DOI] [PubMed] [Google Scholar]
- 34.Shlezinger N, Minz A, Gur Y, Hatam I, Dagdas YF, Talbot NJ, Sharon A. 2011. Anti-apoptotic machinery protects the necrotrophic fungus Botrytis cinerea from host-induced apoptotic-like cell death during plant infection. PLoS Pathog. 7:e1002185. 10.1371/journal.ppat.1002185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berepiki A, Lichius A, Read ND. 2011. Actin organization and dynamics in filamentous fungi. Nat. Rev. Microbiol. 9:876–887 [DOI] [PubMed] [Google Scholar]
- 36.Boyce KJ, Hynes MJ, Andrianopoulos A. 2003. Control of morphogenesis and actin localization by the Penicillium marneffei RAC homolog. J. Cell Sci. 116:1249–1260 [DOI] [PubMed] [Google Scholar]
- 37.Kwon MJ, Arentshorst M, Roos ED, van den Hondel CA, Meyer V, Ram AF. 2011. Functional characterization of Rho GTPases in Aspergillus niger uncovers conserved and diverged roles of Rho proteins within filamentous fungi. Mol. Microbiol. 79:1151–1167 [DOI] [PubMed] [Google Scholar]
- 38.Kokkelink L, Minz A, Al-Masri M, Giesbert SR, Sharon A, Tudzynski P. 2011. The small GTPase BcCdc42 affects nuclear division, germination and virulence of the gray mold fungus Botrytis cinerea. Fungal Genet. Biol. 48:1012–1019 [DOI] [PubMed] [Google Scholar]
- 39.Akbar H, Cancelas J, Williams DA, Zheng J, Zheng Y. 2006. Rational design and applications of a Rac GTPase-specific small molecule inhibitor. Methods Enzymol. 406:554–565 [DOI] [PubMed] [Google Scholar]
- 40.Temme N, Tudzynski P. 2009. Does Botrytis cinerea ignore H2O2-induced oxidative stress during infection? Characterization of botrytis activator protein 1. Mol. Plant Microbe Interact. 22:987–998 [DOI] [PubMed] [Google Scholar]
- 41.Heller J, Ruhnke N, Espino JJ, Massaroli M, Collado IG, Tudzynski P. 2012. The mitogen-activated protein kinase BcSak1 of Botrytis cinerea is required for pathogenic development and has broad regulatory functions beyond stress response. Mol. Plant Microbe Interact. 25:802–816 [DOI] [PubMed] [Google Scholar]
- 42.Fortwendel JR, Fuller KK, Stephens TJ, Bacon WC, Askew DS, Rhodes JC. 2008. Aspergillus fumigatus RasA regulates asexual development and cell wall integrity. Eukaryot. Cell 7:1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fortwendel JR, Zhao W, Bhabhra R, Park S, Perlin DS, Askew DS, Rhodes JC. 2005. A fungus-specific ras homolog contributes to the hyphal growth and virulence of Aspergillus fumigatus. Eukaryot. Cell 4:1982–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Araujo-Palomares CL, Richthammer C, Seiler S, Castro-Longoria E. 2011. Functional characterization and cellular dynamics of the CDC-42–RAC–CDC-24 module in Neurospora crassa. PLoS One 6:e27148. 10.1371/journal.pone.0027148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ha YS, Memmott SD, Dickman MB. 2003. Functional analysis of Ras in Colletotrichum trifolii. FEMS Microbiol. Lett. 226:315–321 [DOI] [PubMed] [Google Scholar]
- 46.Alspaugh JA, Cavallo LM, Perfect JR, Heitman J. 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36:352–365 [DOI] [PubMed] [Google Scholar]
- 47.Waugh MS, Nichols CB, DeCesare CM, Cox GM, Heitman J, Alspaugh JA. 2002. Ras1 and Ras2 contribute shared and unique roles in physiology and virulence of Cryptococcus neoformans. Microbiology 148:191–201 [DOI] [PubMed] [Google Scholar]
- 48.Heasman SJ, Ridley AJ. 2008. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 9:690–701 [DOI] [PubMed] [Google Scholar]
- 49.Sharon A, Finkelshtein A. 2009. Programmed cell death in fungus-plant interactions, p 221–236 In Esser K, Deising H. (ed), Plant relationships: the Mycota, vol 5 Springer, Berlin, Germany [Google Scholar]
- 50.Jaffe A, Hall A. 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21:247–269 [DOI] [PubMed] [Google Scholar]
- 51.Nesher I, Barhoom S, Sharon A. 2008. Cell cycle and cell death are not necessary for appressorium formation and plant infection in the fungal plant pathogen Colletotrichum gloeosporioides. BMC Biol. 6:9. 10.1186/1741-7007-6-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.