Abstract
Mycoplasma hyopneumoniae is the etiological agent of porcine enzootic pneumonia (EP), which is a respiratory disease responsible for huge economic losses in the pig industry worldwide. The commercially available vaccines provide only partial protection and are expensive. Thus, the development of alternatives for the prophylaxis of EP is critical for improving pig health. The use of multiple antigens in the same immunization may represent a promising alternative. In the present study, seven secreted proteins of M. hyopneumoniae were cloned, expressed in Escherichia coli, and evaluated for antigenicity using serum from naturally and experimentally infected pigs. In addition, the immunogenicity of the seven recombinant proteins delivered individually or in protein cocktail vaccines was evaluated in mice. In Western blot assays and enzyme-linked immunosorbent assays, most of the recombinant proteins evaluated were recognized by convalescent-phase serum from the animals, indicating that they are expressed during the infectious process. The recombinant proteins were also immunogenic, and most induced a mixed IgG1/IgG2a humoral immune response. The use of these proteins in a cocktail vaccine formulation enhanced the immune response compared to their use as antigens delivered individually, providing evidence of the efficacy of the multiple-antigen administration strategy for the induction of an immune response against M. hyopneumoniae.
INTRODUCTION
Porcine enzootic pneumonia (EP) is a chronic respiratory disease with a high incidence in pig farms worldwide. Mycoplasma hyopneumoniae, the etiological agent of this disease, colonizes and destroys the main mechanism of innate immunity of the pig respiratory system—the respiratory ciliated epithelium—predisposing the pigs to secondary and opportunistic infections (1). EP prophylaxis comprises the use of antibiotics, management procedures, and vaccination, which is considered the most efficient control measure (2). The commercially available vaccines consist of inactivated whole cells (bacterins) and have high production costs, mainly due to the fastidious growth of this microorganism. In addition, these vaccines do not eliminate M. hyopneumoniae from infected pig herds (1, 3, 4). Thus, efforts have been directed toward the search for new prophylaxis strategies against EP that do not include the use of bacterins.
M. hyopneumoniae does not penetrate host cells, and thus it is believed that the pathogenesis of this microorganism is mediated by a complex and multifactorial process that involves components of the cell membrane and secreted proteins, many of which are still unidentified (5). Data generated from the sequencing of four isolates of M. hyopneumoniae (7448, 168, J, and 232) and the comparative genomic and proteomic analyses of pathogenic strains (7448 and 232) and a nonpathogenic strain (J) of M. hyopneumoniae (6, 7, 8, 9) have allowed the identification of coding sequences (CDS) from secreted antigenic proteins and/or proteins involved in the pathogenicity of the microorganism. Some vaccines including only one of these candidate antigens have shown promising results (10, 11). However, only two single-antigen vaccines have been tested in pigs, and these vaccines have resulted in only partial protection (12, 13). The simultaneous administration of multiple antigens, combining proteins in a single immunization dose (cocktail vaccine), may be an alternative that offers more adequate protection (13, 14).
In the present study, the antigenicity of recombinant secreted antigens of M. hyopneumoniae was verified using serum from naturally and experimentally infected pigs. Additionally, the immunogenicity of the recombinant proteins delivered individually or in protein cocktail vaccines was evaluated in mice, providing evidence of the ability of the multiple-antigen administration strategy to induce an immune response.
MATERIALS AND METHODS
Bacterial strains, plasmids, and serum samples.
M. hyopneumoniae isolates (strains 7448, 7422, and J), serum from specific-pathogen-free (SPF) pigs, and porcine hyperimmune serum against M. hyopneumoniae strain 7448 were obtained from Embrapa (Concórdia, Santa Catarina, Brazil). Porcine convalescent-phase serum was obtained from a commercial herd chronically affected by EP. In this herd, a nonproductive cough was observed during the finishing phase of the pigs, and suggestive lung lesions were observed in the pigs at necropsy. The Champion pET200D/TOPO His tag expression vector and the Escherichia coli TOP10 cloning strain were purchased from Invitrogen. The E. coli BL21(DE3)-RIL expression strain was purchased from Stratagene. The pAE vector was obtained from the Butantan Institute (15).
In silico selection of open reading frames and primer design.
Open reading frames (ORFs) of M. hyopneumoniae 7448 (GenBank access NC_007332) were analyzed using bioinformatics software (Pfam, SignalP, PROSITE, NNPREDICT, and Vector NTI 8.0), as previously described (16). ORFs encoding secreted proteins related to pathogenesis, including up to three tryptophan (TGA) codons, were selected. Predominantly hydrophilic regions of the ORFs were selected for amplification. Primers were designed with the use of Vector NTI 10 (Invitrogen). For site-directed mutagenesis, primers upstream (forward [F]) and downstream (reverse [R]) of the mutation site and two mutagenic primers (forward [FM] and reverse [RM]) were designed according to Simionatto et al. (17). To clone fragments into the pAE vector, a restriction site was added to each primer. To directionally clone fragments into the Champion pET200D/TOPO expression vector (Invitrogen), a CACC sequence was included in the forward primer. The primers used in this study are shown in Table 1.
Table 1.
Selected ORFs of M. hyopneumoniae and primer sequences
| Recombinant protein annotationa | Function | Selected fragment (aa) | Primer sequencec |
|---|---|---|---|
| MHP0107 | Protein P102 (copy 2) | 646–948 | 5′-CACCGGATCCGTATCAAAAGCAGATCGA-3′ (F) |
| 5′-CCCAAGCTTTTATTGTTTTATATAATTACTAAT-3′ (R) | |||
| MHP0272 | Protein P97-paralog | 228–708 | 5′-CACCGGATCCAAAATAAAAGAAAAATTGTTT-3′ (F) |
| 5′-CCGGTACCTCAAGCCCGAACTTT-3′ (R) | |||
| MHP0418 | Hypothetical protein | 145–500 | 5′-CACCGGATCCGAAAATTTAGCGCCATAT-3′ (F) |
| 5′-CCCAAGCTTTCATTTTTTAACTGAAATTGG-3′ (R) | |||
| 5′-AGGCATTGgTATTCTTTATTAG-3′ (FM) | |||
| 5′-AGAATAcCAATGCCTTTCAG-3′ (RM) | |||
| MHP0443 | Hypothetical protein | 32–510 | 5′-CACCGGGTACCGAATGATTTTGTCTTTTTG-3′ (F) |
| 5′-CCCAAGCTTTCAGATTCTACCATTAATCTTT-3′ (R) | |||
| MHP0487 | Putative Mg2+ transporter | 1–280 | 5′-CACCGGATCCGATAAAAAAAGTTCTTTTCTTTT-3′ (F) |
| 5′-CCCAAGCTTTCAAAAAACTCTTGATTTTACGAT-3′ (R) | |||
| MHP0660 | Hypothetical protein | 1–394 | 5′-CACCGGATCCATAAAAAAATCATCAAAAAT-3′ (F) |
| 5′-GGGGTACCTCACAGCCTTAGATTTAAA-3′ (R) | |||
| MHP0372b | Lppt lipoprotein | 497–883 | 5′-CACCTTTGATTTTCTTAATTTTTCAACA-3′ (F) |
| 5′-TTATTCCATATATTCGCTAAGGA-3′ (R) | |||
| 5′-TTCCTTGATTGgCGATCAAATATA-3′ (FM) | |||
| 5′-TGATCGcCAATCAAGGAATAAATC-3′ (RM) |
GenBank annotation of M. hyopneumoniae 7448 (NC_007332).
Directionally cloned into a Champion pET200D/TOPO His-tagged expression vector (Invitrogen).
F, forward primer; R, reverse primer; FM and RM, forward and reverse primers for site-directed mutagenesis of MHP0418 and MHP0372, respectively. Mutated nucleotides are shown in bold lowercase letters. Underlined nucleotides represent restriction sites of BamHI (GGATCC), HindIII (AAGCTT), and KpnI (GGTACC) enzymes.
Amplification and cloning of the coding sequences.
The M. hyopneumoniae coding sequences were amplified and purified according to Simionatto et al. (16). The targets MHP0418 and MHP0372 were subjected to site-directed mutagenesis according to Simionatto et al. (17) and confirmed by DNA sequencing using the DYEnamic ET dye terminator cycle sequencing kit and a MegaBACE 1000 DNA sequencer (GE Healthcare). PCR products and mutated amplicons were cloned into the pAE vector using T4 DNA ligase (Invitrogen). MHP0372 was directionally cloned into the Champion pET200D/TOPO His tag expression vector (Invitrogen) according to the manufacturer's instructions.
Expression, solubility testing, and purification of recombinant proteins.
Recombinant plasmids were transformed into E. coli BL21(DE3)-RIL expression-competent cells. Recombinant protein expression and solubility testing were performed as previously described (16, 18). The purity of the purified recombinant proteins was analyzed on a 12% SDS-PAGE gel, and the protein concentrations were determined using the BCA protein assay kit (Pierce) according to the manufacturer's instructions. The presence of the purified recombinant proteins was verified by Western blotting performed according to Simionatto et al. (18), using a mouse monoclonal anti-histidine-tagged antibody (Sigma-Aldrich) and an anti-mouse IgG peroxidase-conjugated (1:4,000) (Sigma-Aldrich) secondary antibody.
Recombinant protein antigenicity assays.
To assess the antigenicity of M. hyopneumoniae proteins expressed in E. coli, Western blotting and enzyme-linked immunosorbent assays (ELISAs) were performed as previously described (18). For the Western blot analysis, two pools containing five convalescent-phase pig sera and one pool with five SPF pig sera were tested. To perform the ELISA, 36 convalescent-phase pig sera, 4 hyperimmune pig sera (experimentally infected with the M. hyopneumoniae 7448 strain), and 10 SPF pig sera were tested. The levels of antibodies against M. hyopneumoniae in the sera of convalescing pigs were previously determined by ELISA using an extract of M. hyopneumoniae strain 7448. Mean values were calculated from serum samples assayed in triplicate.
Inoculation of mice with recombinant vaccines.
Female BALB/c mice aged 8 to 10 weeks were inoculated intramuscularly (IM) with 50 μg of each recombinant protein in 15% aluminum hydroxide adjuvant on day zero. Eleven groups with five animals each were randomly allocated and immunized, as follows: group 1, MHP0107; group 2, MHP0272; group 3, MHP0418; group 4, MHP0487; group 5, MHP0443; group 6, MHP0660; group 7, MHP0372; group 8, protein cocktail containing MHP0418, MHP0443, and MHP0487 (C1); group 9, protein cocktail containing MHP0418, MHP0660, and MHP0372 (C2); group 10, 100 μl of phosphate-buffered saline (PBS) with 15% aluminum hydroxide adjuvant (negative control); and group 11, 100 μl of the commercial vaccine RespiSure (Pfizer) (positive control). Each animal was boosted with the same dose and formulation 21 days after the first inoculation. Animals from groups C1 and C2 were immunized with 16.6 μg of each protein at each dose. Blood samples were collected from the retro-orbital sinus at 0 (preimmune serum), 21, 42, 63, 84, and 105 days postinoculation (DPI). At day 105, the animals were euthanized. All animal experiments were performed according to the guidelines of the Ethics Committee in Animal Experimentation of the Federal University of Pelotas.
Indirect ELISA to evaluate total IgG antibodies in serum of immunized mice.
In serum from immunized mice, specific antibodies against the recombinant proteins were quantified by ELISA as previously described (18, 19), with some modifications. Microtiter plates were coated with 100 ng of each recombinant protein (indirect ELISA). To evaluate the immune response induced by the C1 and C2 vaccines, mouse sera were tested against 100 ng of each individual protein present in the cocktails and against a pooled sample containing 33.3 ng of each included protein. Proteins were incubated with the serum from immunized mice (diluted 1:50), and IgG anti-mouse antibody conjugated to horseradish peroxidase (Sigma-Aldrich) was used to evaluate the total IgG antibodies.
Double-sandwich ELISA to evaluate IgG subclass (IgG1 and IgG2a) antibodies in serum of immunized mice.
To evaluate IgG subclasses (IgG1 and IgG2a), a double-sandwich ELISA was performed similarly to the indirect ELISA, but using antibodies provided by the IsoQuick kit (Sigma-Aldrich) followed by anti-goat antibody conjugated to horseradish peroxidase (Sigma-Aldrich). Sera from mice were assessed individually, and all reactions were performed in triplicate.
ELISA of cell lysate.
The sera from immunized mice (diluted 1:20) were incubated with 1 μg of crude extract of M. hyopneumoniae 7448, in order to verify if the antibodies induced by the recombinant vaccines recognized the native proteins of M. hyopneumoniae. The ELISA was performed as previously described (18, 19). Sera from mice were assayed individually and all reactions were performed in triplicate.
Western blot assay with whole-cell extracts of Mycoplasma spp.
To verify the ability of sera from mice immunized with the recombinant proteins to recognize native proteins from the M. hyopneumoniae 7448, 7422, and J strains, a whole-cell extract was used in a Western blot assay, as previously described (18, 19). Sera from mice inoculated with PBS and the commercial vaccine were used as negative and positive controls, respectively.
Statistical analysis.
Statistical analyses were performed using the Tukey test because the data presented a normal distribution pattern. Differences were considered significant when P was <0.01, determined using GraphPad Prism 4 (GraphPad Software).
RESULTS
Cloning of targets and expression and purification of recombinant proteins.
According to the criteria used, seven sequences encoding the predicted secreted proteins were selected to be cloned and expressed in E. coli: the C-terminal region of the P102 paralog (copy 2) (MHP0107), the N-terminal region of the P97 paralog (MHP0272), a putative Mg2+ transporter protein (MHP0487), a lipoprotein (LPPT) (MHP0372), and three hypothetical proteins (MHP0418, MHP0443, and MHP0660). The TGA codons from MHP0418 and MHP0372 were replaced by TGG, which was confirmed by sequencing. All amplicons were successfully cloned and characterized by restriction enzymes showing the expected fragment sizes. The results of SDS-PAGE showed that all expressed recombinant proteins were insoluble, making solubilization with 8 M urea necessary. The seven recombinant proteins were purified (Fig. 1A) and recognized by anti-histidine monoclonal antibodies (MAbs) in the Western blot analysis (Fig. 1B).
Fig 1.
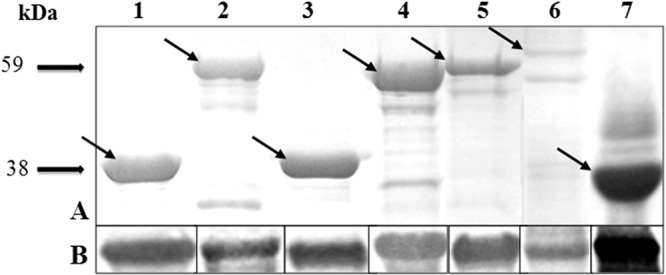
(A) SDS-PAGE (12%) of the seven recombinant proteins purified by affinity chromatography. (B) Western blot analysis of the seven recombinant proteins subjected to 12% SDS-PAGE, electrotransferred to a nitrocellulose membrane, and probed with mouse monoclonal anti-histidine-tagged antibody (diluted 1:10,000) and peroxidase-conjugated anti-mouse IgG (diluted 1:4,000) as the secondary antibody. Lane 1, MHP0107 (38 kDa); lane 2, MHP0272 (59 kDa); lane 3, MHP0418 (37 kDa); lane 4, MHP0443 (54 kDa); lane 5, MHP0372 (44 kDa); lane 6, MHP0660 (48.5 kDa); lane 7, MHP0487 (33 kDa). Arrows indicate the bands corresponding to the recombinant proteins.
Antigenicity of the recombinant proteins.
Six of the seven recombinant proteins tested were recognized by serum from convalescing pigs, according to the Western blot assay (Fig. 2B). The recombinant proteins were not recognized by SPF serum, indicating that they are expressed only during infection with M. hyopneumoniae. In the ELISA, only the MHP0443 and MHP0660 proteins did not significantly react against convalescent-phase pig serum (Fig. 2A).
Fig 2.
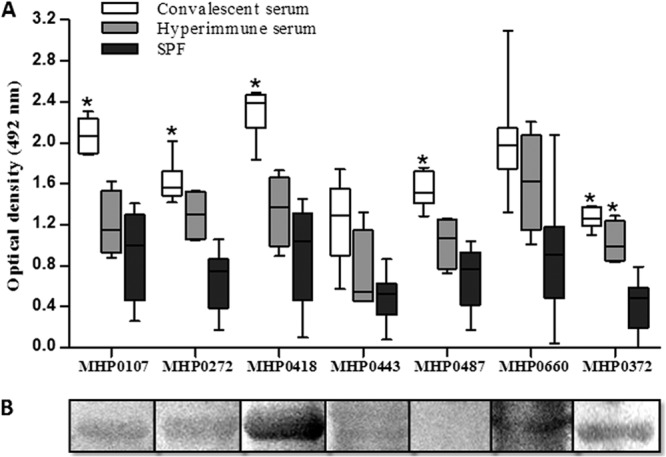
Antigenicity analysis of the recombinant proteins. (A) ELISA of the seven recombinant M. hyopneumoniae proteins against 30 convalescent-phase pig serum samples, four hyperimmune pig serum samples (experimentally infected with M. hyopneumoniae 7448), and 10 SPF pig sera (all sera were diluted 1:100). Peroxidase-conjugated anti-pig IgG (diluted 1:2,000) was used as the secondary antibody. Asterisks indicate significant differences compared to SPF sera using the Tukey test with GraphPad Prism 4 software (P < 0.01). Boxes represent the interquartile range in the middle 50% of the absorbance values. Whiskers represent the minimum and maximum values. (B) Western blot analysis of the recombinant proteins probed with the pooled sera of 5 convalescing pigs (diluted 1:100). Peroxidase-conjugated anti-pig IgG (diluted 1:2,000) was used as the secondary antibody.
Immunogenicity of the recombinant proteins.
The induction of specific antibodies (total IgG; IgG1 and IgG2a subclasses) in mice was verified by ELISA after immunization with the recombinant proteins. All recombinant proteins induced an antibody response that was statistically higher (P < 0.01) at 42 DPI than that of the negative control (PBS) mice and preimmune serum; the antibody titers remained constant through 105 DPI. The recombinant proteins MHP0372 and MHP0443 induced higher optical density (OD) values in mice (Fig. 3A) than the other proteins; however, the differences were not statistically significant. Most of the antigens induced a mixed IgG1/IgG2a response (Fig. 4A). MHP0443 and MHP0372 were also the most immunogenic in animals immunized with the C1 and C2 cocktail vaccines, respectively (Fig. 3B). In mice, the induction of total antigen-specific IgG antibodies against cocktail vaccines was similar to that for most of the proteins administered individually. However, a more sensitive ELISA (the double-sandwich ELISA) using the secondary antibody against the IgG1 and IgG2a subclasses demonstrated that this vaccination strategy resulted in the production of higher levels of these subclasses than those of the proteins administered individually (Fig. 4). Animals inoculated with PBS and the commercial vaccine did not produce antibodies against the recombinant proteins used in this study (Fig. 3A).
Fig 3.
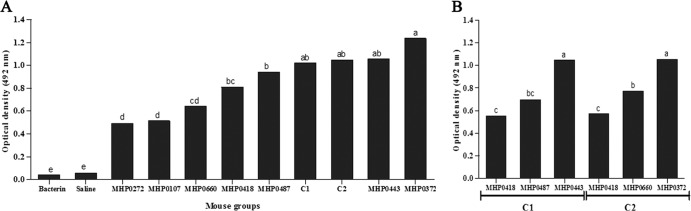
Antibody responses of mice immunized with recombinant vaccines determined by indirect ELISA. Microtiter plates were coated with the following proteins: (A) 100 ng well−1 of recombinant proteins administered individually or a pooled protein sample containing 33.3 ng well−1 of each protein included in the cocktail vaccines, totaling 100 ng, and (B) 100 ng well−1 of each protein from the cocktail vaccines. Recombinant proteins were incubated with sera from immunized mice (diluted 1:50). Sera from mice immunized with PBS (negative control) and bacterin (positive control) were incubated with each protein individually, and a mean value was generated. Peroxidase-conjugated anti-mouse IgG (1:4000) was added as the secondary antibody. The data represent the mean optical density at 492 mm (OD492) (n = 8) of the mouse sera collected at 105 DPI minus the mean OD492 of the mouse sera collected at 0 DPI. All reactions were performed in triplicate. Different letters indicate significant differences (P < 0.01) among treatments according to the Tukey test, which was performed using GraphPad Prism 4 software.
Fig 4.
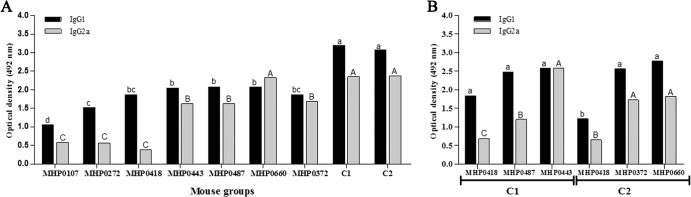
Recombinant protein-specific IgG1 and IgG2a antibodies determined by ELISA. Microtiter plates were coated as follows: (A) 100 ng well−1 of the recombinant proteins administered individually or a pooled protein sample containing 33.3 ng well−1 of each protein included in the cocktail vaccines (C1 and C2), totaling 100 ng, and (B) 100 ng well−1 of each protein from the cocktail vaccines. Recombinant proteins were incubated with sera from immunized mice (diluted 1:50). Goat anti-mouse IgG1 and IgG2a antibodies supplied by the IsoQuick kit for mouse monoclonal isotyping (Sigma) were used as secondary antibodies. The data represent the mean OD492 (n = 8) of the mouse sera collected at 105 DPI minus the mean OD492 of the mouse sera collected at 0 DPI. All reactions were performed in triplicate. Different letters indicate significant differences (P < 0.01) among treatments. Statistical analyses were performed in GraphPad Prism 4 software using the Tukey test.
To determine whether the antibodies produced by mice immunized with the recombinant proteins recognized the native proteins of M. hyopneumoniae, the ELISA and the Western blot assay were performed using cell lysate. In the ELISA, the serum of mice immunized with the recombinant proteins MHP0418 and MHP0372 and with the commercial vaccine (bacterin) showed absorbance values significantly higher (P < 0.05) than those of the preimmune serum (Fig. 5A). The Western blot analysis revealed that the seven recombinant proteins induced antibodies that recognized the native proteins from M. hyopneumoniae strains 7448, 7422, and J (Fig. 5B). Serum from mice inoculated with PBS did not react against the native proteins of these strains, while serum from mice inoculated with the commercial bacterin reacted strongly over the entire length of the nitrocellulose membrane (data not shown).
Fig 5.
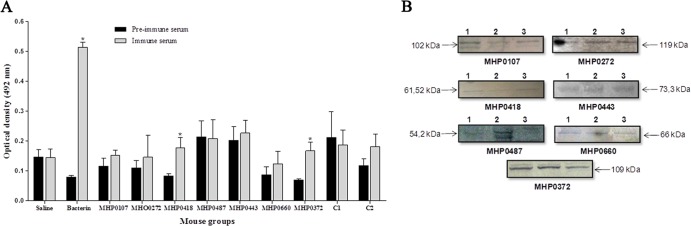
ELISA reactions using M. hyopneumoniae 7448 extract as the antigen (A) and Western blot reactions using M. hyopneumoniae 7448 (1), 7422 (2), and J (3) strains as antigens (B). Detection of antibodies in sera from mice immunized with recombinant proteins and cocktail vaccines (diluted 1:20). Peroxidase-conjugated anti-mouse immunoglobulin was used as the secondary antibody. Sera from mice immunized with PBS and bacterin (RespiSure-ONE) were used as negative and positive controls, respectively. The data from panel A represent the OD492 (mean ± standard deviation [SD]) of sera from five immunized mice. Asterisks indicate significant differences (P < 0.01) between preimmune (0 DPI) and immune (105 DPI) sera, according to the Tukey test.
DISCUSSION
The antigenicity characterizations of M. hyopneumoniae proteins as well as the immunogenicity evaluations in mice represent important steps toward the selection of potential antigens or potential ways to deliver these antigens, which might improve the efficacy of the vaccines to be tested in pigs. In this study, we selected, cloned, and purified seven antigens that were predicted to be secreted proteins in an in silico analysis and therefore are possibly related to the pathogenesis of M. hyopneumoniae because they are exposed on the surface of the microorganism. The antigenicity and immunogenicity of these antigens were characterized when delivered individually or as a protein cocktail, and the results indicated that immunization with multiple antigens is a promising approach for inducing antibodies against M. hyopneumoniae in mice.
The adhesion of M. hyopneumoniae in the respiratory ciliated epithelium of the host is a crucial step in disease pathogenesis. This process is mediated by a protein-receptor interaction, and adhesins involved in this interaction are likely virulence factors. Among the likely adhesins are those belonging to the P97/P102 operon, such as MHP0272, which is a paralog from the best-characterized surface antigen of M. hyopneumoniae (P97 protein), and MHP0107 (second copy of the P102 operon), which is located downstream of the gene encoding P97 (20–22). The proteins MHP0272 and MHP0107, as well as MHP0372, MHP0418, MHP0660, and MHP0487, were recognized by antibodies present in sera from convalescing pigs when assessed by ELISA and Western blot (Fig. 2A and B). The results indicated that these proteins are expressed during infection, making them promising candidates for further studies regarding the development of a new vaccine. However, MHP0272 and MHP0107 were not recognized by serum from pigs experimentally infected with M. hyopneumoniae 7448 (Fig. 2A), although the expression of members of the P97 gene family has been observed in pigs experimentally infected with M. hyopneumoniae 232 (23). The difference in the protein expression profiles of M. hyopneumoniae observed in in vivo and in vitro may explain, at least in part, the partial protection elicited by bacterins. Interestingly, serum from animals immunized with the commercial bacterin did not react with any of the recombinant proteins evaluated. The same result was observed by Marchioro et al. (19) and Simionatto et al. (18) using a wide range of proteins as antigens. The partial protection provided by this vaccine may also be associated with the absence or very low concentrations of these important antigens.
The other proteins evaluated, including the Mg2+ transport protein MHP0487, the surface lipoprotein MHP0372, and the hypothetical proteins MHP0443, MHP0660, and MHP0418, were immunogenic in mice (Fig. 3 and 4). In a study addressing the comparative proteomic analysis between nonpathogenic (J) and pathogenic (7448 and 7422) strains (24, 25), many of the differentially expressed proteins in these strains were hypothetical proteins that have been neglected; therefore, we included these proteins in the present study. In fact, compared to the other recombinant proteins, the hypothetical protein MHP0418 presented the highest reactivity in the ELISA and the Western blot assay when probed with serum from convalescing pigs (Fig. 2A and B). Therefore, this protein was included in both cocktail vaccines evaluated.
In the study by Pinto et al. (24), MHP0372 lipoprotein was associated with M. hyopneumoniae cytoadherence, and although its role in the mechanism of pathogenicity was not evaluated, this protein is well known to show isoforms in two-dimensional (2D) gels, indicating that it is posttranslationally modified. The surface lipopeptides of mycoplasmas that originated through these modifications are known to contribute to the biological processes of adaptation (25, 26) and pathogenicity, including antigenic variation among strains (5, 27), justifying the choice of this protein for immunological studies. In fact, the results show that MHP0372 is expressed in vitro in addition to inducing antibodies during natural infection (Fig. 2). Furthermore, serum from mice immunized with recombinant MHP0372 showed high absorbance values (Fig. 3) and reacted against M. hyopneumoniae cell lysate (Fig. 5).
Although the mechanisms of M. hyopneumoniae pathogenesis have not been completely elucidated, Thacker et al. (3) suggested the participation of both humoral and cellular immune responses during the infectious process. It is commonly accepted that Th1 cells are preferentially involved in cell-mediated immunity by inducing the production of IgG2a antibodies, while Th2 cells are more effective in the regulation and support of B-cell responses, resulting in the production of IgG1 antibodies (28). Despite the fact that the induction of Th1 and/or Th2 immune responses by a vaccine is species dependent and that the induction of antibodies in mice against M. hyopneumoniae is not correlated to protection, the production of IgG1 and IgG2a by mice immunized with the recombinant proteins was evaluated. It is noteworthy that recombinant proteins usually induce Th2 cells (29). Nevertheless, the majority of recombinant proteins evaluated induced both IgG1 and IgG2a, indicating a mixed Th1/Th2 response (Fig. 4), possibly due to the presentation of these proteins on the cell surface.
Evaluation of the antigenicity and immunogenicity of recombinant M. hyopneumoniae proteins has been pursued by many scientists to select antigens for vaccine production. However, among the antigens previously evaluated as protein (18, 19, 30) or DNA vaccines (30), only two have been tested in pig immunoprotection assays, and these vaccines resulted in partial protection (10, 12, 31), possibly because of the administration of a single antigen. Therefore, the use of a multiple-antigen vaccine has gained attention (13, 14). In this study, we evaluated two protein cocktails (C1 and C2) that included antigens that were immunogenic when delivered individually, including MHP0443, MHP0487, MHP0372, and MHP0660, or that were antigenic (according to the Western blot analysis using convalescent-phase pig serum), such as MHP0418 and MHP0372. An ELISA analysis using serum from mice immunized intramuscularly with these protein cocktails demonstrated that this vaccination strategy resulted in significant increases in the total IgG (Fig. 3A), IgG1, and IgG2a levels compared to immunization with individual proteins (Fig. 4A). MHP0443 and MHP0372 were primarily responsible for the immune response induced by C1 and C2, respectively (Fig. 3B and 4B). Interestingly, the administration of only 16.6 μg of each protein from the cocktails, which represents 1/3 of the individually administered dose, was sufficient to induce antibodies against all the proteins in the cocktails. Previous studies showed that the concurrent administration of many proteins may contribute to the modulation of the immune system (14, 32–34). It remains unknown, however, whether the production of antibodies against multiple proteins results in improvement of recognition and recruitment of immune cells and if it increases the likelihood of M. hyopneumoniae recognition in a later infection event.
The present study demonstrated the antigenic and immunogenic properties of seven recombinant secreted proteins of M. hyopneumoniae administered to mice. These data will help identify potential targets for a subunit vaccine against EP. The combined administration of these proteins increased the production of IgG1 and IgG2a antibodies, suggesting that this strategy represents a promising approach to the development of an effective vaccine. Although the mouse immune response cannot be extrapolated to other species and no direct correlation has been found between antibody concentration and protection against M. hyopneumoniae challenge (35), the results warrant further investigation in challenge experiments in pigs.
ACKNOWLEDGMENTS
We thank EMBRAPA Suínos e Aves (Concórdia-SC, Brazil) for providing the M. hyopneumoniae 7448 strain.
V.G. and S.B.M. received scholarships from the CAPES Foundation. Financial support was provided by Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Footnotes
Published ahead of print 26 June 2013
REFERENCES
- 1.Thacker EL, Halbur PG, Ross RF, Thanawongnuwech R, Thacker BJ. 1999. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J. Clin. Microbiol. 37:620–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross RF. 1986. Mycoplasma disease, p 469–483 In Ross RF, Leman AD, Straw B, Glock RD, Mengeling WL, Penny RHC, Scholl E. Diseases of swine. The Iowa State University Press, Ames, IA [Google Scholar]
- 3.Thacker EL, Thacker BJ, Kuhn M, Hawkins PA, Waters WR. 2000. Evaluation of local and systemic immune responses induced by intramuscular injection of a Mycoplasma hyopneumoniae bacterin to pigs. Am. J. Vet. Res. 61:1384–1389 [DOI] [PubMed] [Google Scholar]
- 4.Meyns T, Dewulf J, De Kruif A, Calus D, Haesebrouck F, Maes D. 2006. Comparison of transmission of Mycoplasma hyopneumoniae in vaccinated and non-vaccinated populations. Vaccine 24:7081–7086 [DOI] [PubMed] [Google Scholar]
- 5.Razin S, Yogev D, Naot Y. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasconcelos AT, Ferreira HB, Bizarro CV, Bonatto SL, Carvalho MO, Pinto PM, Almeida DF, Almeida LG, Almeida R, Alves-Filho L, Assuncao EN, Azevedo VA, Bogo MR, Brigido MM, Brocchi M, Burity HA, Camargo AA, Camargo SS, Carepo MS, Carraro DM, Mattos Cascardo JC, Castro LA, Cavalcanti G, Chemale G, Collevatti RG, Cunha CW, Dallagiovanna B, Dambros BP, Dellagostin OA, Falcao C, Fantinatti-Garboggini F, Felipe MS, Fiorentin L, Franco GR, Freitas NS, Frias D, Grangeiro TB, Grisard EC, Guimaraes CT, Hungria M, Jardim SN, Krieger MA, Laurino JP, Lima LF, Lopes MI, Loreto EL, Madeira HM, Manfio GP, Maranhao AQ, Martinkovics CT, Medeiros SR, Moreira MA, Neiva M, Ramalho-Neto CE, Nicolas MF, Oliveira SC, Paixao RF, Pedrosa FO, Pena SD, Pereira M, Pereira-Ferrari L, Piffer I, Pinto LS, Potrich DP, Salim AC, Santos FR, Schmitt R, Schneider MP, Schrank A, Schrank IS, Schuck AF, Seuanez HN, Silva DW, Silva R, Silva SC, Soares CM, Souza KR, Souza RC, Staats CC, Steffens MB, Teixeira SM, Urmenyi TP, Vainstein MH, Zuccherato LW, Simpson AJ, Zaha A. 2005. Swine and poultry pathogens: the complete genome sequences of two strains of Mycoplasma hyopneumoniae and a strain of Mycoplasma synoviae. J. Bacteriol. 187:5568–5577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W, Feng Z, Fang L, Zhou Z, Li Q, Li S, Luo R, Wang L, Chen H, Shao G, Xiao S. 2011. Complete genome sequence of Mycoplasma hyopneumoniae strain 168. J. Bacteriol. 193:1016–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minion FC, Lefkowitz EJ, Madsen ML, Cleary BJ, Swartzell SM, Mahairas GG. 2004. The genome sequence of Mycoplasma hyopneumoniae Strain 232, the agent of swine mycoplasmosis. J. Bacteriol. 186:7123–7133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto PM, Klein CS, Zaha A, Ferreira HB. 2009. Comparative proteomic analysis of pathogenic and non-pathogenic strains from the swine pathogen Mycoplasma hyopneumoniae. Proteome Sci. 7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagan PK, Walker M, Chiun J, Eamens GJ, Djordjevic SP. 2001. Oral immunization of swine with attenuated Salmonella Typhimurium aroA SL3261 expressing a recombinant antigen of Mycoplasma hyopneumoniae (NrdF) primes the immune system for a NrdF specific secretory IgA response in the lungs. Microb. Pathog. 30:101–110 [DOI] [PubMed] [Google Scholar]
- 11.Chen YL, Wang SN, Yang WJ, Chen YJ, Lin HH, Shiuan D. 2003. Expression and immunogenicity of Mycoplasma hyopneumoniae heat shock protein antigen P42 by DNA vaccination. Infect. Immun. 71:1155–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimoji Y, Oishi E, Muneta Y, Nosaka H, Mori Y. 2003. Vaccine efficacy of the attenuated Erysipelothrix rhusiopathiae YS-19 expressing a recombinant protein of Mycoplasma hyopneumoniae P97 adhesin against mycoplasmal pneumonia of swine. Vaccine 21:532–537 [DOI] [PubMed] [Google Scholar]
- 13.Okamba FR, Arella M, Music N, Ji JJ, Gottschalk M, Gagnon CA. 2010. Potential use of a recombinant replication-defective adenovirus vector carrying the C-terminal portion of the P97 adhesin protein as a vaccine against Mycoplasma hyopneumoniae in swine. Vaccine 28:4802–4809 [DOI] [PubMed] [Google Scholar]
- 14.Chen AY, Fry SR, Daggard GE, Mukkur TK. 2008. Evaluation of immune response to recombinant potential protective antigens of Mycoplasma hyopneumoniae delivered as cocktail DNA and/or recombinant protein vaccines in mice. Vaccine 26:4372–4378 [DOI] [PubMed] [Google Scholar]
- 15.Ramos CRR, Abreu PAE, Nascimento ALTO, Ho PL. 2004. A high-copy T7 Escherichia coli expression vector for the production of recombinant proteins with a minimal N-terminal His-tagged fusion peptide. Braz. J. Med. Biol. Res. 37:1103–1109 [DOI] [PubMed] [Google Scholar]
- 16.Simionatto S, Marchioro SB, Galli V, Hartwig DD, Carlessi RM, Munari FM, Laurino JP, Conceição FR, Dellagostin OA. 2010. Cloning and purification of recombinant proteins of Mycoplasma hyopneumoniae expressed in Escherichia coli. Protein Expr. Purif. 69:132–136 [DOI] [PubMed] [Google Scholar]
- 17.Simionatto S, Marchioro SB, Galli V, Luerce TD, Hartwig DD, Moreira AN, Dellagostin OA. 2009. Efficient site-direct mutagenesis using an overlap extension-PCR method for expressing Mycoplasma hyopneumoniae genes in Escherichia coli. J. Microbiol. Methods 79:101–105 [DOI] [PubMed] [Google Scholar]
- 18.Simionatto S, Marchioro SB, Galli V, Brum CB, Klein CS, Rebelatto R, Silva EF, Borsuk S, Conceição FR, Dellagostin AO. 2012. Immunological characterization of Mycoplasma hyopneumoniae recombinant proteins. Comp. Immunol. Microbiol. Infect. Dis. 35:209–216 [DOI] [PubMed] [Google Scholar]
- 19.Marchioro SB, Simionatto S, Galli V, Conceição FR, Brum CB, Fisch A, Gomes CK, Dellagostin AO. 2012. Production and characterization of recombinant transmembrane proteins from Mycoplasma hyopneumoniae. Vet. Microbiol. 155:44–52 [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Young TF, Ross RF. 1994. Glycolipid receptors for attachment of Mycoplasma hyopneumoniae to porcine respiratory ciliated cells. Infect. Immun. 62:4367–4373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Young TF, Ross RF. 1995. Identification and characterization of a Mycoplasma hyopneumoniae adhesion. Infect. Immun. 63:1013–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu T, Minion C. 1998. Identification of the cilium binding epitope of the Mycoplasma hyopneumoniae P97 adhesin. Infect. Immun. 66:4762–4766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams C, Pitzer J, Minion FC. 2005. In vivo expression analysis of the P97 and P102 paralog families of Mycoplasma hyopneumoniae. Infect. Immun. 73:7784–7787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinto PM, Chemale G, De Castro LA, Metz AP, Kich J, Vainstein MH, Zaha A, Ferreira HB. 2007. Proteomic survey of the pathogenic Mycoplasma hyopneumoniae strain 7448 and identification of novel post-translationally modified and antigenic proteins. Vet. Microbiol. 121:83–93 [DOI] [PubMed] [Google Scholar]
- 25.Behrens A, Heller M, Kirchhoff H, Yogev D, Rosengarten R. 1994. A family of phase- and size-variant membrane surface lipoprotein antigens (Vsps) of Mycoplasma bovis. Infect. Immun. 62:5075–5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhugra B, Voelker LR, Zou N, Yu H, Dybvig K. 1995. Mechanism of antigenic variation in Mycoplasma pulmonis: interwoven, site-specific DNA inversions. Mol. Microbiol. 18:703–714 [DOI] [PubMed] [Google Scholar]
- 27.Djordjevic SP, Cordwell SJ, Djordjevic MA, Wilton J, Minion FC. 2004. Proteolytic processing of the Mycoplasma hyopneumoniae cilium adhesion. Infect. Immun. 72:2791–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosmann TR, Coffman RI. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145–173 [DOI] [PubMed] [Google Scholar]
- 29.Liljeqvist S, Ståhl S. 1999. Production of recombinant subunit vaccines: protein immunogens, live delivery systems and nucleic acid vaccines. J. Biotechnol. 73:1–33 [DOI] [PubMed] [Google Scholar]
- 30.Galli V, Simionatto S, Marchioro SB, Fisch A, Gomes CK, Conceição FR, Dellagostin AO. 2012. Immunisation of mice with Mycoplasma hyopneumoniae antigens P37, P42, P46 and P95 delivered as recombinant subunit or DNA vaccines. Vaccine 31:135–140 [DOI] [PubMed] [Google Scholar]
- 31.Okamba FR, Moreau E, Cheikh Saad Bouh K, Gagnon CA, Massie B, Arella M. 2007. Immune responses induced by replication-defective adenovirus expressing the C-terminal portion of the Mycoplasma hyopneumoniae P97 adhesin. Clin. Vaccine Immunol. 14:767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fachado A, Rodriguez A, Angel SO, Pinto DC, Vila I, Acosta A, Amendoeira RR, Lannes-Vieira J. 2003. Protective effect of a naked DNA vaccine cocktail against lethal toxoplasmosis in mice. Vaccine 21:1327–1335 [DOI] [PubMed] [Google Scholar]
- 33.Hooper JW, Custer DM, Thompson E. 2003. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 306:181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur T, Sobti RC, Kaur S. 2011. Cocktail of gp63 and Hsp70 induces protection against Leishmania donovani in BALB/c mice. Parasite Immunol. 33:95–103 [DOI] [PubMed] [Google Scholar]
- 35.Djordjevic SP, Eamens GJ, Romalis LF, Nicholls PJ, Taylor V, Chin J. 1997. Serum and mucosal antibody responses and protection in pigs vaccinated against Mycoplasma hyopneumoniae with vaccines containing a denatured membrane antigen pool and adjuvant. Aust. Vet. J. 75:504–511 [DOI] [PubMed] [Google Scholar]


