Abstract
Skin vaccination with influenza virus-like particles (VLPs) using microneedles has been shown to induce protection similar to or better than that induced by intramuscular immunization. In this study, we examined the long-term protective efficacy of influenza (H1N1 A/PR/8/34) VLPs after skin vaccination using microneedle patches coated with the vaccine. Microneedle vaccination of mice in the skin induced 100% protection against lethal challenge infection with influenza A/PR/8/34 virus 14 months after a single vaccine dose. Influenza virus-specific total IgG response and hemagglutination inhibition (HAI) titers were maintained at high levels for over 1 year after microneedle vaccination. Microneedle vaccination also induced substantial levels of lung IgG and IgA antibody responses, and antibody-secreting plasma cells from spleen and bone marrow, as well as conferring effective control of lung viral loads, resulting in complete protection 14 months after vaccination. These strong and long-lasting immune responses were enabled in part by stabilization of the vaccine by formulation with trehalose during microneedle patch fabrication. Administration of the stabilized vaccine using microneedles was especially effective at enabling strong recall responses measured 4 days after lethal virus challenge, including increased HAI and antibody-secreting cells in the spleen and reduced viral titer and inflammatory response in the lung. The results in this study indicate that skin vaccination with VLP vaccine using a microneedle patch provides long-term protection against influenza in mice.
INTRODUCTION
Influenza is a serious respiratory disease spreading around the world, causing seasonal epidemics and recurrent outbreaks, resulting in more than 220,000 hospitalizations. Approximately 36,000 people die in the United States every year (1, 2). The experience with the 2009 H1N1 pandemic demonstrated that conventional vaccination showed a significant delay in controlling the new pandemic spread. Significant shortages and delays happened in the supply of the 2009 pandemic vaccine, due in part to lower growth in egg substrates compared to those observed with seasonal vaccines. New approaches are therefore needed to develop an effective influenza vaccine that can be rapidly produced on a large scale with low production costs.
Virus-like particles (VLPs) are noninfectious, thus requiring no exceptional biosafety containment, and can be manufactured rapidly. They present structurally native, immunologically relevant viral antigens. Influenza VLPs, as a promising vaccine candidate, have been shown to induce high neutralizing antibody titers and strong protective immunity (3–7). Influenza VLP vaccines were shown to be more immunogenic and to provide better protection than a commercial split vaccine in ferrets (8) or a soluble hemagglutinin (HA) protein vaccine (9), indicating the possibility that influenza VLPs could be a new vaccine platform (10, 11).
Skin is considered an important peripheral immune organ rich in potent immune-inducing cells, including Langerhans cells (LCs), dermal dendritic cells (DCs), and keratinocytes (12–15). Thus, vaccine delivery via skin has been suggested to be an attractive approach for vaccination, especially using a microneedle patch (16–23). Microneedles are micrometer-scale needles that can be coated with vaccine for simple, painless, and targeted delivery of the vaccine to the skin (24). It was also reported that microneedle vaccination induces protective immunity at a lower dose and provides vaccine dose-sparing effects (25). In addition, skin immunization with microneedles coated with influenza VLPs or inactivated viral vaccines in the presence of a stabilizer, trehalose, was shown to induce better protection than intramuscular immunization (19, 20, 26, 27). However, protective immunity longer than 6 months has received only limited attention after microneedle vaccination (28).
In this study, we determined the protective efficacy of influenza VLP vaccine delivered to the skin using coated microneedles. Microneedle vaccine effects after over a year of immunization were compared in formulations with and those without trehalose as a stabilizer. We found that stabilized microneedle vaccination in the skin provided improved efficacy of protection after 14 months of vaccination.
MATERIALS AND METHODS
Virus and cells.
Influenza virus, A/PR/8/1934 (H1N1, abbreviated A/PR/8), was grown in 10-day-old embryonated hen's eggs for 2 days at 36 to 37°C. Allantoic fluids were harvested from infected eggs after storage overnight at 4°C and centrifuged to remove cell debris. The virus was purified from allantoic fluids by using a discontinuous sucrose gradient (15%, 30%, and 60% layers) and ultracentrifugation (at 28,000 rpm for 60 min). The purified virus was inactivated by mixing the virus with formalin at a final concentration of 1:4,000 (vol/vol). For use in challenge experiments, mouse-adapted A/PR/8 was prepared as lung homogenates of infected mice as described previously (6). Spodoptera frugiperda Sf9 cells were maintained in suspension in serum-free SF900II medium (Gibco-BRL). MDCK cells were grown and maintained in Dulbecco's modified Eagle's medium (DMEM).
Preparation of influenza VLPs and microneedle patches.
Influenza VLPs containing hemagglutinin (HA) and matrix M1 derived from A/PR/8 were prepared as described previously (6). Briefly, Sf9 insect cells were coinfected with recombinant baculoviruses expressing HA and M1 at multiplicities of infection of 2 and 1, respectively. Influenza VLPs released into culture medium were purified using (15%, 30%, and 60% layers) ultracentrifugation (28,000 rpm, 60 min). The purified VLPs were characterized by Western blot and hemagglutination activity analysis (6, 29). The content of HA was approximately 10% of total proteins of influenza VLPs, which is similar to that found in a previous report (6).
Microneedle preparations and coatings were performed as described previously (27, 30). Briefly, rows of solid metal microneedles were fabricated by cutting needle structures from stainless sheets (SS304, 75-μm thickness; McMaster-Carr, Atlanta, GA) using an infrared laser (Resonetics Maestro, Nashua, NH), and the microneedles measured 700 μm in length and 160 μm in width. In order to apply a vaccine as a coating on the surface of microneedles, microneedles were dipped six times at 25°C into coating solution using a dip-coating device. The coating solution was composed of 1% (wt/vol) carboxymethyl cellulose (CMC) sodium salt (Carbo-Mer, San Diego, CA) and 0.5% (wt/vol) Lutrol F-68 NF (BASF, Mount Olive, NJ) with or without 15% (wt/vol) d-(+)-trehalose dihydrate (Sigma-Aldrich, St. Louis, MO). Four micrograms of influenza VLPs (total proteins) was applied as a coating onto a microneedle array with 5 needles in the presence or absence of a trehalose disaccharide stabilizer (15% [wt/vol]; Sigma-Aldrich). Although trehalose may slightly improve delivery kinetics due to its solubility characteristics, the main function of trehalose is to stabilize the HA of VLPs (20, 25, 26, 30). Microneedles coated with VLP vaccines were used to vaccinate animals. Mock vaccination was carried out using microneedles without VLP vaccine.
Immunization and challenge infection.
Female inbred BALB/c mice (Charles River) aged 6 to 8 weeks were used. Groups of mice (12 mice per group) were immunized with a microneedle array coated with VLP vaccine (4 μg total VLP proteins) for delivery to the skin. For microneedle delivery, mice were anesthetized with ketamine (110 mg/kg of body weight, Abbott Laboratories) mixed with xylazine (11 mg/kg; Phoenix Scientific). Hair on the dorsal surface of mice was removed with hair-removing cream (Nair) with a moisturized cotton stick. After cleaning with a soaked cotton ball (70% ethanol) and drying with a hair dryer, an array of vaccine-coated microneedles was inserted into the skin and held for 10 min for release of the vaccine antigen from the coated microneedle.
For challenge infections, mice lightly anesthetized with isoflurane were intranasally infected with a lethal dose of A/PR8 virus (10× 50% lethal dose [LD50]) in 50 μl of phosphate-buffered saline (PBS) at 14 months after a single VLP vaccine dose. Mice were observed daily to monitor changes in body weight and to record mortality. We followed an approved Emory University Institutional Animal Care and Use Committee (IACUC) protocol for this study, in which animals losing more than 25% body weight were euthanized.
Antibody responses and HAI titer.
Influenza virus-specific total IgG or IgA antibody responses were determined on enzyme-linked immunosorbent assay (ELISA) plates coated with inactivated A/PR8 viral antigen and by using anti-mouse IgG isotype-specific secondary antibodies as described previously (6). ELISA titers were defined as the reciprocal of the highest dilution with an optical density (OD) value that is twice the OD background. For determination of hemagglutination inhibition (HAI) titers, serum samples were first treated with receptor-destroying enzyme (Denka Seiken) by incubation overnight at 37°C and then incubated for 30 min at 56°C. Sera were serially diluted, mixed with 4 HA units (HAU) of influenza A/PR8 virus, and incubated for 30 min at room temperature prior to adding 0.5% chicken red blood cells. The reciprocal of the highest serum dilution preventing hemagglutination was scored as the HAI titer.
Lung viral titer and lung inflammatory cytokine assays.
Lung viral titers at day 4 postchallenge were determined by counting plaques formed on the MDCK cells as described previously (6). The whole-lung extracts prepared as homogenates using frosted glass slides were centrifuged at 1,000 rpm for 10 min to collect supernatants. The lung supernatants were frozen and kept at −70°C until used for immunoglobulin and virus titers and cytokine assays. Inflammatory cytokines gamma interferon (IFN-γ), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) in lungs and bronchoalveolar lavage fluid (BALF) collected at day 4 postchallenge were analyzed by the Ready-Set-Go cytokine kit (eBioscience) according to the manufacturer's procedure as previously described (31, 32).
Analysis of antibody-secreting cell response in vitro and antibody responses postchallenge.
Virus-specific antibody responses were determined from serum, lung, bone marrow, and spleen at day 4 postchallenge using ELISA (6, 20, 29). To determine antibody-producing cell responses in vitro, bone marrow and spleen cells were cultured in multiscreen 96-well filtration plates (Millipore) coated with inactivated A/PR/8 viral antigen with RPMI medium for 2 and 4 days (5 × 105 cells per well), and levels of virus-specific antibodies secreted into the culture medium were determined (6, 20, 29).
Histopathology.
Mice were challenged with A/PR/8/34 virus after 6 months of immunization with 4 μg of influenza VLPs. For histological analysis of lung tissue, mice were anesthetized with isoflurane and exsanguinated from the abdominal aorta. Lung samples were fixed in 10% neutral buffered formalin for 48 h, transferred to 70% ethanol, embedded in paraffin blocks, sectioned into a thickness of 5 μm, and stained with hematoxylin and eosin (H&E) as described previously (33, 34).
Statistics.
All parameters were recorded for individuals within all groups. Three independent experiments have been performed, and the data shown in the figures consist of the averages of several independent experiments. Statistical comparisons were carried out using the analysis of variance (ANOVA) and Npar one-way Kruskal-Wallis tests of the PC-SAS system. P values of 0.05 or less were considered significant.
RESULTS
High levels of long-lasting virus-specific antibodies were achieved after microneedle VLP vaccination.
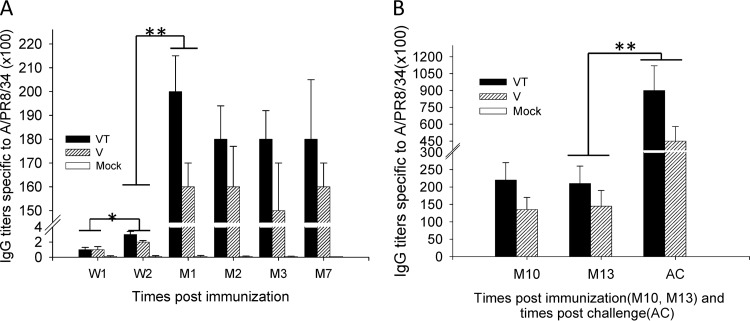
Our previous studies demonstrated that trehalose-stabilized microneedle influenza vaccines were effective in inducing higher recall immune responses and enhanced immunity (20, 26, 30). In the present study, to determine long-term antibody responses after skin delivery of vaccines, groups of mice were immunized in the skin with influenza VLPs using coated microneedles with and without trehalose stabilizer. Levels of total IgG antibody titers specific to influenza virus (A/PR/8) were determined in serum samples collected at 1 and 2 weeks and 1, 2, 3, 7, 10, and 13 months after a single vaccination and at month 14 after challenge (Fig. 1). At an early time point of 1 week, virus-specific IgG antibody levels were detected at very low levels (Fig. 1A). At week 2 after microneedle vaccination, IgG antibodies were detected at increased levels (Fig. 1A; *, P < 0.05). At month 1 after microneedle vaccination, both the trehalose-stabilized microneedle group (VT) and the trehalose-free microneedle group (V) showed high levels of virus-specific antibodies compared to that at week 2 (Fig. 1A; **, P < 0.01) and then maintained high levels at months 2, 3, 7, and 10 and up to month 13 after microneedle vaccination (Fig. 1A and B). IgG titers in the VT group were significantly higher than those in the V group at all time points except week 1 (P < 0.05). Interestingly, the trehalose-stabilized VT group showed a trend of inducing higher levels of IgG2a isotype antibody levels (see Fig. S1 in the supplemental material).
Fig 1.
Influenza A/PR8 virus-specific IgG responses. Groups of mice (n = 12) immunized with a single dose of VLPs. Mice (n = 12 per group) were immunized with microneedles coated with 4 μg of influenza VLPs. At weeks 1 and 2 (W1 and W2) and months 1, 2, 3, 7, 10, and 13 (M1, M2, M3, M7, M10, and M13) after a single-dose vaccination, blood samples were collected. At month 14, mice were challenged with a high lethal dose of A/PR8 virus (10× LD50) and IgG levels were measured 4 days after challenge (AC). The groups of immunized mice were designated V (microneedle vaccine without trehalose), VT (microneedle vaccine with trehalose as a stabilizer), and Mock (placebo microneedles with coating buffer only). Significant differences were detected between W1 and W2 (*, P < 0.05), W2 and M1 (**, P < 0.01), and M13 and AC (**, P < 0.01). Three independent experiments have been performed, and the data shown in the figures consist of the averages of several independent experiments. Data show averages ± standard errors of the means from 6 mice.
Groups of mice were challenged 14 months after microneedle vaccination. A significant increase in levels of virus-specific antibodies was observed at day 4 postchallenge in both stabilized (VT) and nonstabilized (V) microneedle groups over that prior to challenge (Fig. 1B; **, P < 0.01). In particular, we observed a greater increase in virus-specific antibody titers from the trehalose-stabilized microneedle group (VT) compared to that in the trehalose-free microneedle group (V) as well as higher levels of IgG2a isotype antibodies in the VT group at day 4 postchallenge (see Fig. S1 in the supplemental material). Taken together, stabilized microneedle immunization could induce effective memory B cells that differentiate rapidly into antibody-secreting cells upon exposure to viral infection. The results also suggest that microneedle immunizations in the skin with VLP vaccines (VT and V) were effective in inducing virus-specific antibodies for a long period of time.
Stabilized microneedle vaccine contributes significantly in enhancing recall HAI activity compared to trehalose-free microneedle vaccine.
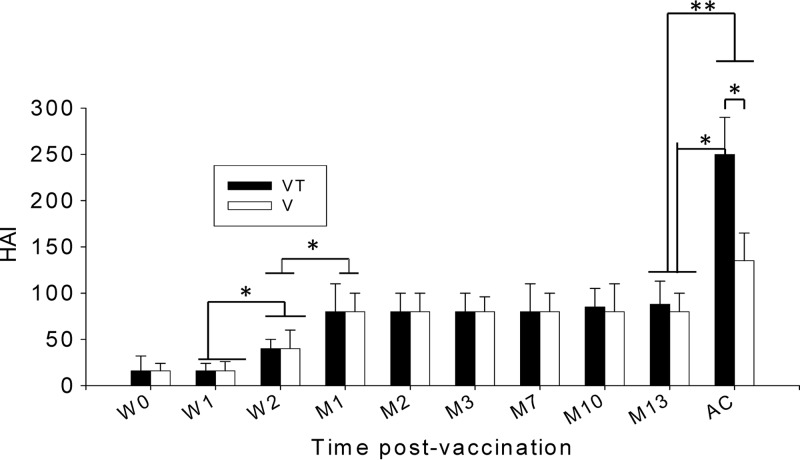
Titers of hemagglutination-inhibiting activity are used as a criterion in assessing vaccine efficacy. We determined HAI titers in serum samples collected at different time points after microneedle vaccination. At week 2 after microneedle vaccination, an increased HAI titer was observed (Fig. 2; *, P < 0.05) compared to week 1 and week 0. At 1 month after microneedle vaccination, an HAI titer of approximately 80 was observed and maintained up to 13 months (Fig. 2; *, P < 0.05). No significant differences were found between the trehalose-stabilized (VT) and nonstabilized (V) microneedle influenza VLP vaccines. However, when serum samples were collected at day 4 postchallenge, significant increases of HAI titers were detected in both trehalose-stabilized and nonstabilized groups (Fig. 2; *, P < 0.05, and **, P < 0.01). Interestingly, the trehalose-stabilized group showed much higher increases in HAI titer (Fig. 2; *, P < 0.05) than did the nonstabilized group. These results suggest that stabilization of microneedles coated with influenza vaccines using trehalose may be important in inducing a high level of recall HAI activities, which are functional and protective antibody responses to influenza virus.
Fig 2.
Hemagglutination inhibition titers. HAI titers were determined at weeks 0, 1, and 2 (W0, W1, and W2) and months 1, 2, 3, 7, 10, and 13 (M1, M2, M3, M7, M10, and M13) postimmunization and at day 4 postchallenge (AC). Significantly higher HAI titers were found from week 2, and higher titers were found from month 1 postvaccination and maintained until month 13 compared to week 1 or week 2 (*, P < 0.05; **, P < 0.01). V, microneedle vaccine without trehalose; VT, microneedle vaccine with trehalose. Data show averages ± standard errors of the means from 6 mice.
Stabilized microneedle influenza VLP vaccine confers improved protection.
To determine and compare the long-term protective efficacies after delivery of influenza VLP vaccines, groups of mice, including those microneedle treated with (VT) or without (V) trehalose, were challenged with a lethal dose of A/PR/8 virus (10 LD50) 14 months after a single microneedle vaccination. All mice in the mock-vaccinated negative-control group rapidly lost body weight and were euthanized when body weight loss was over 25%. Mice vaccinated with nonstabilized microneedle vaccine exhibited substantial body weight losses up to approximately 10%, whereas mice vaccinated with stabilized microneedle vaccine showed no body weight loss (Fig. 3A). Both groups of mice showed 100% survival protection (Fig. 3B).
Fig 3.
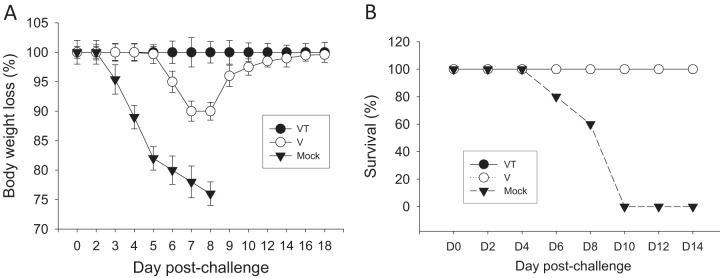
Body weight changes and survival. At month 14 after microneedle vaccination, mice were challenged with a lethal dose (A/PR8 virus, 10 LD50) and were monitored daily to record body weight changes (A) and survival (B). V, microneedle vaccine without trehalose; VT, microneedle vaccine with trehalose; Mock, microneedles with coating buffer only. Data show averages ± standard errors of the means from 6 mice out of 12 mice per group.
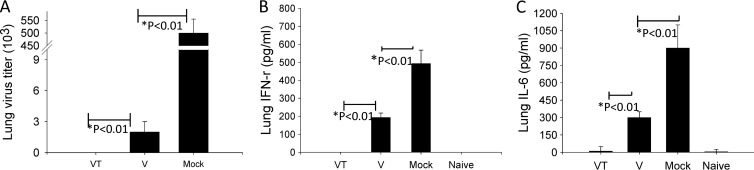
To better assess the efficacy of vaccines, we determined viral titers in lungs at day 4 postchallenge. The mock control group showed the highest viral titers of 5 × 105 PFU. Virus titers were below the limit of detection in the mice vaccinated with stabilized microneedle vaccine (Fig. 4A). The nonstabilized microneedle vaccination group showed 2 × 103 PFU/ml (Fig. 4A; *, P < 0.01), which is 200-fold less than the mock-vaccinated control group. To determine whether microneedle vaccination would diminish an inflammatory response due to influenza viral replication in lungs, we measured levels of inflammatory cytokines, IFN-γ and IL-6, in lung extracts at day 4 postchallenge (Fig. 4B). IFN-γ was not detected in lung extracts of mice vaccinated with stabilized microneedle vaccine, while mice immunized with nonstabilized microneedle vaccine and the mock-vaccinated control group showed 200 and 500 pg/ml IFN-γ, respectively (Fig. 4B; *, P < 0.05). IL-6 levels showed a similar patterns of IFN-γ (Fig. 4C). These results indicate that stabilized microneedle influenza VLP vaccination was more effective in inducing protection and in controlling lung viral replication than nonstabilized microneedle VLP vaccination.
Fig 4.
Lung virus titers and lung IFN-γ and IL-6 responses. Lungs from individual mice in each group were collected on day 4 postchallenge, and lung virus titers (PFU) and lung IFN-γ and IL-6 responses were determined in the lung extracts at day 4 postchallenge. V, microneedle vaccine without trehalose; VT, microneedle vaccine with trehalose; Mock, microneedles with coating buffer only; Naïve, normal mice. Either no virus or lower virus titers were detected in VT and V groups (*, P < 0.01). No inflammatory cytokines IFN-γ and IL-6 were determined in the VT group compared to the V or mock control group (*, P < 0.01). Data show averages ± standard errors of the means from 6 mice.
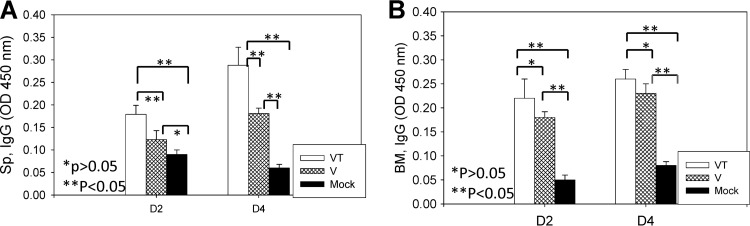
Levels of lung IgG antibodies were detected at higher levels than IgA antibodies (Fig. 5A and B). In particular, the stabilized microneedle group showed significantly higher levels of virus-specific lung IgG and IgA antibodies than those in the nonstabilized microneedle group (Fig. 5; *, P < 0.01). Thus, these results further indicate that trehalose-mediated stabilization of microneedle influenza VLP vaccines is important for improving long-term protective immunity.
Fig 5.
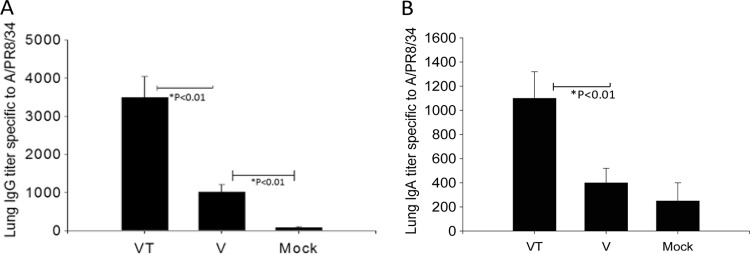
Lung IgG (A) and IgA (B) responses after lethal challenge. Lung IgG and IgA responses were determined from the lung extracts collected at day 4 postchallenge. Significantly higher IgG and IgA antibody responses were found in the VT group than in the V (*, P < 0.01) or mock challenge control (*, P < 0.01) group. V, microneedle vaccine without trehalose; VT, microneedle vaccine with trehalose; Mock, microneedles with coating buffer only. Data show averages ± standard errors of the means from 6 mice.
Microneedle vaccination induces long-lived antibody-secreting cell responses.
In general, vaccine antigen-specific cells such as memory B cells and plasma cells are present at low levels at the memory state after vaccination. Therefore, we determined recall antibody-secreting cell responses shortly after challenge. Spleen and bone marrow cells were harvested at day 4 postchallenge, which was a time point of 14 months after microneedle vaccination. The stabilized microneedle vaccination elicited higher levels of recall antibody-secreting cells from spleens after 2 and 4 days of in vitro cultures in the presence of inactivated A/PR/8 viral antigen than did nonstabilized microneedle or placebo vaccination (Fig. 6A; P < 0.05). Similar levels of antibody-secreting cell responses were detected from bone marrow cell cultures in both microneedle vaccine groups (Fig. 6B). These results indicate that microneedle vaccination in the skin induces long-lived cells capable of producing antibodies specific to virus.
Fig 6.
Antibody-secreting cells (ASC) induced by microneedle VLP vaccination. Spleen (Sp) (A) and bone marrow (BM) (B) samples were collected from individual mice in each group at day 4 postchallenge. Significantly higher numbers of ASC were found in VT groups in both spleen and bone marrow than in V and mock control groups (P < 0.05). V, microneedle vaccine without trehalose; VT, microneedle vaccine with trehalose; Mock, microneedles with coating buffer only. Data show averages ± standard errors of the means from 6 mice.
DISCUSSION
Our previous studies showed that skin vaccination with influenza VLP-coated microneedles provided higher short-term efficacy of protection than did intramuscular immunization. Thus, the capability of inducing long-term protective immunity by vaccination using VLP-coated microneedles would be an important added strength of this approach. In this study, we focused on long-term protective immunity after single microneedle vaccination with influenza VLPs in the presence and absence of a trehalose stabilizer. Results indicate that complete virus clearance was found 14 months after stabilized microneedle vaccination without any body weight loss.
Immune parameters such as IgG response, HAI titer, lung virus titer, lung IgG or IgA response, lung inflammatory cytokine IFN-γ response, cellular response or memory response, body weight change, and survival rate are informative in assessing the influenza vaccine-induced efficacy. All these immune parameters are likely contributing to clearing lung viral loads and protection against lethal influenza virus challenge. Inflammatory cytokines (IFN-γ, IL-6, and TNF-α) in lung homogenates (lungs) and BALF at an early time point of virus challenge probably were induced nonspecifically and measured as a result of inflammation due to influenza virus infection (Fig. 5; see also Fig. S2 in the supplemental material). We have not determined what cells produce inflammatory cytokines, although it is assumed that natural killer cells and nonspecifically activated CD4 and CD8 T cells would contribute to the excess production of inflammatory cytokines as natural innate immune responses to viral infection. The levels of inflammatory cytokines in lung extracts were highest in unvaccinated naive mice that were infected with A/PR8 virus (see Fig. S2) in contrast to those in the VLP-vaccinated and A/PR8 virus-infected mice (vaccine plus virus; see Fig. S2). Peripheral bronchioles of lung sections after H&E staining showed necrotizing bronchiolitis, and alveolar space contained infiltrates of mixed inflammatory cells after A/PR8 virus infection (see Fig. S3). There was a correlation between levels of IFN-γ in lung homogenates and infiltrates in H&E staining of lung histology (see Fig. S3).
In particular, clearance of virus in lungs is considered to be an important and sensitive barometer for evaluating vaccine efficacy. Different types of microneedle influenza vaccines and efficacies have been demonstrated. Substantial amounts of viral loads with a range of 104- to 105-PFU titers were detected in mice that were immunized on the skin using microneedles coated with 0.4 to 2 μg H5 HA VLPs derived from influenza A/Vietnam/1203/04 virus (21, 35). Also, 102- to 103-PFU lung viral titers and weight losses of 5 to 10% were observed in mice immunized with microneedle vaccines coated with 3 μg of split A/Brisbane/59/2007 vaccine, which are approximately 10- to 50-fold less than the infected naive control group (36). A low level of lung viral titers (102-PFU titers) was reported in mice after 5 weeks of microneedle vaccination with 0.4 μg inactivated A/PR/8/34 H1N1 virus (20). In summary, previous studies demonstrated a certain level of lung virus titers and body weight loss. Also, microneedle vaccine-induced efficacies in mice are different depending on influenza virus strains, vaccine doses, prime-boost immunizations, and timing of postimmunization at the time of challenge. In this study, immunization with a high dose (4 μg) of microneedle vaccines induced high levels of IgG antibody responses (before challenge) regardless of trehalose stabilization, which is consistent with an early study of microneedle vaccination without trehalose (23). Dosage effects and higher immunogenicity of microneedle VLP vaccines were previously demonstrated when a low dose of 0.4 μg of vaccines was used to immunize mice (20, 25, 26). Nevertheless, we observed that stabilized microneedle vaccination with 4 μg HA VLPs (derived from A/PR/8/34) was able to provide lung virus clearance and prevent weight loss in lethal challenge infection for a long period of 14 months.
Influenza hemagglutinin (HA) stability is likely to play an important role in providing long-term protective immunity. Our previous studies demonstrated that inclusion of 15% trehalose in the microneedle coating formulation resulted in retaining the HA activity of VLPs over 60% after a 1-day drying process in contrast to less than 10% HA activity of VLPs from coated microneedles without trehalose (26, 30). HAI titers were maintained at similar levels for 13 months after vaccination between stabilized and nonstabilized microneedle vaccination groups. Interestingly, HAI titers were found to increase rapidly in the stabilized microneedle vaccine group upon challenge. Differences in host protective immune responses of two microneedle vaccine groups were found to be more evident after challenge. The experimental data for improved protection include no detectable lung viral titers, no loss in body weight, and higher levels of protective host responses such as significant increases in HAI titers and vaccine antigen-specific recall antibody-secreting cell responses. We have shown that IgG2a isotype antibody and Th1 dominant immune responses were due to increased stability of the HA in the trehalose-stabilized microneedle vaccination (20). There was no significant difference in bone marrow antibody-secreting cell responses between stabilized VT and nonstabilized V groups. However, higher levels of splenic B cells secreting antibodies were detected in the stabilized microneedle vaccination at an early time point after challenge, which was also reflected by enhanced serum total IgG antibodies (Fig. 1) and IgG2a isotype antibody levels (see Fig. S1 in the supplemental material). Stabilized microneedle vaccination was highly effective in inducing improved protection. Therefore, maintaining influenza virus HA antigen stability in vaccines is important in providing long-term protective immunity.
The present study implies an important aspect in evaluating the efficacy of experimental vaccines. Immunogenicity itself may not reflect a good correlation with protective efficacy. At month 13 after vaccination and at an early time point of challenge, levels of binding antibodies did not show obvious differences between the two groups of microneedle-vaccinated mice. Even the HAI titers, a measure of functional antibodies for predicting protection against influenza virus, did not show a significant difference at 13 months after vaccination. However, protective efficacy was significantly improved in the stabilized microneedle group after challenge (Fig. 3). It is likely that applying influenza vaccines as a coating onto microneedles in the absence of stabilizer might have resulted in exposing nonneutralizing domains of influenza virus HA proteins in a denatured conformation. These conformational changes are inferred from a result of decreased hemagglutination activity of coated microneedle vaccines (25, 27). This might explain similar levels of binding antibodies in both stabilized and nonstabilized groups. An insignificant difference in qualitative antibody levels was rapidly expanded upon infectious virus challenge, as shown by a significant increase in HAI titers. Therefore, developing an appropriate challenge-protection animal model should be an essential feature of evaluating the vaccine efficacy.
Another important arm of host immunity to pathogens is T cell immune responses. Although we have not investigated T cell immunity in this study, there is a possibility that T cell immune responses induced by stabilized microneedle vaccination might have contributed to improved protection observed. In previous studies, we observed enhanced T cell responses after stabilized microneedle vaccination compared to intramuscular immunization or nonstabilized microneedle vaccines (20, 26). Also, intradermal vaccination in the elderly population was reported to be more effective in inducing protective immune responses, including CD4 or CD8 T cell responses (37–42). As a future direction, it is important to study long-term T cell immune responses after microneedle skin vaccination.
Overall, the literature suggests that microneedle skin vaccination can offer important advantages. Influenza VLP microneedle vaccination generated dose-sparing effects and robust HAI titers (20, 26, 43). Microneedle skin delivery of inactivated influenza vaccine induced better control of viral replication and reduced inflammatory responses in the lungs (43). In addition, skin microneedle vaccination provides important logistic advantages. Microneedle skin vaccination can probably be administered by patients themselves, significantly increasing the coverage of vaccination. It can also eliminate or reduce the risk of needle-associated injury and reuse of needles and syringes (43). These advantages from both immunologic and logistic benefits, combined with long-term protective immunity as presented in this study, indicate that microneedle delivery to the skin may offer a strategy for improved influenza vaccination.
In conclusion, this study demonstrated that highly effective long-term immunity to influenza was induced by skin vaccination using microneedles coated with VLPs. Stabilized microneedle VLP vaccine conferred rapid increases in functional antibody responses and effective viral clearance resulting in improved long-term protection after lethal challenge. Thus, microneedle delivery to the skin using nonreplicating influenza VLPs has the potential to become an effective vaccine for inducing long-term protective immunity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Kyung Hee University in 2012 (KHU-20120459) and by grants from NIH, AI105170 (S.-M.K.), AI093772 (S.-M.K.), AI087782 (S.-M.K.), AI068003 (R.W.C.), and EB006369 (M.R.P.). This work was carried out at the Center for Inflammation, Immunity & Infection and Department of Biology, Georgia State University, at the Emory Vaccine Center, and at the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Bioscience at Georgia Tech.
Footnotes
Published ahead of print 17 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00251-13.
REFERENCES
- 1.Viboud C, Miller M, Olson D, Osterholm M, Simonsen L. 2010. Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons. PLoS Curr. 2:RRN1153. 10.1371/currents.RRN1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osterholm MT. 2005. Preparing for the next pandemic. N. Engl. J. Med. 352:1839–1842 [DOI] [PubMed] [Google Scholar]
- 3.Galarza JM, Latham T, Cupo A. 2005. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 18:244–251 [DOI] [PubMed] [Google Scholar]
- 4.Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. 2005. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 23:5751–5759 [DOI] [PubMed] [Google Scholar]
- 5.Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, Massare M, Pushko P, Mytle N, Rowe T, Smith G, Ross TM. 2007. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 25:3871–3878 [DOI] [PubMed] [Google Scholar]
- 6.Quan FS, Huang C, Compans RW, Kang SM. 2007. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 81:3514–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haynes JR, Dokken L, Wiley JA, Cawthon AG, Bigger J, Harmsen AG, Richardson C. 2009. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine 27:530–541 [DOI] [PubMed] [Google Scholar]
- 8.Hossain MJ, Bourgeois M, Quan FS, Lipatov AS, Song JM, Chen LM, Compans RW, York I, Kang SM, Donis RO. 2011. Virus-like particle vaccine containing hemagglutinin confers protection against 2009 H1N1 pandemic influenza. Clin. Vaccine Immunol. 18:2010–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, Chen LM, Hogan RJ, Donis RO, Compans RW, Kang SM. 2010. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology 405:165–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang SM, Song JM, Compans RW. 2011. Novel vaccines against influenza viruses. Virus Res. 162:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang SM, Song JM, Quan FS, Compans RW. 2009. Influenza vaccines based on virus-like particles. Virus Res. 143:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. 1991. Keratinocytes as initiators of inflammation. Lancet 337:211–214 [DOI] [PubMed] [Google Scholar]
- 13.Enioutina EY, Visic D, Daynes RA. 2000. The induction of systemic and mucosal immune responses to antigen-adjuvant compositions administered into the skin: alterations in the migratory properties of dendritic cells appears to be important for stimulating mucosal immunity. Vaccine 18:2753–2767 [DOI] [PubMed] [Google Scholar]
- 14.Flacher V, Bouschbacher M, Verronese E, Massacrier C, Sisirak V, Berthier-Vergnes O, de Saint-Vis B, Caux C, Dezutter-Dambuyant C, Lebecque S, Valladeau J. 2006. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J. Immunol. 177:7959–7967 [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Garmise RJ, Crowder TM, Mar K, Hwang CR, Hickey AJ, Mikszta JA, Sullivan VJ. 2004. A novel dry powder influenza vaccine and intranasal delivery technology: induction of systemic and mucosal immune responses in rats. Vaccine 23:794–801 [DOI] [PubMed] [Google Scholar]
- 16.Kendall M, Mitchell T, Wrighton-Smith P. 2004. Intradermal ballistic delivery of micro-particles into excised human skin for pharmaceutical applications. J. Biomech. 37:1733–1741 [DOI] [PubMed] [Google Scholar]
- 17.Vrdoljak A, McGrath MG, Carey JB, Draper SJ, Hill AV, O'Mahony C, Crean AM, Moore AC. 2012. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J. Control. Release 159:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Maaden K, Jiskoot W, Bouwstra J. 2012. Microneedle technologies for (trans)dermal drug and vaccine delivery. J. Control. Release 161:645–655 [DOI] [PubMed] [Google Scholar]
- 19.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. 2009. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine 27:6932–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. 2009. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One 4:e7152. 10.1371/journal.pone.0007152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song JM, Kim YC, Barlow PG, Hossain MJ, Park KM, Donis RO, Prausnitz MR, Compans RW, Kang SM. 2010. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antiviral Res. 88:244–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi SO, Murthy N, Compans RW, Skountzou I, Prausnitz MR. 2010. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 16:915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Q, Zarnitsyn VG, Ye L, Wen Z, Gao Y, Pan L, Skountzou I, Gill HS, Prausnitz MR, Yang C, Compans RW. 2009. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl. Acad. Sci. U. S. A. 106:7968–7973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YC, Park JH, Prausnitz MR. 2012. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 64:1547–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan FS, Kim YC, Compans RW, Prausnitz MR, Kang SM. 2010. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J. Control. Release 147:326–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, Compans RW, Kang SM. 2010. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J. Virol. 84:7760–7769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. 2010. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Control. Release 142:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, Jacob J, Prausnitz MR, Compans RW, Skountzou I. 2011. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. J. Infect. Dis. 204:582–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quan FS, Vunnava A, Compans RW, Kang SM. 2010. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PLoS One 5:e9161. 10.1371/journal.pone.0009161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. 2010. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech 11:1193–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan FS, Compans RW, Cho YK, Kang SM. 2007. Ginseng and Salviae herbs play a role as immune activators and modulate immune responses during influenza virus infection. Vaccine 25:272–282 [DOI] [PubMed] [Google Scholar]
- 32.Quan FS, Compans RW, Nguyen HH, Kang SM. 2008. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J. Virol. 82:1350–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, Lee S, Goleniewska K, Pretto C, Williams JV, Hotard A, Sherrill TP, Peebles RS, Jr, Moore ML. 2011. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J. Virol. 85:5782–5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Heaton PM, Fraire AE, Morrison TG. 2010. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J. Virol. 84:1110–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song JM, Kim YC, Lipatov AS, Pearton M, Davis CT, Yoo DG, Park KM, Chen LM, Quan FS, Birchall JC, Donis RO, Prausnitz MR, Compans RW, Kang SM. 2010. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice. Clin. Vaccine Immunol. 17:1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koutsonanos DG, Vassilieva EV, Stavropoulou A, Zarnitsyn VG, Esser ES, Taherbhai MT, Prausnitz MR, Compans RW, Skountzou I. 2012. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci. Rep. 2:357. 10.1038/srep00357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ansaldi F, Durando P, Icardi G. 2011. Intradermal influenza vaccine and new devices: a promising chance for vaccine improvement. Expert Opin. Biol. Ther. 11:415–427 [DOI] [PubMed] [Google Scholar]
- 38.Arnou R, Eavis P, Pardo JR, Ambrozaitis A, Kazek MP, Weber F. 2010. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18–60 years: randomized, controlled, phase III trial. Hum. Vaccines 6:346–354 [DOI] [PubMed] [Google Scholar]
- 39.Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, McKeirnan M, Salem H, Mills G, Reid J, Weber F, Saville M. 2008. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J. Infect. Dis. 198:650–658 [DOI] [PubMed] [Google Scholar]
- 40.Haynes L, Swain SL. 2006. Why aging T cells fail: implications for vaccination. Immunity 24:663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liard C, Munier S, Joulin-Giet A, Bonduelle O, Hadam S, Duffy D, Vogt A, Verrier B, Combadiere B. 2012. Intradermal immunization triggers epidermal Langerhans cell mobilization required for CD8 T-cell immune responses. J. Invest. Dermatol. 132:615–625 [DOI] [PubMed] [Google Scholar]
- 42.Liu WM, van der Zeijst BA, Boog CJ, Soethout EC. 2011. Aging and impaired immunity to influenza viruses: implications for vaccine development. Hum. Vaccines 7(Suppl):94–98 [DOI] [PubMed] [Google Scholar]
- 43.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. 2010. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J. Infect. Dis. 201:190–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.