Abstract
Severe fever with thrombocytopenia syndrome virus (SFTSV), a newly discovered member of the Bunyaviridae family, is the causative agent of an emerging hemorrhagic fever, SFTS, in China. Currently, there are no vaccines or effective therapies against SFTS. In this study, a combinatorial human antibody library was constructed from the peripheral lymphocytes of 5 patients who had recovered from SFTS. The library was screened against purified virions for the production of single-chain variable-region fragments (ScFv). Of the 6 positive clones, one clone (monoclonal antibody [MAb] 4-5) showed neutralizing activity against SFTSV infection in Vero cells. MAb 4-5 was found to effectively neutralize all of the clinical isolates of SFTSV tested, which were isolated from patients in China from 2010 to 2012. MAb 4-5 was found to bind a linear epitope in the ectodomain of glycoprotein Gn. Its neutralizing activity is attributed to blockage of the interactions between the Gn protein and the cellular receptor, indicating that inhibition of virus-cell attachment is its main mechanism. These data suggest that MAb 4-5 can be used as a promising candidate molecule for immunotherapy against SFTSV infection.
INTRODUCTION
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging fatal hemorrhagic fever with fatality of up to 30% of all cases (1). The disease is caused by a newly identified bunyavirus, SFTS virus (SFTSV) (1), and it is characterized by sudden onset of fever, respiratory or gastrointestinal symptoms, and a decrease in whole white blood cell and platelet counts that gradually progresses into hemorrhage and multiorgan failure at the end stage (2). This disease has been reported across a broad geographic area in eastern and central China, including Jiangsu, Anhui, Shandong, Henan, Hubei, and Liaoning Provinces (1). Heightened surveillance of acute febrile illness has led researchers to add Zhejiang, a southeastern province, to the list of regions where SFTSV is endemic (3). This indicates that this disease is continuing to spread in China. Recently, a bunyavirus named Heartland virus (HLV) has been isolated from patients from Missouri in the United States. HLV has 70% homology to the Chinese virus based on amino acid sequences (4). The clinical symptoms of HLV infection are similar to those caused by SFTSV. One case of human SFTS outside China has been reported (5). This demonstrates that SFTSV or a virus similar to SFTSV probably has worldwide distribution.
Although most human SFTS cases in China are sporadic, and the patients tend to have histories of arthropod bites, person-to-person transmissions through blood contact have been reported (2, 6, 7, 8). Despite the medical importance of this disease, no clinical treatment for SFTSV infection other than supportive care has been developed. Prophylactic and therapeutic measures, including therapeutic antibodies and vaccines that would protect susceptible individuals and those at high risk of complications of infection, are urgently needed.
SFTSV is a member of the Phlebovirus genus in the Bunyaviridae family (1). Like all bunyaviruses, SFTSV has a trisegmented, single-stranded RNA genome with negative (L and M segments) or ambisense (S segment) polarity, and it encodes seven proteins (9). The two glycoproteins, Gn and Gc, which are produced by cleavage of a precursor encoded by the M segment, are highly antigenic envelope proteins. They are responsible for receptor binding and membrane fusion (10). For this reason, viral surface glycoproteins may be targets for neutralizing antibody responses.
Antibody has played a critical role in the treatment of a wide variety of viral diseases, such as those caused by Hantaan virus, cytomegalovirus, rabies virus, and respiratory syncytial virus infection (11–14). The mechanisms of antibody protection include neutralization, complement activation, antibody-dependent cellular cytotoxicity, and opsonization (15). Patients infected with SFTSV, like those infected with other systemic arboviruses, can remain viremic for up to 12 days (unpublished data). The administration of neutralizing antibodies can conceivably reduce viral load, prevent viral dissemination into other systems, and likely reduce the risk of severe outcome of the disease. They could also be used for prophylactics in high-risk persons, such as hospital personnel and family members of patients, who are at risk for person-to-person transmission, and immunocompromised patients, who might not respond well to vaccines.
In this study, we developed a human monoclonal antibody (MAb), called MAb 4-5, isolated from a phage antibody library using whole SFTSV virions. Its binding and neutralizing properties were investigated. MAb 4-5 was found to bind a linear epitope in the ectodomain of Gn. This unidentified epitope was found to be conserved among disparate geographic virus isolates within China, since MAb 4-5 shows a cross-neutralizing activity. The mode of inhibition was also characterized, indicating that MAb 4-5 mediates neutralization by blocking the binding of Gn to the cellular receptor. These data suggest that MAb 4-5 could be developed into a therapeutic agent in passive immunotherapy.
MATERIALS AND METHODS
Virus strains and virion preparation.
The SFTSVs used in this study are listed in Table 1. They were propagated at 37°C in Vero cells at a multiplicity of infection (MOI) of 1.0 and cultivated for 10 days. Supernatants containing viral particles were harvested, aliquoted, and stored at −70°C until use. Fifty-percent tissue culture infective doses (TCID50) of working stocks of each strain were titrated on Vero cells. For virion purification, culture supernatant of the JS-2010-003 virus was successively treated with β-propiolactone inactivation, removal of cell debris, ultracentrifugation, and gel filtration chromatography as described previously (16). The purified virions were analyzed by SDS-PAGE to confirm purity. All operations involving SFTSV were performed under biosafety level 2 containment conditions.
Table 1.
SFTS virus strains used in this study
| Virus strain | Areaa | Yr |
|---|---|---|
| JS-2010-014 | Jiangsu | 2010 |
| JS-2011-004 | Jiangsu | 2011 |
| JS-2011-013-1 | Jiangsu | 2011 |
| JS-2011-027 | Jiangsu | 2011 |
| JS-2012-020 | Jiangsu | 2012 |
| JS-2012-035 | Jiangsu | 2012 |
| JS-2010-006 | Anhui | 2010 |
| JS-2010-018 | Anhui | 2010 |
| JS-2011-034 | Anhui | 2011 |
| JS-2010-004 | Shandong | 2010 |
| HN01 | Henan | 2010 |
| HN69 | Henan | 2010 |
| ZJWHX | Zhejiang | 2010 |
| ZJZYT | Zhejiang | 2010 |
Anhui and Shandong SFTS patients were admitted to hospitals in Jiangsu Province, and the pathogens were isolated by Jiangsu medical institutions.
Construction, panning, and screening of the human single-chain variable-region fragment (ScFv) antibody library.
Human lymphocytes were collected from 5 convalescent SFTS patients in Jiangsu Province. The research protocol was approved by the Human Bioethics Committee of Jiangsu Center for Disease Prevention and Control (Jiangsu CDC), and all participants provided written informed consent. Total RNA was extracted from lymphocytes using an RNA purification kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized from total RNA by using a first-strand cDNA synthesis kit (Invitrogen, Carlsbad, CA) with oligo(dT). The variable-region genes of heavy (VH) and light (VL) chains were amplified from cDNA using PCR and sequentially cloned into the phagemid vector pComb3X as described elsewhere (17). The human antibody library was constructed after electrotransformation of the ScFv gene reservoir into Escherichia coli XL1-Blue (Stratagene, La Jolla, CA).
Costar 96-well EIA/RIA Stripwell immunoplates (Corning, NY) were coated with SFTSV virions diluted to a concentration of 8 μg/ml by carbonate-bicarbonate buffer, pH 9.6 (50 μl/well) and incubated overnight at 4°C. Phages were incubated with SFTSV at 37°C for 2 h. Bound phages were eluted and used to reinfect XL1-Blue E. coli and reamplified as described previously (18). After the 3rd round of panning, phages from individual colonies were tested for binding to SFTSV in an enzyme-linked immunosorbent assay (ELISA). For the positive clones, the genes of VH and VL chains were sequenced, and their corresponding amino acid sequences were aligned.
Expression of soluble ScFv and IgG1 MAbs.
Soluble ScFv were expressed in Top10 E. coli (Invitrogen, La Jolla, CA) and purified from the periplasmic fractions. All ScFv contained a 6×His tag at the carboxy terminus that facilitated purification through immobilized metal affinity chromatography. The engineering and production of the human immunoglobulin G1 (IgG1) MAb was performed largely as described previously (19). Briefly, the VH and VL regions of the ScFv-positive clone were PCR amplified by using specific primers and cloned into pAc-K-CH3 baculovirus vector via XhoI/NheI and SacI/HindIII sites. IgG1 MAb was expressed by cotransfection with a recombinant IgG1 plasmid and linearized AcNPV baculovirus DNA (BD, San Jose, CA) into SF9 insect cells according to the manufacturer's instructions. The supernatant containing recombinant human IgG1 was purified through protein G columns (GE Healthcare, Uppsala, Sweden) and kept at −20°C until use.
Neutralization assays.
First, 50 μl of SFTSV-specific ScFv (100 μg/ml) was mixed with an equal volume of suspension of 100 TCID50 of SFTSV strain JS-2010-003 and incubated at 37°C for 1 h. The virus-antibody mixture was then transferred onto monolayers of Vero cells in 96-well plates and incubated at 37°C for 1 h. After being washed with minimal essential medium (MEM), a maintenance medium, the samples were incubated at 37°C in a 5% CO2 incubator. Cytopathic effects (CPE) were observed every 24 h for 6 days. Each antibody was considered to have neutralizing capacity if it could inhibit ≥90% of viral CPE. For the positive clone, here designated MAb 4-5, ScFv was reformatted into full-length IgG1. This molecule was used to further characterize its neutralizing potency and cross-binding activity, as described above. Individual measurements were performed in triplicate, and the relative neutralization was calculated in the presence of patient convalescent-phase sera and an irrelevant human IgG1 (or its ScFv format, enterovirus 71 specific) as positive and negative controls, respectively.
Western blot analysis.
To determine the binding target of the selected MAb 4-5 IgG1, purified SFTSV strain JS-2010-003 virions were lysed with sample buffer and proteins were fractionated by 10% SDS-PAGE. The separated proteins were electrotransferred to a nitrocellulose (NC) membrane and incubated consequently with MAb 4-5 IgG1 and horseradish peroxidase (HRP)-conjugated goat anti-human immunoglobulin (ZHGB-BIO, Beijing, China). The blots were visualized by 3,3′diaminobenzidine (DAB) (Boster Bio, Wuhan, China) according to the manufacturer's instructions.
Immunofluorescence assay.
Reactivity between human MAb 4-5 IgG1 and SFTSV-infected cells was assessed using indirect immunofluorescence assay (IFA). The infected and noninfected (SFTSV, JS-2010-003) Vero cells were grown on an 8-well Millicell EZ slide (Millipore, Billerica, MA) for 36 h at 37°C, and cells were fixed by treatment with acetone for 10 min at −20°C. Human MAb 4-5 IgG1or an irrelevant human IgG1 (described above) were incubated with the fixed cells for 30 min at 37°C. Bound antibodies were detected using fluorescein isothiocyanate (FITC)-conjugated anti-human antibodies (KPL, Gaithersburg, MD; diluted by phosphate-buffered saline PBS containing 0.01% [wt/vol] Evens blue)[] and observed under an immunofluorescence microscope.
Electron microscopy.
Immunoelectron microscopy (immune-EM) of the SFTSV virion was performed essentially as described previously (20). SFTSV JS-2010-003 from the supernatant of infected Vero cells was adsorbed to copper grids coated with carbon and Pioloform. These were incubated with MAb 4-5 IgG1 by floating on droplets for 30 min at room temperature, and an irrelevant human IgG1 (described above) was used as a negative control. Bound monoclonal antibodies were detected by incubation on droplets of anti-human IgG gold 10-nm conjugates (Jieyi Biotech, Shanghai, China). The grids were negatively contrasted with 2% phosphotungstic acid and assessed with a Hitachi 7650A transmission electron microscope.
Glycoprotein ectodomain mapping for MAb 4-5's binding.
The amino acid sequences of SFTSV JS-2010-003 Gn and Gc glycoproteins were separately submitted to the TMHMM server, version 2.0 (http://www.cbs.dtu.dk/services/TMHMM/), for the prediction of protein transmembrane helices. One ectodomain was identified for Gn (amino acids [aa] 20 to 452) and Gc (aa 1 to 473). Their corresponding genes were generated by PCR and cloned into a pXJ40-HA expression vector. All sequences were confirmed by DNA sequencing. These functional domains were expressed in 293 cells by transient transfection, and the recombinant protein-containing supernatants were analyzed by Western blotting as described above.
Mechanism of MAb 4-5 neutralizing activity.
The recombinant Gn ectodomain-containing culture supernatant (100 μl) was mixed with excess MAb 4-5 ScFv (4.0 μg) (another selected clone from the library, called A5, was used as a negative control) and incubated overnight at 4°C. The protein-antibody mixture was added to the Vero cell suspension, mixed, and incubated at room temperature for 2 h. The cells were washed and then lysed with sample buffer. Proteins were analyzed by Western blotting, with an anti-HA-HRP conjugate.
RESULTS
Generation of MAbs against the SFTSV virion.
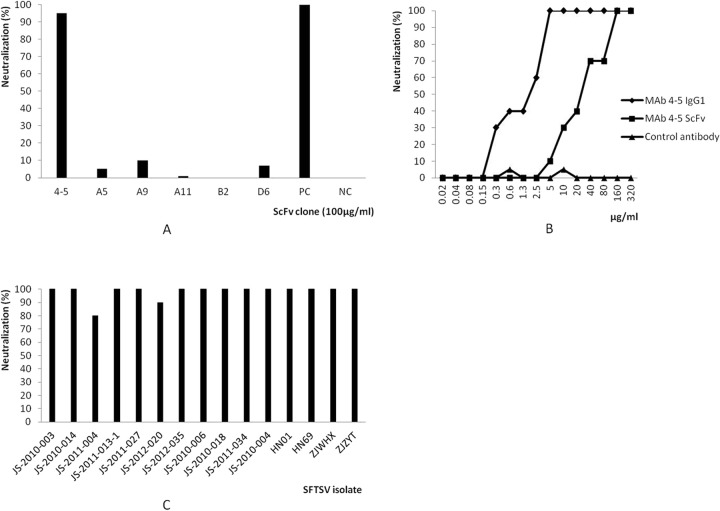
The human ScFv library was panned against the purified inactivated SFTSV virion coated on plastic plates. After 3 rounds of panning, 90 randomly picked clones were screened, and 14 clones were found to recognize SFTSV by ELISA (data not shown). Sequence analysis of positive ScFv clones revealed the presence of 6 unique clones (Fig. 1). The selected ScFv were encoded by 6 different VH and VL sequences. The gene families were VH5 and VH1 for VH and Vκ1, Vκ2, and Vκ3 for VL. The 6 clones were expressed in E. coli and purified by immobilized metal affinity chromatography.
Fig 1.
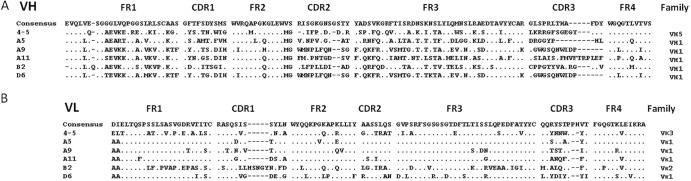
The deduced protein sequences of VH (A) and VL (B) domains of positive clones to SFTSV virion. FR, framework region; CDR, complementary determining region. Dots indicate sequence identity to the consensus sequence. Dashes represent gaps.
In vitro neutralizing activity of antibodies to SFTSV.
The neutralizing activity of the purified soluble ScFv for SFTSV (JS-2010-003) was tested using Vero cells. Of the 6 ScFv clones tested, only clone 4-5 (VH5/Vκ3) showed neutralizing activity (Fig. 2A). This clone was then converted into intact IgG1 and expressed in a baculovirus/insect cell system. To determine its neutralization potency, MAb 4-5 IgG1 was subjected to serial dilutions with 2-fold increments and titrated against the JS-2010-003 strain. As shown in Fig. 2B, MAb 4-5 IgG1 exhibited far more potent neutralization activity against SFTSV than its ScFv format. This was consistent with its superior avidity. The concentration required to obtain 50% neutralization of 100 TCID50 SFTSV was about 2.0 μg/ml, but the same activity was achieved by MAb 4-5 ScFv at a concentration of 25 μg/ml. The control antibody did not exhibit any neutralization activity against SFTSV. The neutralization breadth of MAb 4-5 IgG1was investigated by using a panel of different SFTSV isolates obtained from regions where SFTSV is endemic, including Jiangsu, Anhui, Shandong, Henan, and Zhejiang, between 2010 and 2012 (Table 1). MAb 4-5 IgG1 was separately incubated in the presence of 100 TCID50 of SFTSV isolates for 1 h at 37°C before incubation with Vero cells. Complete protection from most SFTSV strains was achieved at a dose of 5 μg/ml MAb 4-5 IgG1. The two exceptions were both Jiangsu isolates, JS-2011-004 and JS-2012-020, for which MAb 4-5 IgG1showed neutralization rates of 80% and 90%, respectively (Fig. 2C). This indicates that MAb 4-5 recognizes a conserved epitope within SFTSV structural proteins shared by disparate geographic virus isolates in China.
Fig 2.
Neutralization activity of SFTSV-specific MAbs. (A) Of the 6 antibody clones in ScFv format selected from the human phage antibody library, MAb 4-5 readily neutralized SFTSV strain JS-2010-003. Patient convalescent-phase sera were used as positive controls (PC), and an irrelevant ScFv molecule (enterovirus 71 specific) was used as a negative control (NC). (B) Titration of MAb 4-5 IgG1 neutralization potency. MAb 4-5 IgG1 exhibited a higher neutralization activity against SFTSV strain JS-2010-003 than did its ScFv format. A human antibody IgG1 against enterovirus 71 was used as a negative control. (C) The ability of MAb 4-5 IgG1 to neutralize the indicated viral strains at a concentration of 5 μg/ml was measured using a neutralization assay as described in Materials and Methods.
Characterization of MAb 4-5 binding specificity.
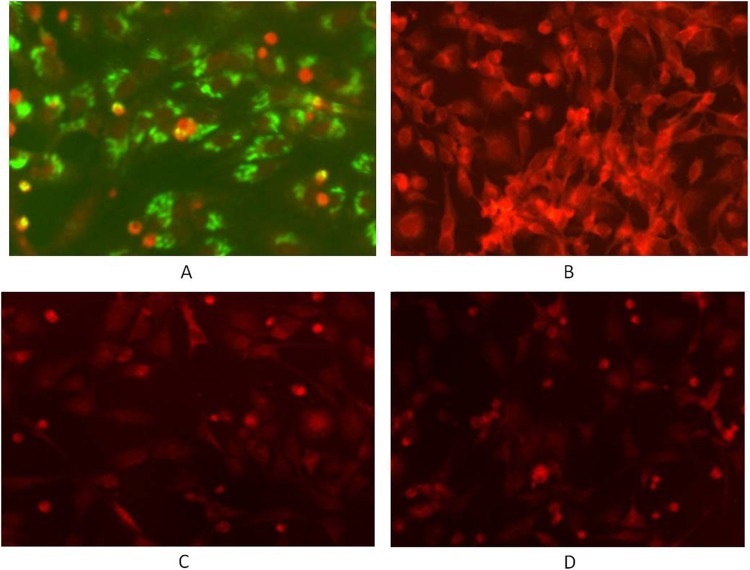
Western blot showed that MAb 4-5 is immunoreactive with two bands with molecular masses of 72 kDa and 170 kDa, respectively, under reducing conditions (Fig. 3A). They probably represent the Gn glycoprotein and the homo- or hetero-oligomer of Gn or Gn/Gc from the observed molecular mass, compared to the purified virion SDS-PAGE profile (Fig. 3B), indicating that it recognizes a continuous linear epitope within Gn. To confirm the specificity of MAb 4-5 for the virus, immunofluorescence was performed on SFTSV-infected and uninfected Vero cells. The SFTSV-infected cells fixed to slides with acetone were moderately stained (Fig. 4A), whereas no fluorescence could be detected with uninfected cells when treated by MAb 4-5 IgG1 (Fig. 4C). The control human IgG1 didn't stain either infected (Fig. 4B) or uninfected (Fig. 4D) cells. Immune-EM was used to investigate the binding of MAb 4-5 to glycoproteins in whole virions. Incubation with MAb 4-5 caused localization of the gold label to the outer peplomer region of the SFTSV JS-2010-003 strain, but a control human IgG1 did not induce any labeling (Fig. 5A and B).
Fig 3.
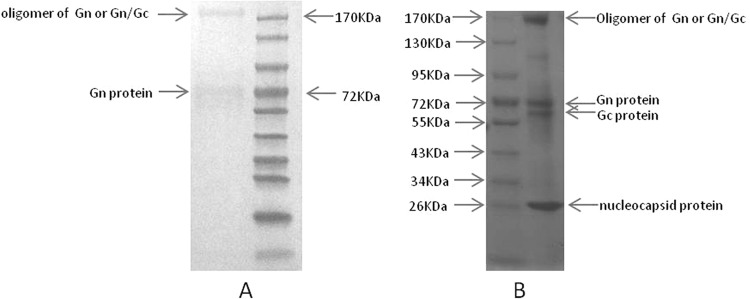
Western blot. Purified SFTSV JS-2010-003 strain virions were electrophoresed on a 10% SDS-PAGE gel and transferred on an NC membrane. MAb 4-5 IgG1 binds two bands with molecular masses of 72 kDa and 170 kDa under reducing conditions (Fig. 3A). They probably represent Gn glycoprotein and homo- or hetero-oligomer of Gn or Gn/Gc compared to the purified virion SDS-PAGE profile (Fig. 3B).
Fig 4.
Characterization of MAb 4-5 IgG1 in IFA. Infected (A, B) and noninfected (SFTSV, JS-2010-003) (C, D) Vero cells were fixed on slides and incubated with MAb 4-5 IgG1 (A, C) or an irrelevant human IgG1 (B, D). Bound antibodies were detected by using FITC-conjugated anti-human antibody with PBS dilution buffer (pH 7.4) containing 0.01% (wt/vol) Evens blue counterstain.
Fig 5.
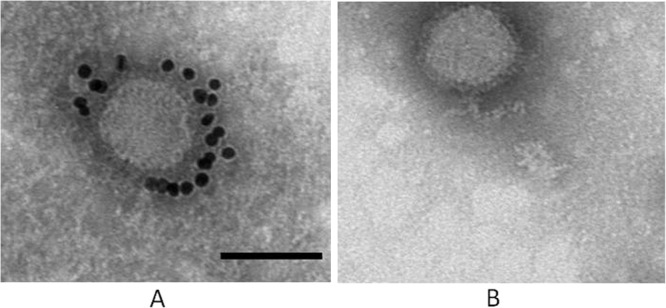
Analysis of glycoprotein binding by immune-EM. Gold immunolabeling of glycoproteins in SFTSV viral peplomers with MAb 4-5 IgG1 (A) or a negative control IgG1 (B) was carried out. This was followed by incubation with anti-human IgG gold 10-nm conjugates. The bar indicates 100 nm.
Mapping of MAb 4-5 binding to SFTSV glycoprotein domains.
Protein transmembrane helices were predicted using TMHMM server version 2.0. One ectodomain was identified for Gn (aa 20 to 452) and Gc (aa 1 to 473). To map the region bound by MAb 4-5, Gn and Gc ectodomains were expressed separately in 293 cells, and Western blot analysis showed that it reacted with the Gn but not with the Gc ectodomain (Fig. 6A and B). This suggested that MAb 4-5 recognized an exposed loop (aa 20 to 452) in the N terminus of the Gn protein.
Fig 6.

Identification of an MAb 4-5 binding target in SFTSV glycoproteins. (A) Of the 2 predicted ectodomains expressed in 293 cells, MAb 4-5 reacted only with the Gn domain 50 kDa in size. (B) The recombinant Gn and Gc ectodomains were hybridized by an anti-HA-HRP conjugate.
Inhibition of binding of the Gn1 domain to cellular receptor by MAb 4-5.
As shown in Fig. 7, when Vero cells were incubated with the recombinant Gn ectodomain in the presence of MAb 4-5 ScFv and analyzed by Western blotting, MAb 4-5 ScFv completely inhibited the binding of Gn to Vero cells, but the nonneutralizing antibody A5 did not inhibit binding under the same conditions. This demonstrated that the mechanism of the neutralizing activity of MAb 4-5 involves blocking binding of Gn to its cellular receptor.
Fig 7.
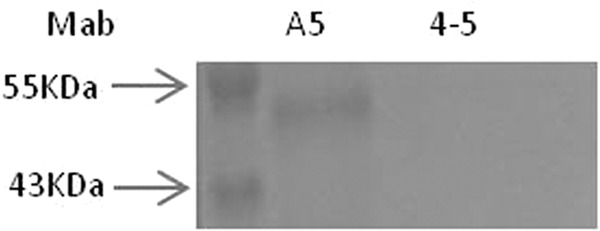
Inhibition of binding of the Gn ectodomain to the cellular receptor by MAb 4-5. The recombinant Gn-HA fusion protein was unable to bind to Vero cells after incubation with MAb 4-5 ScFv. A nonneutralizing antibody, A5, was used as a negative control.
DISCUSSION
In China, SFTS is a severe emerging hemorrhagic fever that causes thousands of people to be hospitalized each year, with an average case fatality of 12% and up to 30% (8). Vaccination is the most effective countermeasure against viral disease (21, 22). However, the low incidence (0.8 to 0.94%) (23, 24) and sporadic nature (9) of SFTSV infection makes it difficult to target the human population most in need of vaccination and to access the vaccine's economic feasibility. This severely influences vaccine development. An antibody-mediated therapeutic may be a useful contributor to treat and prevent SFTS. It can be used in susceptible individuals and those at high risk of complication of infection. In this study, a human antibody against SFTSV was described. The actual breadth of the neutralizing spectrum, functional activity, and binding target were also investigated.
The viral envelope glycoproteins of SFTSV, such as Gn and Gc, mediate receptor attachment and the fusion of viral and cellular membranes, giving the viral genome access to the host cell's cytoplasm. Antibodies can inhibit viral infection through two distinct mechanisms that occur in parallel with the stages of viral entry into the cells. They can directly block viral attachment to target cells by interfering with virus-receptor interactions. These include antibodies against the S1 protein of severe acute respiratory syndrome coronavirus (SARS-CoV) (25) and antibodies against the envelope protein of West Nile virus (26). Antibodies can also target viral receptors, as exemplified by treatment of arenavirus infection with an anti-human transferring receptor antibody (27). During viral entry via endocytosis, antibodies may block the conformational changes in envelope protein required for fusion between viral and endosomal membranes. For example, some antihemagglutinin MAbs neutralize the infectivity of the influenza virus by interfering with the low pH-induced structural rearrangements in the HA2 domain, inhibiting of fusion during viral replication (21, 28). In this study, consistent with the first mechanism described above, MAb 4-5 generated from the memory B cell repertoire of SFTS convalescent patients was found to neutralize SFTSV infectivity by interfering with the interactions between the virus and its receptor, because the recombinant Gn ectodomain cannot bind susceptible Vero cells after incubation with the MAb 4-5 ScFv format (Fig. 7). Structurally, the Gn and Gc of viruses in the Bunyaviridae family are typical class I transmembrane proteins. They have amino termini exposed on the surfaces of the virions and the carboxy terminus anchored in the membrane (10). A functional domain has been predicted for Gn of SFTSV, and the result of the domain mapping for MAb 4-5 showed that the Gn domain (aa 20 to 452), which is located at the amino terminus, is bound by the antibody, demonstrating that this ectodomain is the primary domain for cellular membrane attachment, corresponding to the putative exposed loop for receptor binding in bunyavirus. As reported for other members of Bunyaviridae (29–32), viral neutralization sites are located on both the Gn and Gc. In the present study, due to the limited number of clones selected, the antibody described here was against Gn, and the identification of potential neutralizing antibodies against Gc merits further exploration.
The most important finding of this study may be that one such antibody, 4-5, neutralized SFTSV strains that were found in most of the areas where SFTSV is endemic (Jiangsu, Anhui, Shandong, Henan, and Zhejiang) and that it did so very potently. This indicates that the epitope for MAb 4-5 is conserved among diverse SFTSV strains. It has a single-stranded RNA genome, so the genetic evolution of SFTSV may have accrued in terms of mutation and reassortment (33). However, very little is known about the details of this process. This uncharacterized epitope can resist the host immune pressure and plays a critical role in the receptor binding that takes place during viral transmission. MAb 4-5 may tolerate the antigenic variability of circulating SFTSV variants and maintain its ability to neutralize the viruses. Another unique feature of MAb 4-5 is that it reacts with a linear epitope rather than a conformational one (Fig. 3A). This may facilitate rational vaccine design. These data underscore the importance of broadly cross-reactive, surface-exposed epitopes on the N-terminal domain of Gn in the design of protective SFTSV vaccines.
Human MAbs have been generated through phage/yeast display, immortalization of human B cells from convalescent patients, and immunization of transgenic mice (34). Combinations of antibodies against a variety of structural epitopes on glycoproteins may expand the breadth of protection offered and limit the number of potential immune escape variants (35). However, screening for neutralizing antibodies can isolate only one clone per study. There are several reasons that may account for this. Although phage display-based selections are currently the most commonly used method of developing human MAbs, its inherent selection biases are difficult to control during panning because antibody folding efficiency, posttranslational modification, and epitope accessibility to different display hosts can all influence the outcome of selection (34). Second, the method of antigen presentation during panning may affect the number and distribution of available neutralizing epitopes. In this study, it is postulated that the exposed epitope within the receptor binding domain of the Gn protein on the surface of virion should be easily recognized by antibodies, such as MAb 4-5, but the epitopes exposed only during viral fusion cannot be accessed by antibodies under these selection conditions. Third, there is still a possibility that antibody clones against other abundant viral antigens (e.g., nucleocapsid protein) may overshadow those against glycoproteins during panning, which might otherwise be screened out. New strategies may be required to deplete the library of phages that can bind irrelevant targets to enhance specific clone enrichment ratios.
In summary, MAb 4-5 is a newly discovered human neutralizing antibody capable of recognizing a broadly cross-reactive, surface-exposed epitope on the N-terminal domain of the SFTSV Gn glycoprotein. Further work will be required to delineate the precise structure of this epitope, which may have important implications for the design of vaccines capable of eliciting a protective MAb 4-5-like antibody response. Since the results reported here were generated in vitro, antiviral activities of MAb 4-5 in vivo are under way in our laboratory. Overall, MAb 4-5 may be used as a promising candidate molecule for emergency prophylaxis and treatment of SFTSV infection.
ACKNOWLEDGMENTS
This work was supported by grants from Jiangsu Province community development projects (BE2012768), Jiangsu Province Outstanding Medical Academic Leader Program (RC2011082), and National Mega-Projects for Infectious Diseases (2011ZX10004-902, 2013ZX09102029) from Ministry of Science and Technology. We are grateful to Henan and Zhejiang CDCs for the donation of SFTSV strains.
Footnotes
Published ahead of print 17 July 2013
REFERENCES
- 1.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 364:1523–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao CJ, Guo XL, Qi X, Hu JL, Zhou MH, Varma JK, Cui LB, Yang HT, Jiao YJ, Klena JD, Li LX, Tao WY, Li X, Chen Y, Zhu Z, Xu K, Shen AH, Wu T, Peng HY, Li ZF, Shan J, Shi ZY, Wang H. 2011. A family cluster of infections by a newly recognized bunyavirus in eastern China, 2007: further evidence of person-to-person transmission. Clin. Infect. Dis. 53:1208–1214 [DOI] [PubMed] [Google Scholar]
- 3.Li S, Xue C, Fu Y, Wang J, Ding X, Liu R, Lin Z, Chai N, Yang X, Wang Y, Li Y, Zhang Z, Cheng X, Zhang W. 2011. Sporadic case infected by severe fever with thrombocytopenia syndrome bunyavirus in a nonepidemic region of China. Biosci. Trends 5:273–276 [DOI] [PubMed] [Google Scholar]
- 4.McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albariño CG, Zaki SR, Rollin PE, Nicholson WL, Nichol ST. 2012. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 367:834–841 [DOI] [PubMed] [Google Scholar]
- 5.Denic S, Janbeih J, Nair S, Conca W, Tariq WU, Al-Salam S. 2011. Acute thrombocytopenia, leucopenia, and multiorgan dysfunction: the first case of SFTS bunyavirus outside China? Case Rep. Infect. Dis. 2011:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao CJ, Qi X, Wang H. 2011. A novel bunyavirus in China. N. Engl. J. Med. 365:862–863 [DOI] [PubMed] [Google Scholar]
- 7.Gai Z, Liang M, Zhang Y, Zhang S, Jin C, Wang SW, Sun L, Zhou N, Zhang Q, Sun Y, Ding SJ, Li C, Gu W, Zhang F, Wang Y, Bian P, Li X, Wang Z, Song X, Wang X, Xu A, Bi Z, Chen S, Li D. 2012. Person-to-person transmission of severe fever with thrombocytopenia syndrome bunyavirus through blood contact. Clin. Infect. Dis. 54:249–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L, Walker DH, Ren J, Wang Y, Yu XJ. 2012. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis. 12:156–160 [DOI] [PubMed] [Google Scholar]
- 9.Li DX. 2011. An outline of severe fever with thrombocytopenia syndrome virus. Chin. J. Exp. Clin. Virol. 25:81–84 [Google Scholar]
- 10.Schamljohn CS, Nichol ST. 2007. Bunyaviridae, p 1741–1789 In Knipe DM. (ed), Fields virology. Lippincott/Williams &Wilkins, Philadelphia, PA [Google Scholar]
- 11.Xu R, Yang XY, Yang DF, Zou CY, Gong PL, Zeng FD. 2009. Phase I evaluation of the safety and pharmacokinetics of a single-dose intravenous injection of a murine monoclonal antibody against Hantaan virus in healthy volunteers. Antimicrob. Agents Chemother. 53:5055–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winston DJ, Ho WG, Lin CH, Bartoni K, Budinger MD, Gale RP, Champlin RE. 1987. Intravenous immune globulin for prevention of cytomegalovirus infection and interstitial pneumonia after bone marrow transplantation. Ann. Intern. Med. 106:12–18 [DOI] [PubMed] [Google Scholar]
- 13.Lang J, Gravenstein S, Briggs D, Miller B, Froeschle J, Dukes C, Le Mener V, Lutsch C. 1998. Evaluation of the safety and immunogenicity of a new, heat-treated human rabies immune globulin using a sham, post-exposure prophylaxis of rabies. Biologicals 26:7–15 [DOI] [PubMed] [Google Scholar]
- 14.Groothuis JR, Simoes E, Levin MJ, Hall CB, Long CE, Rodriguez WJ, Arrobio J, Meissner HC, Fulton DR, Welliver RC, Tristram DA, Siber GR, Prince GA, Raden MV, Hemming VG. 1993. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. N. Engl. J. Med. 329:1524–1530 [DOI] [PubMed] [Google Scholar]
- 15.Casadevall A, Dadachova E, Pirofski LA. 2004. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2:695–703 [DOI] [PubMed] [Google Scholar]
- 16.Yu L, Zhang L, Sun L, Lu J, Wu W, Li C, Zhang Q, Zhang F, Jin C, Wang X, Bi Z, Li D, Liang M. 2012. Critical epitopes in the nucleocapsid protein of SFTS virus recognized by a panel of SFTS patients derived human monoclonal antibodies. PLoS One 7:e38291. 10.1371/journal.pone.0038291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim KP, Li H, Nathan S. 2004. Expression and purification of a recombinant ScFv towards the exotoxin of the pathogen, Burkholderia pseudomallei. J. Microbiol. 42:126–132 [PubMed] [Google Scholar]
- 18.Jiao Y, Zhao P, Zhu J, Grabinski T, Feng Z, Guan X, Skinner RS, Gross MD, Hay RV, Tachibana H, Cao B. 2005. Construction of human naïve Fab library and characterization of anti-met Fab fragment generated from the library. Mol. Biotechnol. 31:41–54 [DOI] [PubMed] [Google Scholar]
- 19.Liang M, Dubel S. 2010. Produciton of recombinant human IgG antibodies in the baculovirus expression system, p 453–470 In Kontermann R, Dubel S. (ed), Antibody engineering, 2nd ed, vol 1 Springer, Heidelberg, Germany [Google Scholar]
- 20.Traggiai E, Becker S, Subbarao K, Kolesnikova L, Uematsu Y, Gismondo MR, Murphy BR, Rappuoli R, Lanzavecchia A. 2004. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 10:871–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li OT, Poon LL. 2009. One step closer to universal influenza epitopes. Expert Rev. Anti Infect. Ther. 7:687–690 [DOI] [PubMed] [Google Scholar]
- 23.Zhao L, Zhai S, Wen H, Cui F, Chi Y, Wang L, Xue F, Wang Q, Wang Z, Zhang S, Song Y, Du J, Yu X. 2012. Severe fever with thrombocytopenia syndrome virus, Shandong Province, China. Emerg. Infect. Dis. 18:963–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Zeng X, Zhou M, Jiao Y, Wen T, Guo X, Qi X, Shi Z. 2011. Seroepidemiology of severe fever with thrombocytopenia syndrome virus in Jiangsu Province. Dis. Surveill. 26:676–678 [Google Scholar]
- 25.Sui J, Li W, Murakami A, Tamin A, Matthews LJ, Wong SK, Moore MJ, Tallarico AS, Olurinde M, Choe H, Anderson LJ, Bellini WJ, Farzan M, Marasco WA. 2004. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human MAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. U. S. A. 101:2536–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Throsby M, Geuijen C, Goudsmit J, Bakker AQ, Korimbocus J, Kramer RA, Clijsters-van der Horst M, de Jong M, Jongeneelen M, Thijsse S, Smit R, Visser TJ, Bijl N, Marissen WE, Loeb M, Kelvin DJ, Preiser W, ter Meulen J, de Kruif J. 2006. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile virus. J. Virol. 80:6982–6992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helguera G, Jemielity S, Abraham J, Cordo SM, Martinez MG, Rodríguez JA, Bregni C, Wang JJ, Farzan M, Penichet ML, Candurra NA, Choe H. 2012. An antibody recognizing the apical domain of human transferrin receptor 1 efficiently inhibits the entry of all new world hemorrhagic fever arenaviruses. J. Virol. 86:4024–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Carvalho Nicacio C, Lundkvist A, Sjölander KB, Plyusnin A, Salonen EM, Björling E. 2000. A neutralizing recombinant human antibody Fab fragment against Puumala hantavirus. J. Med. Virol. 60:446–454 [DOI] [PubMed] [Google Scholar]
- 30.Guttieri MC, Bookwalter C, Schmaljohn C. 2000. Expression of a human, neutralizing monoclonal antibody specific to Puumala virus G2-protein in stably-transformed insect cells. J. Immunol. Methods 246:97–108 [DOI] [PubMed] [Google Scholar]
- 31.Koch J, Liang M, Queitsch I, Kraus AA, Bautz EK. 2003. Human recombinant neutralizing antibodies against Hantaan virus G2 protein. Virology 308:64–73 [DOI] [PubMed] [Google Scholar]
- 32.Liang M, Mahler M, Koch J, Ji Y, Li D, Schmaljohn C, Bautz EK. 2003. Generation of an HFRS patient-derived neutralizing recombinant antibody to Hantaan virus G1 protein and definition of the neutralizing domain. J. Med. Virol. 69:99–107 [DOI] [PubMed] [Google Scholar]
- 33.Russell CJ, Webster RG. 2005. The genesis of a pandemic influenza virus. Cell 123:368–371 [DOI] [PubMed] [Google Scholar]
- 34.Marasco WA, Sui J. 2007. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat. Biotechnol. 25:1421–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ter Meulen J, van den Brink EN, Poon LL, Marissen WE, Leung CS, Cox F, Cheung CY, Bakker AQ, Bogaards JA, van Deventer E, Preiser W, Doerr HW, Chow VT, de Kruif J, Peiris JS, Goudsmit J. 2006. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 3:e237. 10.1371/journal.pmed.0030237 [DOI] [PMC free article] [PubMed] [Google Scholar]