Abstract
Diagnosis of active tuberculosis by detection of urinary lipoarabinomannan (uLAM) from Mycobacterium tuberculosis is an attractive approach. Concentrating urine 100-fold allowed quantitation of uLAM at levels equal to picograms/ml of nonconcentrated urine. The approach of concentrating urine 100-fold improved the clinical sensitivity of the Clearview TB enzyme-linked immunosorbent assay (ELISA) from 7% to 57% yet impaired its specificity from 97% to 89%. (This study has been registered at University Hospital of Turku under registration no. 47/180/2009, Helsinki University Central Hospital under 149/2010, University Hospital of Kuopio under 105/2010, and China Medical University Hospital, Taichung, under DMR-99-IRB-075-2.)
TEXT
Measuring microbial antigens excreted into urine offers an attractive approach to diagnose acute infections (1–3). While the diagnostics of tuberculosis (TB) is challenging, an appealing approach is to detect urinary lipoarabinomannan (uLAM), the major structural component of the outer cell wall, shed into the environment by replicating, metabolically active, or degrading mycobacteria (4–6). Several publications have reported the use of the Clearview TB enzyme-linked immunosorbent assay (ELISA) (Inverness Medical Innovations, Bedford, United Kingdom) (7–12) or MTB LAM ELISA (Chemogen, Portland, ME) (13–17) to detect uLAM. The assay has almost invariably been found to have better sensitivity for cases with advanced HIV infection than for cases without HIV (7, 8, 11, 12, 16, 17). This has been explained by the progressively increasing bacillary burden in TB-positive and HIV-positive (TB+/HIV+) patients after the profound loss of CD4+ T cells and the inability to restrict mycobacterial growth, which results in heavy antigenemia and excretion of larger amounts of LAM into urine (8, 11, 12, 15).
The present study was carried out to (i) examine whether concentrating urine will improve the analytical sensitivity of the Clearview TB ELISA in TB+/HIV− patients, (ii) estimate the quantities of LAM excreted, (iii) look into the factors affecting analytical performance, and (iv) investigate the correlation of LAM detection rates in concentrated and nonconcentrated urine with sputum staining.
Midstream urine samples were collected in Finland (F) and Taiwan (T) from adult patients with active pulmonary TB (PF-TB, n = 28, and PT-TB, n = 17) or extrapulmonary TB (EPF-TB, n = 7, and EPT-TB, n = 3), miliary TB (n = 2), latent TB (LTBI; n = 15), or treated TB (n = 4) infections, from disease control groups (n = 60), and from healthy volunteers (n = 101). The clinical and demographic details on enrolled groups are presented in Text S1 and Table S2 in the supplemental material; the regents and the procedure are described in Text S3. Ethical clearances were as follows: University Hospital of Turku, 47/180/2009; Helsinki University Central Hospital, 149/2010; University Hospital of Kuopio, 105/2010; China Medical University Hospital, Taichung, DMR-99-IRB-075-2.
When optimizing the assay, we found that LAM dissolved in urine produced higher optical densities (ODs) than that dissolved in water, and a wide range of pHs (>3) was tolerable without deterioration. The calibration curves were prepared as described in Text S3 in the supplemental material; the effect of concentration on the ODs is shown in Fig. S4. The theoretical analytical sensitivities (8 replicates) were 320 and 15 pg/ml for the nonconcentrated and concentrated urine samples, respectively.
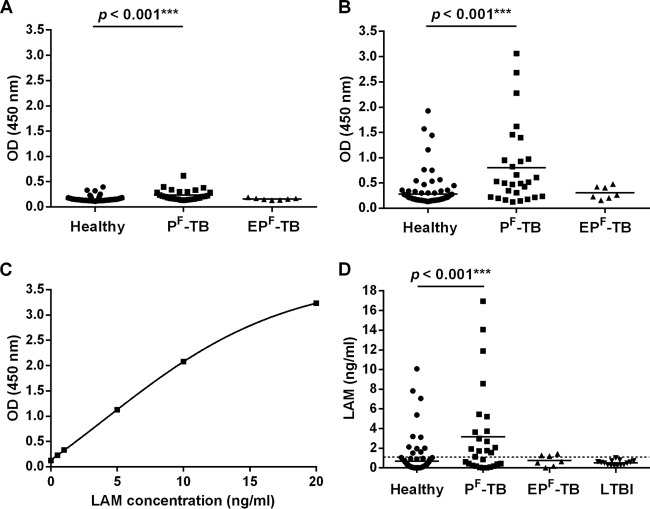
The urine samples were analyzed in both nonconcentrated and 100-fold-concentrated forms. As shown in Fig. 1A and B, the ODs were higher and the dynamic range wider for the 100-fold-concentrated (OD range, 0.132 to 3.060) than for the nonconcentrated (OD range, 0.132 to 0.395) samples. Although a statistically significant difference was reached for patient groups with both the concentration method (P < 0.001) and the original method (P < 0.001), practical discrimination between the groups seemed possible only with the modified approach. Generally, assay imprecision tends to be higher at low OD values, which leads to inconsistent and inaccurate interpretations. By the modified method with calibration curves constructed for each run (see, e.g., Fig. 1C) and receiver operating characteristic (ROC) analysis (Fig. S5), the cutoff level was estimated at 1.1 ng/ml (Table S6). In these settings, uLAM was detectable in 16/28 (57%) PF-TB patients (Fig. 1D). When taking into account the 100-fold-concentration coefficient, the estimated range of excreted uLAM in native samples of the PF-TB group proved to be 0 to 170 pg/ml. In the EPF-TB group, uLAM excretion was at a maximum of 14 pg/ml, whereas in a patient with miliary TB, uLAM excretion was at 166 pg/ml. In a sample from another patient with miliary TB, uLAM was still detectable at a concentration of 24 pg/ml after 3 months of specific therapy.
Fig 1.
Analysis of urine samples from patient groups and healthy volunteers. ODs of nonconcentrated (A) and concentrated (B) samples from healthy (n = 101), PF-TB (n = 28), and EPF-TB (n = 7) groups. (C) Calibration curve of uLAM samples (range, 0 to 20 ng/ml, nonconcentrated). (D) uLAM concentrations estimated from the calibration curve. Healthy (n = 101), PF-TB (n = 28), EPF-TB (n = 7), and LTBI (n = 15) groups. Horizontal bars show the mean concentrations. The cutoff (1.1 ng/ml) is presented with a dashed line.
Overall, the concentration approach improved the sensitivity of the assay from 7% to 57% but decreased its specificity from 97% to 89% (see Table S6 in the supplemental material). An analysis of the false-positivity rate showed that concentration did not always augment false reactivity. Interestingly, in some samples, false positivity disappeared after concentration but occurred at the same time in some others; the ODs were, however, close to the cutoff. The majority (12/15 [80%]) of samples in the urinary tract infection (UTI) group gave a false-positive result when analyzed as a 100-fold concentrate, while only 1 sample without concentration proved positive (7%) (Table S6 and Fig. S7). On the other hand, spiking urine with Escherichia coli, Enterococcus faecalis, and Candida albicans at 104 to 106 CFU/ml did not cause false positivity (Fig. S7). With Corynebacterium sp., it did, yet in a dose-dependent manner (Fig. S7). Spiking with Mycobacterium tuberculosis produced high ODs already at 10 CFU/ml (Fig. S7). Samples from pneumococcal (PNC) pneumonia and non-PNC groups showed false-positive results in 2/7 (29%), 3/7 (43%), and 0/7 (0%) cases when they were concentrated 75-fold and in 1/7 (14%) cases when they were native (Table S6 and Fig. S7). d-Mannose and l-arabinose at concentrations of 1 mg/ml each had no effect on the results. uLAM was not detectable in a sample from a patient with pulmonary M. avium infection, but with a modified method, uLAM was detectable at 12 pg/ml (nonconcentrated). It appears probable that polyclonal antibodies of the assay cannot discriminate AraLAM from ManLAM of the cell wall structures of nontuberculous and pathogenic mycobacteria, respectively (18).
Compared with sputum staining, uLAM detection identified some but not all patients; thus, the techniques are supplemental (Table 1). There were only two cases with simultaneously positive uLAM (nonconcentrated) and sputum smear staining results, but more samples became so when the modified method was used.
Table 1.
Comparison of positive results by the uLAM detection method and sputum staininga
| Sample type | No. (%) of samples that were positive or negative by each method |
Total no. of LAM samples (%) | |
|---|---|---|---|
| AFB+ samples | AFB− samples | ||
| 100-fold-concentrated urine | |||
| LAM+ | 16 (57) | 1 (4) | 17 (61) |
| LAM− | 8 (29) | 3 (11) | 11 (39) |
| Total no. of AFB+ samples | 24 (86) | 4 (14) | |
| Nonconcentrated urine | |||
| LAM+ | 2 (7) | 0 (0) | 2 (7) |
| LAM− | 22 (79) | 4 (14) | 26 (93) |
| Total no. of AFB+ samples | 24 (86) | 4 (14) | |
AFB, acid-fast bacillus.
uLAM excretion kinetics could be exploited in treatment monitoring, as they are utilized for some other infections (2, 19, 20). Samples from a patient with PF-TB were analyzed at 11, 14, and 15 days and 9 months later. When used in a concentrated form, the acute-phase samples were clearly above the cutoff, but the convalescent-phase sample was negative. In contrast, when the samples were tested intact, uLAM was not detectable at any time point (see Fig. S8 in the supplemental material).
The reported sensitivities and specificities of the Clearview TB ELISA method were 13% to 93% and 87% to 99%, respectively (4–6). As far as we are aware, this is the first study to demonstrate that urine concentration, a simple preanalytical step, can improve the detection of small amounts of uLAM in TB+/HIV− patients and raise clinical sensitivity, yet, as a trade-off, it somewhat decreases specificity. Impairment of specificity was most evident in the UTI group. These results were consistent with earlier findings (13), but false positivity might not be caused by cross-reactivity with bacterial components per se. Rather, nonspecificity might be attributed to inflammation, an issue to be studied further. The specificity of polyclonal antibodies in the Clearview TB ELISA is not reported. LAM molecules are not confined to mycobacteria (18), and therefore, the antibodies may recognize LAM-like structures from actinomycetes. Our data corroborated earlier findings that small amounts of Corynebacterium spp. belonging to microbiota of mucous membranes can potentially be hazardous. Antigens from several species of fungi were reported to produce cross-reactivity (8). In the present study, Candida albicans, an opportunistic yeast found often in the female genitourinary tract, had no such effect.
Qualitative analysis allows only a dichotomous distribution to positive and negative results. It has been reported that uLAM is excreted in variable concentrations between 100 pg/ml and several hundred ng/ml (9, 13, 15, 21). If 100 pg/ml were to be a clinically relevant concentration, the cutoff should be well below this value. However, using the Clearview TB ELISA without the concentrating step, 100 pg/ml is not distinguishable from the background values (see Fig. S4 in the supplemental material). The analytical sensitivity of an in-house enzyme immunoassay (EIA) was 1 ng/ml of 50-fold-concentrated LAM, which corresponds to 20 pg/ml of the untreated urine (22). This method, with 450 pg/ml as a cutoff, was evaluated later on Ethiopian TB patients and attained a clinical sensitivity and specificity of 74% and 90%, respectively (21); the HIV status of the patients was not reported, however. In another study, only a modest improvement (from 33% to 38%) was reached with the MTB LAM ELISA when the samples from HIV+/TB+ patients were concentrated; however, the concentration coefficient was not reported (15). In a study where the Clearview TB ELISA was used as instructed, the median concentrations of excreted uLAM were 1.25 (interquartile range [IQR], 0.2 to 7.1) ng/ml and 0.1 (IQR, 0 to 0.5) ng/ml in patients with and without immune reconstitution inflammatory syndrome (IRIS), respectively (9). In our opinion, if the manufacturer's instructions are followed, uLAM in 0.1 ng/ml can hardly be distinguished from the zero calibrator (Fig. S4B).
It has recently been hypothesized that the negative results obtained with the Clearview TB ELISA for some TB patients could be explained by the formation of immune complexes between the excreted uLAM and anti-LAM immunoglobulins (11). The molecular sizes of these complexes may be too big for these molecules to pass through the glomerular basement membrane. This hypothesis seems far too theoretical, because anti-LAM antibodies might be found in the circulation of all TB patients (23). As TB is, in fact, a chronic condition, the immune system is continuously being sensitized to alien antigens. We incline to believe that negative results are more likely to be attributed to low assay sensitivity. Furthermore, when the excretion of M. tuberculosis DNA into the urine of TB+/HIV+ patients was tested with the Xpert MTB/RIF assay (11), a positive correlation with the Clearview TB ELISA was found. This may mean that mycobacteriuria, which might occur due to disseminated or urogenital TB of HIV+ individuals, accounts for the better sensitivity of the Clearview TB ELISA for samples from these patients.
To conclude, the present study is the first to show that a preanalytic 100-fold-concentrating step increases significantly the sensitivity of the Clearview TB ELISA in the TB+/HIV− group. Our data suggest that the detection of uLAM from a concentrated sample may serve as a viable tool for TB diagnostics in various types of TB patients, yet this method still needs to be refined.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge financial support from the following private Finnish foundations: the Tuberculosis Association of the University of Tampere, the Nummela Sanatorium Association, the Foundation of the Finnish Anti-Tuberculosis Association, the Research Foundation of Pulmonary Diseases, and the Väinö and Laina Kivi Foundation. We declare that we have no financial relationship with any commercial entity that has any interest in the subject of this paper.
We thank the clinicians from Peijas Hospital (Vantaa, Finland) and the Department of Mycobacteriology, HUSLAB, for help in recruiting TB patients.
T.T., L.S., and B.S. designed the study. T.V., A.K., T.T., B.S., and C.-L.K. submitted applications for ethical permission. A.K., L.S., T.V., L.P., R.E.-P., H.V., R.T., T.T., A.K., and C.-Y.C. collected clinical samples. L.S., B.S., and M.S. performed the study. T.T. and L.S. interpreted the results and drafted the paper. All authors contributed to critically revising the manuscript.
Footnotes
Published ahead of print 3 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00375-13.
REFERENCES
- 1.Alere Inc 2013. BinaxNOW Legionella test manual. Alere Inc., Waltham, MA [Google Scholar]
- 2.Alere Inc 2013. BinaxNOW S. pneumoniae test manual. Alere Inc., Waltham, MA [Google Scholar]
- 3.Tam FC, Ling TK, Wong KT, Leung DT, Chan RC, Lim PL. 2008. The TUBEX test detects not only typhoid-specific antibodies but also soluble antigens and whole bacteria. J. Med. Microbiol. 57:316–323 [DOI] [PubMed] [Google Scholar]
- 4.Minion J, Leung E, Talbot E, Dheda K, Pai M, Menzies D. 2011. Diagnosing tuberculosis with urine lipoarabinomannan: systematic review and meta-analysis. Eur. Respir. J. 38:1398–1405 [DOI] [PubMed] [Google Scholar]
- 5.Peter J, Green C, Hoelscher M, Mwaba P, Zumla A, Dheda K. 2010. Urine for the diagnosis of tuberculosis: current approaches, clinical applicability, and new developments. Curr. Opin. Pulm. Med. 16:262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawn SD. 2012. Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: a state of the art review. BMC Infect. Dis. 12:103. 10.1186/1471-2334-12-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah M, Variava E, Holmes CB, Coppin A, Golub JE, McCallum J, Wong M, Luke B, Martin DJ, Chaisson RE, Dorman SE, Martinson NA. 2009. Diagnostic accuracy of a urine lipoarabinomannan test for tuberculosis in hospitalized patients in a high HIV prevalence setting. J. Acquir. Immune Defic. Syndr. 52:145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dheda K, Davids V, Lenders L, Roberts T, Meldau R, Ling D, Brunet L, van Zyl Smit R, Peter J, Green C, Badri M, Sechi L, Sharma S, Hoelscher M, Dawson R, Whitelaw A, Blackburn J, Pai M, Zumla A. 2010. Clinical utility of a commercial LAM-ELISA assay for TB diagnosis in HIV-infected patients using urine and sputum samples. PLoS One 5:e9848. 10.1371/journal.pone.0009848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conesa-Botella A, Loembe MM, Manabe YC, Worodria W, Mazakpwe D, Luzinda K, Mayanja-Kizza H, Miri M, Mbabazi O, Koole O, Kestens L, Colebunders R, for the Group TB IRIS 2011. Urinary lipoarabinomannan as predictor for the tuberculosis immune reconstitution inflammatory syndrome. J. Acquir. Immune Defic. Syndr. 58:463–468 [DOI] [PubMed] [Google Scholar]
- 10.Gounder CR, Kufa T, Wada NI, Mngomezulu V, Charalambous S, Hanifa Y, Fielding K, Grant A, Dorman S, Chaisson RE, Churchyard GJ. 2011. Diagnostic accuracy of a urine lipoarabinomannan enzyme-linked immunosorbent assay for screening ambulatory HIV-infected persons for tuberculosis. J. Acquir. Immune Defic. Syndr. 58:219–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood R, Racow K, Bekker LG, Middelkoop K, Vogt M, Kreiswirth BN, Lawn SD. 2012. Lipoarabinomannan in urine during tuberculosis treatment: association with host and pathogen factors and mycobacteriuria. BMC Infect. Dis. 12:47. 10.1186/1471-2334-12-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talbot E, Munseri P, Teixeira P, Matee M, Bakari M, Lahey T, von Reyn F. 2012. Test characteristics of urinary lipoarabinomannan and predictors of mortality among hospitalized HIV-infected tuberculosis suspects in Tanzania. PLoS One 7:e32876. 10.1371/journal.pone.0032876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehme C, Molokova E, Minja F, Geis S, Loscher T, Maboko L, Koulchin V, Hoelscher M. 2005. Detection of mycobacterial lipoarabinomannan with an antigen-capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Trans. R. Soc. Trop. Med. Hyg. 99:893–900 [DOI] [PubMed] [Google Scholar]
- 14.Daley P, Michael JS, Hmar P, Latha A, Chordia P, Mathai D, John KR, Pai M. 2009. Blinded evaluation of commercial urinary lipoarabinomannan for active tuberculosis: a pilot study. Int. J. Tuberc. Lung Dis. 13:989–995 [PMC free article] [PubMed] [Google Scholar]
- 15.Lawn SD, Edwards DJ, Kranzer K, Vogt M, Bekker LG, Wood R. 2009. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS 23:1875–1880 [DOI] [PubMed] [Google Scholar]
- 16.Mutetwa R, Boehme C, Dimairo M, Bandason T, Munyati SS, Mangwanya D, Mungofa S, Butterworth AE, Mason PR, Corbett EL. 2009. Diagnostic accuracy of commercial urinary lipoarabinomannan detection in African tuberculosis suspects and patients. Int. J. Tuberc. Lung Dis. 13:1253–1259 [PMC free article] [PubMed] [Google Scholar]
- 17.Reither K, Saathoff E, Jung J, Minja LT, Kroidl I, Saad E, Huggett JF, Ntinginya EN, Maganga L, Maboko L, Hoelscher M. 2009. Low sensitivity of a urine LAM-ELISA in the diagnosis of pulmonary tuberculosis. BMC Infect. Dis. 9:141. 10.1186/1471-2334-9-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra AK, Driessen NN, Appelmelk BJ, Besra GS. 2011. Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS Microbiol. Rev. 35:1126–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sopena N, Sabria M, Pedro-Botet ML, Reynaga E, Garcia-Nunez M, Dominguez J, Matas L. 2002. Factors related to persistence of Legionella urinary antigen excretion in patients with Legionnaires' disease. Eur. J. Clin. Microbiol. Infect. Dis. 21:845–848 [DOI] [PubMed] [Google Scholar]
- 20.Dominguez JA, Manterola JM, Blavia R, Sopena N, Belda FJ, Padilla E, Gimenez M, Sabria M, Morera J, Ausina V. 1996. Detection of Legionella pneumophila serogroup 1 antigen in nonconcentrated urine and urine concentrated by selective ultrafiltration. J. Clin. Microbiol. 34:2334–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tessema TA, Hamasur B, Bjun G, Svenson S, Bjorvatn B. 2001. Diagnostic evaluation of urinary lipoarabinomannan at an Ethiopian tuberculosis centre. Scand. J. Infect. Dis. 33:279–284 [DOI] [PubMed] [Google Scholar]
- 22.Hamasur B, Bruchfeld J, Haile M, Pawlowski A, Bjorvatn B, Kallenius G, Svenson SB. 2001. Rapid diagnosis of tuberculosis by detection of mycobacterial lipoarabinomannan in urine. J. Microbiol. Methods 45:41–52 [DOI] [PubMed] [Google Scholar]
- 23.Kanaujia GV, Lam PK, Perry S, Brusasca PN, Catanzaro A, Gennaro ML. 2005. Integration of microscopy and serodiagnostic tests to screen for active tuberculosis. Int. J. Tuberc. Lung Dis. 9:1120–1126 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.