Abstract
The poor immunogenicity of the meningococcal serogroup B (MenB) capsule has led to the development of vaccines targeting subcapsular antigens, in particular the immunodominant and diverse outer membrane porin, PorA. These vaccines are largely strain specific; however, they offer limited protection against the diverse MenB-associated diseases observed in many industrialized nations. To broaden the scope of its protection, the multicomponent vaccine (4CMenB) incorporates a PorA-containing outer membrane vesicle (OMV) alongside relatively conserved recombinant protein components, including factor H-binding protein (fHbp), Neisseria adhesin A (NadA), and neisserial heparin-binding antigen (NHBA). The expression of PorA is unique to meningococci (Neisseria meningitidis); however, many subcapsular antigens are shared with nonpathogenic members of the genus Neisseria that also inhabit the nasopharynx. These organisms may elicit cross-protective immunity against meningococci and/or occupy a niche that might otherwise accommodate pathogens. The potential for 4CMenB responses to impact such species (and vice versa) was investigated by determining the genetic distribution of the primary 4CMenB antigens among diverse members of the common childhood commensal, Neisseria lactamica. All the isolates possessed nhba but were devoid of fhbp and nadA. The nhba alleles were mainly distinct from but closely related to those observed among a representative panel of invasive MenB isolates from the same broad geographic region. We made similar findings for the immunogenic typing antigen, FetA, which constitutes a major part of the 4CMenB OMV. Thus, 4CMenB vaccine responses may impact or be impacted by nasopharyngeal carriage of commensal neisseriae. This highlights an area for further research and surveillance should the vaccine be routinely implemented.
INTRODUCTION
The genus Neisseria includes several species that are obligate commensals of the human upper respiratory tract. Neisseria meningitidis (the meningococcus) is unique among these in that it occasionally invades the previously healthy host to cause life-threatening diseases and is a leading cause of meningitis and septicemia (1). The obligate human pathogen Neisseria gonorrhoeae is associated with human reproductive tract infections and may disseminate to cause, e.g., bacteremia and septic joints (1). The virulence potential of meningococci is multifactorial; however, the expression of a protective capsule, the meningococcal serogroup determinant, is almost always a prerequisite for survival during dissemination and is unique to meningococci among human-associated Neisseria species.
The prevalence of invasive meningococcal disease (IMD) is greatest among infants following the decline of maternal antibody levels (2). Nasopharyngeal carriage of meningococci is thought to be a naturally immunizing process (3), but it is uncommon in infancy and early childhood. It increases with age, peaking in late adolescence or early adulthood when there is also a secondary peak in the prevalence of IMD (4). Nasopharyngeal carriage of nonpathogenic Neisseria spp., of which Neisseria lactamica is the most widely studied species, is also thought to generate a degree of natural immunity against meningococci, owing to cross-protective responses against surface antigens (3). Carriage of N. lactamica, in contrast with that of meningococci, is prevalent in young children, and so it is possible that this plays a pertinent role in priming the immune system against the meningococcus (4). N. lactamica-based vaccine strategies to prevent IMD have thus been proposed and investigated (5, 6).
The capsular polysaccharide of capsular group B meningococci (MenB), the most prevalent cause of IMD in high-income countries (7), is poorly immunogenic in humans (8). MenB vaccine development has therefore focused on relatively heterogeneous subcapsular antigens. Outer membrane vesicle (OMV) preparations, of which porin A (PorA) constitutes the immunodominant antigen, are effective against PorA-homologous strains (9). They offer limited protection, however, especially in infants, against the diverse MenB diseases that dominate IMD in many industrialized nations (7, 9). Recent efforts have focused on broadening the scope of protection afforded by MenB vaccines by using multiple components and/or relatively conserved antigens that elicit broader protection against heterologous strains (10–12). The recently licensed 4CMenB vaccine (formerly rMenB-OMV [Bexsero]; Novartis Vaccines and Diagnostics) is based on four main antigens, including two lipoproteins (factor H-binding protein [fHbp] and neisserial heparin-binding antigen [NHBA]) and two outer membrane proteins (Neisseria adhesin A [NadA] and PorA) (13).
fHbp is presented in 4CMenB as a fusion protein with the protein GNA2091 (the function of which has not been determined) (13, 14). The vaccine contains a variant 1 fHbp subvariant that may afford cross-protection against other variant 1 subvariants, but not those of the remaining two variant groups (variants 2 and 3) (15). A further investigational vaccine, Pfizer's bivalent recombinant (r)fHbp investigational vaccine, comprises one subvariant each from variants 1 and 3 as its sole constituents (10). NHBA is presented in 4CMenB as a fusion protein with the protein GNA1030 (the function of which has not been determined) (13, 14). The potential to elicit relatively broad cross-protective responses has been demonstrated for NHBA (16), which, unlike fHbp, does not fall into discrete immunologically distinct groups. The NadA component belongs to variant group 3 (of five) and may provide protection against subvariants of variant groups 1, 2, and 3 (17–19). The possession of nadA is largely strain-specific among meningococci. Clonal complexes (CCs) CC32, CC11, and CC8 typically possess alleles for nadA variant 1, 2, or 3, CC213 typically possesses variant 5 alleles, and CC269 and CC41/44 are typically devoid of nadA (19, 20). Variant 4 alleles are usually associated with carriage strains (18). The final major 4CMenB component is a PorA P1.4-containing outer membrane vesicle that affords specific protection against PorA-homologous strains in infants (21). Some protection may also be afforded by other antigens that are abundant within the OMV preparation. For example, the meningococcal enterobactin receptor FetA (formerly known as FrpB), a prevalent OMV component (22) and fine-typing antigen (23), has been proposed as a potential subcapsular vaccine candidate in its own right (24) and possesses a surface-exposed variable loop (VR) similar to those of PorA.
The use of subcapsular antigens raises the prospect that 4CMenB may impact other members of the genus Neisseria. Such an effect may be beneficial in the case of N. gonorrhoeae. A recent study investigating the genetic distribution of the 4CMenB antigens among temporally and geographically diverse N. gonorrhoeae isolates indicated the widespread presence of nhba and fhbp alleles and the absence of nadA (25). Frameshifting among significant proportions of fhbp and nhba alleles and doubts over the surface location of fHbp, however, led the authors to speculate that the potential beneficial impact of 4CMenB against N. gonorrhoeae is likely to be limited. Conversely, antibodies raised against the class 4 outer membrane protein (OMP), RmpM, may actually serve to block gonococcal killing (26, 27). Of particular interest, however, is the potential for responses to such vaccines to impact or be impacted by commensal neisseriae in the nasopharynx. For example, the targeting of subcapsular antigens may serve to eliminate nonpathogenic and potentially beneficial neisserial species inhabiting the nasopharynx. This may in turn vacate a niche for pathogens. Conversely, the carriage of these organisms may serve to prime or even boost responses to the vaccine.
With the exception of porA, which in its active form is exclusive to meningococci (28), our current knowledge of the genetic distribution and diversity of 4CMenB antigen genes among nonpathogenic commensal Neisseria is limited by small numbers of isolates of unspecified diversity (15, 17, 29, 30) or, in the case of fhbp, methodologies that have yielded inconclusive or conflicting results. For instance, none of the N. lactamica genomes currently (as of 17 May 2013) in GenBank (GenBank accession no. NC_014752 [31], NZ_CACL01000000, NZ_ACEQ02000000, and AEPI01000000) or PubMLST.org (Neisseria isolate database ids 1771, 1772, 1787, and 1790) (32) contain fhbp alleles, while only one fhbp gene (partial) has been amplified among 30 (out of 31) N. lactamica isolates yielding positive results in either Western or Southern blot experiments (15, 29, 33). In the present study, we aimed to assess the distribution and diversity of fhbp, nhba, and nadA from a recent diverse set of N. lactamica isolates from England, Wales, Northern Ireland, and Ireland. The diversity of the FetA VR, as obtained for fine-typing purposes, was also considered in terms of its potential immunogenic contribution.
MATERIALS AND METHODS
Isolates.
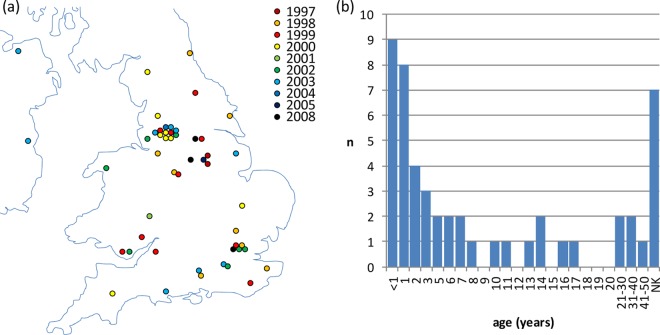
Fifty recently isolated N. lactamica isolates (received by the Health Protection Agency Meningococcal Reference Unit [MRU] between 1997 and 2008) were chosen to represent geographically diverse locations in England, Wales, Northern Ireland, and Ireland, at diverse time points (Fig. 1a). They were classified as N. lactamica on the basis of being Gram-negative oxidase-positive diplococci that were able to produce acid from glucose, maltose, and lactose, but not sucrose. In addition, they did not react with the comprehensive meningococcal serotyping panel employed at the MRU (34). Sources included IMD, suspected IMD, and other patients (n = 30; in the majority of these cases, N. lactamica was obtained via throat swab sample as a “bystander” organism), case contacts (n = 6), carriage or outbreak study participants (n = 7), and unspecified (n = 6). A further isolate was from a patient displaying possible meningitis symptoms (stiff neck and fever) who was also an IMD case contact. The isolates were collected by throat swab (n = 43), blood sample (n = 3), bronchial wash (n = 1), vaginal swab (n = 1), eye swab (n = 1), and unspecified (outbreak investigation, n = 1) (see Table S1 in the supplemental material). The age profile of the sources was typical of that seen in N. lactamica carriage, i.e., the highest prevalence was in infants and young children (Fig. 1b).
Fig 1.
(a) Temporal and geographic distributions of N. lactamica isolates included in the study. The indicated geographic locations are approximate, and the corresponding towns and cities are listed in Table S1 in the supplemental material. (b) Age distribution of individuals from whom N. lactamica study isolates were obtained. NK, not known.
eBURST analysis of N. lactamica population structure.
eBURST v3 (35) was used to investigate the diversity and population structure of N. lactamica. Multilocus sequence type (MLST) profiles for the analysis were collected as follows: (i) available ST profiles were obtained for all N. lactamica entries in the PubMLST isolate database (http://pubmlst.org/neisseria/) and added to an empty database, the “ST bin,” (ii) the entire repertoire of ST profiles was downloaded from the PubMLST database and purged of all STs not attributed to an N. lactamica CC. The remaining STs were added to the ST bin, (iii) the entire repertoire of Neisseria ST profiles was reobtained and subjected to eBURST analysis, in which the minimal number of identical loci for group definition was set to the most stringent level of six. The resulting list of groups was then purged of all groups not containing an N. lactamica ST and of all singletons not pertaining to an N. lactamica isolate. The remaining STs were added to the ST bin, and (iv) the ST bin was purged of duplicates.
The final list of STs was subjected to eBURST analysis, in which the minimal number of identical loci for group definition was set to the least stringent level of zero to provide a population snapshot for the known diversity of N. lactamica.
Molecular analyses.
Multilocus sequence typing and characterization of FetA were adapted from the PubMLST website and previous publications (36, 37). Where appropriate, the absence of fetA was investigated by PCR using the primers fetAnegf2 [5′-(A/G)CCGTCTTCAACGGTAATCAC-3′] and fetAnegr2 (5′-CATACCCAAATCGTAAACGGCGA-3′), which targeted the upstream (NLA19290, groEL) and downstream (NLA19320, fetB) genes, respectively. The latter PCRs were performed with a final MgCl2 concentration of 1.5 mM, using 30 cycles of 96°C for 30 s, 61°C for 30 s, and 72°C for 120 s. The resulting PCR products underwent sequence analyses using the PCR primers and the internally directed primers fetAnegs1 (5′-GGTTTGGCCGCTGTATTTG-3′) and fetAnegs4 (5′-AGCCGCAGACAGTACAAATAG-3′).
The presence and, where applicable, the diversity of nhba and nadA were investigated using PCR and sequence analysis as previously described (19, 38). The corresponding meningococcal fhbp locus, i.e., the region between nla18140 (putative fructose-1,6-bisphosphate aldolase; nmb1869 in N. meningitidis isolate MC58) and nla18160 (putative peptidase; nmb1871 in MC58), was amplified using primers targeting the upstream and downstream flanking genes, respectively, in accordance with Lucidarme et al. (39). The genome-wide absence of fhbp was confirmed by two internally directed variant-specific PCRs using the common forward primer variant1and2F (5′-GCCCTGATTCTGACCGCCTG-3′) and the variant-specific reverse primer variant1R2 (variant 1; 5′-GAACCGCTGATGACGGCATGG-3′) or variant2R2 (variant 2; 5′-TATCTTCACGGTTGCCGAGCCG-3′). These PCRs were performed concurrently (in separate tubes), each with a final MgCl2 concentration of 1.5 mM, incorporating 30 cycles of 96°C for 30 s, 70°C for 30 s, and 72°C for 40 s.
The presence or absence of fhbp in published N. lactamica genomes was determined by BLAST searches using alleles from each of the three variant groups (alleles 1, 16, and 28).
Phylogenetic analyses.
Antigenic subvariants were translated in silico and aligned using BioEdit (version 6.0.8.0) (40). Phylogenetic network analysis was performed using SplitsTree4 (version 4.12.8) (41).
GenBank accession numbers.
nhba alleles were deposited in GenBank under the accession no. KC480197 to KC480246. Alleles corresponding to nla18150 (31) were deposited in GenBank under the accession no. KC495573 to KC495616.
RESULTS
Figure 2 provides a population snapshot of the known diversity of N. lactamica, across which the study isolates were well distributed. Half of the study isolates belonged to STs that were assigned to four (out of six) designated N. lactamica CCs, including CC613 (n = 4), CC624 (n = 4), CC640 (n = 9), and CC1494 (n = 8). eBURST analysis revealed that several unassigned STs among the study isolates were single-locus variants (SLVs) of assigned STs (or SLVs thereof). These were regarded as putative members of the respective CCs (designated by a capital “P,” e.g., CC613P) and included CC613P (n = 4; 2× ST-4406, 1× ST-609, and 1× ST-9872) and CC624P (n = 1; ST-9873). Four of the study isolates (3× ST-631 and 1× ST-9877) were SLVs within a small cluster of unassigned STs (n = 4) centered on ST-631 (cluster 631). Two study isolates (ST-4192 and ST-9866) were SLVs within a cluster of unassigned STs (n = 3) centered on ST-4192 (cluster 4192), and a further four study isolates (all ST-9864) were SLVs within a cluster of STs (n = 6) centered on ST-4194 (cluster 4194). The remaining 10 isolates belonged to unassigned singleton STs. Owing to the apparent diversity of N. lactamica and the relatively small number of N. lactamica STs in the PubMLST database, the designated clonal complexes and clusters should be regarded as preliminary.
Fig 2.
Population snapshot of the known diversity of N. lactamica. Numbers indicate multilocus sequence types (STs). Lines connect single-locus variants with respect to the ST loci and are arranged to centralize the predicted group or subgroup founder. STs represented by isolates that were included in the present study are displayed in bold red type. The shading highlights the N. lactamica clonal complexes as designated by the PubMLST database as of 11 January 2013.
fhbp.
Intragenically directed PCRs indicated the genome-wide absence of fhbp alleles among all of the N. lactamica isolates. In the corresponding meningococcal fhbp locus, they each were found to possess nla18150 alleles for an unrelated putative opacity protein (31), and this gene was orientated in the opposite direction from that of fhbp. The nla18150 alleles possessed between 2 and ≥22 octanucleotide GCGTTCCT repeats within the open reading frame (the tract length of six isolates was indeterminate, owing to strand slippage during PCR, heterogeneous plate population, or both), which was shortly preceded by a homopolymeric A repeat tract of between four and six repeats. Thus, the predicted phase-variable status of the corresponding alleles was determined by the combination of the numbers of A and GCGTTCCT repeats. Among the 44 isolates with known tract lengths, 30 and 14 were in the “off” and “on” configurations, respectively. One isolate (N. lactamica M02 241586) had an A-to-T substitution (corresponding to residue 698 in nla18150 of the N. lactamica isolate 020-06; GenBank accession no. NC_014752 [31]), creating a premature stop codon site 42 bp upstream from the prototypical stop codon.
BLAST searches of alleles representing the three variant groups among the published N. lactamica genomes failed to return any significant hits.
nadA.
Intragenically directed PCRs were indicative of the genome-wide absence of nadA alleles among all of the study isolates. PCR amplification of the corresponding meningococcal nadA locus, i.e., between nla19360 (encoding a putative ABC transporter ATP-binding subunit) and nla19370 (encoding a nitrogen regulatory protein, P-II 1), yielded typical nadA-negative products of approximately 400 bp (17) in 38 of the isolates. Sequence analysis of one of these products (for the isolate N. lactamica M08 240567) confirmed the presence of a typical nadA-negative locus (18). The remaining 12 isolates (8/8 CC613[P] isolates and 4/11 singletons) yielded larger products of approximately 800 to 900 bp. Sequence analyses of these products indicated the presence of an insertion of 542 bp as previously described (18).
nhba.
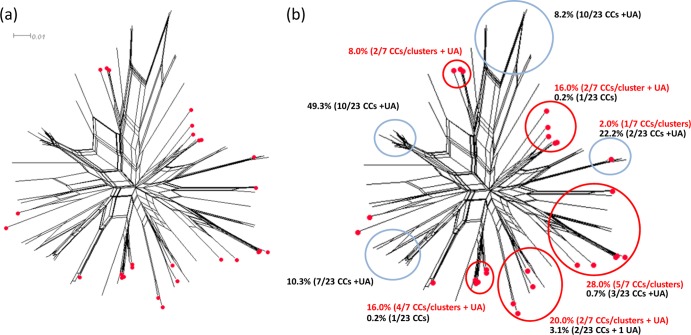
Translated NHBA peptide subvariants are named according to the NHBA database at PubMLST.org. All of the isolates were found to possess alleles for full-length NHBA peptides. Twenty-eight peptide subvariants were represented in total. The pairwise amino acid identities ranged from 67.5% to 99.7%. None of the isolates possessed alleles for the 4CMenB subvariant (peptide 2). The peptide subvariants were compared with those of all English, Welsh, and Northern Irish invasive meningococcal isolates from the epidemiological year of July 2007 to June 2008, inclusive (2007 and 2008) (19, 42). The pairwise amino acid sequence identities among the meningococcal isolates were comparable, ranging from 66.4% to 99.7% across 59 subvariants. Among both isolate panels combined, the pairwise amino acid sequence identities ranged from 65.3% to 99.7%. The pairwise amino acid sequence identities between the N. lactamica subvariants and the vaccine subvariant ranged from 71.4% to 98.3% (versus 66.9% to 99.7% among the meningococci). A single peptide subvariant (peptide 34) was shared between the two sets of isolates. This subvariant was relatively common among the N. lactamica isolates (14% [7/50]) compared with the meningococcal isolates (0.2% [1/613]). Using the SplitsTree analysis (Fig. 3; see also Fig. S1 in the supplemental material), the corresponding clade also contained peptides 47, 188, and 120 (pertaining to 1.1% [n = 7], 0.32% [n = 2], and 1.5% [n = 9] of the meningococci, respectively) and a further “N. lactamica peptide” (peptide 497; 2% [n = 1] of N. lactamica isolates). It is interesting to note that peptide 120 constitutes a major meningococcal CC32 NHBA subvariant, and so the corresponding clade contains a major subvariant for CCs from each of the two species. The remainder of the SplitsTree analysis was similar, interspersing N. lactamica and N. meningitidis subvariants and providing evidence of interspecies recombination with respect to nhba. In terms of numbers, however, it was noteworthy that certain clades were largely dominated by one of the two species (N. meningitidis or N. lactamica). For example, 49.4% of the meningococci, spanning 10/23 CCs, fell into a clade comprising 14 (out of 59) meningococcal peptide subvariants, none of which were found among the N. lactamica isolates. Similarly, approximately 88% of the N. lactamica isolates fell into five distinct clades that included 22/27 N. lactamica peptide subvariants and the mutual subvariant, peptide 34. The corresponding clades accounted for only 4.1% (n = 25) of the meningococci (across 12 peptide subvariants) (Fig. 3).
Fig 3.
Phylogenetic network analysis of translated nhba subvariants among N. lactamica and invasive meningococci collected in England, Wales, Northern Ireland, and Ireland. (a) Phylogenetic network of translated nhba subvariants from meningococci and N. lactamica, in which peptide subvariants identified among N. lactamica isolates are highlighted with red circles. (b) Overall distribution of meningococci and N. lactamica isolates among the most-populous clades. Blue and red circles highlight clades dominated by meningococci and N. lactamica, respectively. The text indicates the proportion of isolates and the number of clonal complexes represented by each highlighted clade. Red text refers to N. lactamica and black text refers to meningococci. NHBA peptide identifications (IDs) (in accordance with PubMLST.org) can be viewed in Fig. S1 in the supplemental material. UA, unassigned ST.
Despite these small numbers for the respective CCs, a possible degree of nhba-CC association was apparent; e.g., 4/8 of the CC613(P) isolates were found to possess alleles for peptide 313, 4/8 CC1494 isolates possessed alleles for peptide 485, 7/9 CC640 isolates possessed alleles for peptide 34, and all four cluster 4194 isolates possessed alleles for peptide 92. Peptide 313 was represented by >1 CC/cluster (CC613[P] and cluster 4192), while alleles for peptides 272, 88, and 91 were each found among both singletons and CCs or clusters (Fig. 4).
Fig 4.
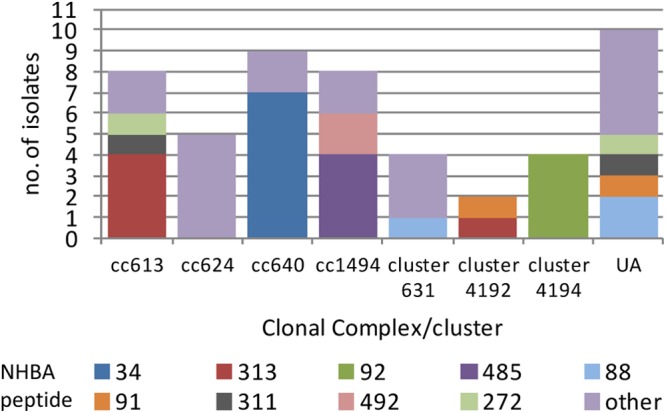
Clonal complex distribution of nhba subvariants among N. lactamica isolates collected in England, Wales, Northern Ireland, and Ireland. UA, unassigned STs.
FetA.
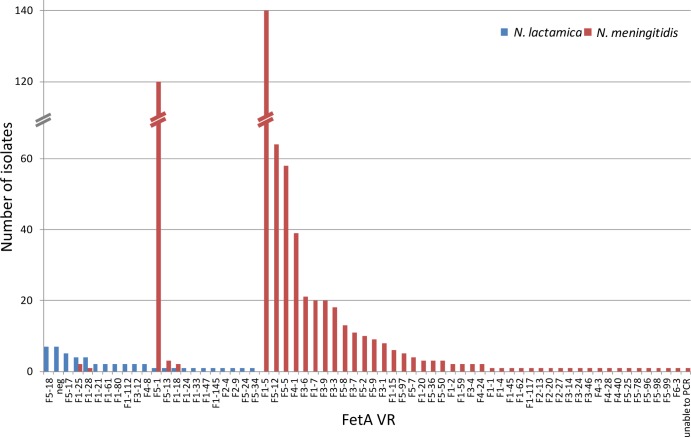
All but seven of the isolates yielded FetA VR data, representing 21 FetA VRs in total. Clusters 4192 and 4194 were each homogeneous with respect to FetA VRs, representing F1-21 and F1-28, respectively. Similarly, cluster 631 was predominantly (3/4) F5-17 and CC640 was predominantly (5/9) F5-18. However, these associations were based on small numbers, and the remaining CCs yielding FetA VRs (CC613[P] and CC1494) were relatively heterogeneous for FetA VRs, as were the isolates belonging to singleton STs (Fig. 5). Six of the 10 isolates belonging to singleton STs shared their FetA VRs with isolates belonging to CCs or clusters. Sequence analyses confirmed that the fetA allele had been deleted from the remaining seven isolates, which included 5/5 CC624(P), 1/9 CC640, and 1/11 singleton-ST isolates (data not shown). The meningococcal isolates from 2007 and 2008 represented 49 VRs, of which five were found from the N. lactamica isolates. Four of these collectively accounted for just 1.3% of the meningococci. The remaining one (F5-1) accounted for 19.6% of the meningococci; however, this only occurred in a single N. lactamica isolate (Fig. 6).
Fig 5.
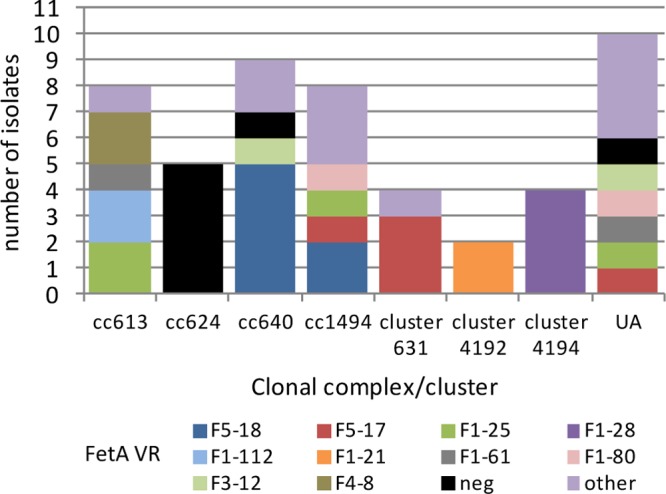
Clonal complex distribution of FetA VRs among N. lactamica isolates collected in England, Wales, Northern Ireland, and Ireland. Other, VR occurred only once among the entire isolate panel; UA, unassigned STs; neg, fetA negative.
Fig 6.
Distribution of FetA VRs among the 43 fetA+ N. lactamica study isolates and all (n = 613) invasive meningococcal isolates received by the Health Protection Agency Meningococcal Reference Unit in 2007 and 2008. neg, fetA negative.
DISCUSSION
The multicomponent vaccine 4CMenB has recently been granted European licensure and shows promise as a broadly protective vaccine against MenB. The vaccine targets subcapsular antigens that may be present in commensal Neisseria organisms. This raises the possibility that vaccine responses and the carriage of these organisms may influence one another. Such an effect may be positive in terms of vaccine impact, in that carriage of commensal Neisseria organisms may prime or boost vaccine responses. It might also, however, be negative in that carriage may be eliminated or prevented, thereby abrogating potentially cross-protective natural immunization against invasive meningococcal disease and vacating a niche for potential pathogens. Previous studies have investigated 4CMenB antigens among limited numbers of N. lactamica isolates of unspecified diversity, e.g., regarding ST, and/or have yielded ambiguous results. GenBank currently contains only four N. lactamica genomes. The present study aimed to genotypically assess the presence and diversity of the 4CMenB antigens among a genetically and geographically diverse recent panel of N. lactamica isolates from England, Wales, Northern Ireland, and Ireland in order to assess the potential impact of 4CMenB.
In common with the published N. lactamica genomes (31, 32) and previous studies involving small numbers of isolates (17, 18) or less-definitive (Southern blot) detection methods (33), none of the isolates were found to possess nadA alleles. Similarly, in common with the published genomes, the isolates were all devoid of fhbp alleles. Interestingly, a previous study identified a partial (in which internally directed primers were used for amplification) fhbp sequence in the sole N. lactamica isolate tested, and this was supported by Western blotting of whole-cell lysates using polyclonal antiserum (29). A further study also detected a putative fHbp band by Western blotting of the sole N. lactamica isolate tested (15). Failure to amplify the gene from the same isolate, however, raises possible doubt over the true fhbp status of this isolate. Litt et al. (33) detected putative fhbp bands in 28/29 N. lactamica isolates (at least 27 of which were collected in the United Kingdom between 1997 and 2000) by Southern blotting. The latter report was inconsistent with the findings of the present study, which suggest that such isolates are likely to be unusual among contemporaneous N. lactamica isolates collected in England, Wales, Northern Ireland, and Ireland. The putative fhbp+ isolates identified by Fletcher et al. (29) and Masignani et al. (15) may have represented isolated recombinants or specific strains that have adopted fhbp but that were not included in the present study. The latter was unlikely to be true of the 28 isolates studied by Litt et al. (33), however, since these were diverse in terms of ST and CC. In addition, four and six of the isolates matched those of the present study in terms of ST and CC, respectively. While it is possible that the internal fhbp PCRs of the present study missed divergent alleles, this is unlikely, since the primers target highly conserved regions in each variant group (42). Furthermore, the published N. lactamica genomes, including that of isolate 020-06 (31), and those of isolates 030-24, 014-24, 09002S1, and 8206 (32) (all of which were included in the Litt study), do not contain fhbp, according to BLAST searches. To resolve this discrepancy, a further nine (unpublished) whole-genome sequences of isolates used by Litt et al. (33) (those of N. lactamica isolates 028-12, 039-03, 012-12, 224, 4116, 017-02, 004-12, 016-24, and 049-12) underwent BLAST searches using alleles representing each of the three fhbp variant groups (alleles 1, 22, and 65). No significant matches were returned (data not shown), further indicating that the isolates were indeed devoid of fhbp and that the Southern blots of the previous study may have yielded false-positive results, probably due to unrelated, though partially homologous, DNA elsewhere in the genome.
Sequence analyses of the respective N. lactamica fhbp loci indicated that all of the isolates possessed alleles for a phase-variable putative opacity protein, NLA18150 (31), the function of which is currently unknown. This too was consistent with the published genomes. Interestingly, a previous study identified a putative meningococcal CC (centered on ST-286) of which approximately half of the isolates tested possessed nla18150 alleles that had putatively replaced fhbp in an ancestral horizontal transfer event (39). It is possible that the fhbp+ N. lactamica isolate described by Fletcher et al. (29) acquired the gene in a similar fashion but in the opposite direction.
Also consistent with the published genomes and a previous report of a single N. lactamica isolate (30) is the fact that each of the isolates tested possessed intact nhba alleles. A comparison with all nhba subvariants identified among all invasive meningococcal isolates from 2007 to 2008, including the 4CMenB subvariant (peptide 2), indicated that major subvariants among the two species are largely nonoverlapping. The single subvariant that was found among both isolate panels (peptide 34) was relatively rare among the meningococci, occurring in just 1/613 isolates for the whole year. The isolate in question belongs to a rare CC (CC35; n = 8 for 2007 and 2008); however, the predominant subvariant for this CC was peptide 21 (5/8 isolates). Therefore, it seems plausible that peptide 34 is characteristically an N. lactamica subvariant and that the meningococcal isolate bearing it was a transient recombinant. Such transients are common with respect to immunodominant antigens, such as PorA, owing to frequent horizontal transfer between meningococci (43), and between meningococci and related species (44). The majority of isolates within a given lineage, however, tend to conform to characteristic, temporally enduring, and nonoverlapping antigenic repertoires. It has been proposed that this occurs because the sharing of dominant antigens confers disadvantageous immunological pressures on the respective strains (45). Since they each occupy the same ecological niche, it is possible that the same should apply between the two species considered herein, and it is consistent with the idea that N. lactamica carriage has the potential to afford some protection against IMD. Despite this apparent nonoverlapping distribution of nhba subvariants, isolates of the two species appeared to be well dispersed across the collective nhba diversity observed. Nonetheless, large proportions of each species tended to dominate particular clades, and so the remainder was likely, for the most part, to constitute the aforementioned transient recombinants along with rare lineages.
In the present study, the complete absence of the fetA gene has been confirmed as a characteristic trait of United Kingdom CC624 and occasionally other N. lactamica isolates. This has previously been proposed following a large-scale study of N. lactamica FetA VRs (44) and has been demonstrated among small but diverse proportions of meningococcal isolates elsewhere (46, 47). Consistent with the observations of Bennett et al. (44), several VRs occurred in both the N. lactamica isolates and a comprehensive panel of meningococci received by the MRU in the latter part of the N. lactamica sample period. This supports the concept of a common gene pool for fetA (44). Only one of these VRs occurred in a relatively large proportion of meningococcal isolates, however, and this was found in just a single N. lactamica isolate. Therefore, it is possible that the N. lactamica isolate was a transient recombinant and that, by and large, the fetA VRs are nonoverlapping between the two species.
No trends were noted among the three invasive isolates obtained from blood in terms of lineage or any of the targets investigated. Similarly, none of these possessed allelic variants that are characteristic of meningococci.
Taken together, these data suggest that vaccine responses against three of the four main 4CMenB antigens (PorA, fHbp, and NadA) are unlikely to interact with N. lactamica that is carried in the nasopharynx. The response against NHBA does, however, have this potential given (i) the possession of nhba in diverse N. lactamica lineages, (ii) the similarities in nhba between N. lactamica and N. meningitidis, and (iii) the reported cross-protection of NHBA (14). This may manifest in beneficial priming or boosting of the immune response to the vaccine. It may also, however, serve to eliminate N. lactamica carriage and the potential benefits that this provides (cross-protective immunity to IMD and the occupation of a niche that may otherwise accommodate potential pathogens). The ability of the vaccine to eliminate meningococcal carriage (or the acquisition thereof) is not currently known and is the subject of an ongoing investigation (see ClinicalTrials.gov identifier NCT01214850). In addition, sufficient surface expression of NHBA by N. lactamica during carriage is a prerequisite to any such interactions and is a topic for further investigation. Furthermore, despite the observed similarities among N. lactamica and meningococcal nhba alleles, the full extent of cross-protection among NHBA subvariants is still relatively poorly understood.
In addition to NHBA, this and other studies (44) show that FetA is potentially expressed by the majority of N. lactamica CCs. As with nhba, however, the FetA VR repertoires of the two species appear to be mainly nonoverlapping. In addition, none of the isolates were found to possess the vaccine variant F1-5 (48), while a previous study of functional and specific antibody responses to the PorA P1.4 OMV failed to correlate elicited FetA IgG levels with serum bactericidal antibody (SBA) activity against the homologous vaccine strain (49). Therefore, immune responses to any meningococcal FetA component, against which immunogenicity tends to be variant specific (36), are unlikely to influence N. lactamica carriage and vice versa. Nonetheless, there are a plethora of other antigens in the OMV component, not to mention GNA1030 and GNA2091 (presented in fusion with NHBA and fHbp, respectively), which may have an effect, especially given the acapsulate status of N. lactamica. The presence of gna1030 and gna2091 in N. lactamica is not currently known.
The potential strain coverage of 4CMenB among meningococci is assessed using the Meningococcal Antigen Typing System (MATS), a qualitative and quantitative enzyme-linked immunosorbent assay (ELISA) that assigns a relative potency for each of fHbp, NHBA, and NadA (50). This is then compared to a defined threshold value to determine the likelihood that the isolate would be killed by immune sera. MATS does not, however, account for minor antigens in the vaccine, nor is it validated for use with N. lactamica or predicting the clearance or blocking of carriage. Thus, given the overall complexity of the issue, in the event that 4CMenB is adopted into infant vaccination schedules, it may ultimately be necessary to include postimplementation carriage studies to understand the effect of the vaccine on N. lactamica and other nasopharyngeal flora in order to fully understand both the beneficial and potentially detrimental effects of the vaccine. Such effects may be reduced in older age groups, in whom N. lactamica carriage is relatively rare (4).
Finally, it should be noted that the immunity raised against the Pfizer rfHbp vaccine containing only fHbp (51) would not be expected to interact with N. lactamica carriage. Both vaccines may, however, affect other potentially beneficial neisserial commensals that are yet to be investigated in meaningful numbers for the presence of these antigens. Studies of limited numbers of isolates and a small number of available genomes and partial genomes indicate the possible presence of fhbp among Neisseria cinerea (15) and Neisseria polysaccharea (GenBank accession no. ADBE01000014 and AEPH01000008), the possible presence of nadA among some N. cinerea and Neisseria sicca isolates (33), and the absence of nhba in N. cinerea (30).
Supplementary Material
ACKNOWLEDGMENTS
We thank Daniel Hungerford and Matt Bull for their assistance with production of the geographic figure.
Footnotes
Published ahead of print 26 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00090-13.
REFERENCES
- 1.Fallon RJ, Slack RCB. 2000. Neisseria and Moraxella (branhamella), p 243–251 In Greenwood D, Slack, RCB, Peutherer, JF (ed), Medical microbiology, 15th ed. Churchill Livingstone, London, United Kingdom [Google Scholar]
- 2.Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartwright KA, Stuart JM, Jones DM, Noah ND. 1987. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol. Infect. 99:591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiss JM, Yamasaki R, Estabrook M, Kim JJ. 1991. Meningococcal molecular mimicry and the search for an ideal vaccine. Trans. R. Soc. Trop. Med. Hyg. 85(Suppl 1):32–36. 10.1016/0035-9203(91)90338-Y [DOI] [PubMed] [Google Scholar]
- 6.Gorringe AR, Taylor S, Brookes C, Matheson M, Finney M, Kerr M, Hudson M, Findlow J, Borrow R, Andrews N, Kafatos G, Evans CM, Read RC. 2009. Phase I safety and immunogenicity study of a candidate meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Clin. Vaccine Immunol. 16:1113–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison LH, Trotter CL, Ramsay ME. 2009. Global epidemiology of meningococcal disease. Vaccine 27(Suppl 2):B51–B63. 10.1016/j.vaccine.2009.04.063 [DOI] [PubMed] [Google Scholar]
- 8.Wyle FA, Artenstein MS, Brandt BL, Tramont EC, Kasper DL, Altieri PL, Berman SL, Lowenthal JP. 1972. Immunologic response of man to group B meningococcal polysaccharide vaccines. J. Infect. Dis. 126:514–521 [DOI] [PubMed] [Google Scholar]
- 9.Tappero JW, Lagos R, Ballesteros AM, Plikaytis B, Williams D, Dykes J, Gheesling LL, Carlone GM, Høiby EA, Holst J, Nøkleby H, Rosenqvist E, Sierra G, Campa C, Sotolongo F, Vega J, Garcia J, Herrera P, Poolman JT, Perkins BA. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520–1527 [DOI] [PubMed] [Google Scholar]
- 10.Jiang HQ, Hoiseth SK, Harris SL, McNeil LK, Zhu D, Tan C, Scott AA, Alexander K, Mason K, Miller L, DaSilva I, Mack M, Zhao XJ, Pride MW, Andrew L, Murphy E, Hagen M, French R, Arora A, Jones TR, Jansen KU, Zlotnick GW, Anderson AS. 2010. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 28:6086–6093 [DOI] [PubMed] [Google Scholar]
- 11.Pinto VB, Moran EE, Cruz F, Wang XM, Fridman A, Zollinger WD, Przysiecki CT, Burden R. 2011. An experimental outer membrane vesicle vaccine from N. meningitidis serogroup B strains that induces serum bactericidal activity to multiple serogroups. Vaccine 29:7752–7758 [DOI] [PubMed] [Google Scholar]
- 12.Kaaijk P, van Straaten I, van de Waterbeemd B, Boot EP, Levels LM, van Dijken HH, van den Dobbelsteen GP. 2013. Preclinical safety and immunogenicity evaluation of a nonavalent PorA native outer membrane vesicle vaccine against serogroup B meningococcal disease. Vaccine 31:1065–1071 [DOI] [PubMed] [Google Scholar]
- 13.Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. 2012. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 30(Suppl 2):B87–B97. 10.1016/j.vaccine.2012.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giuliani MM, Adu-Bobie J, Comanducci M, Aricó B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, Cartocci E, Ciucchi L, Di Marcello F, Ferlicca F, Galli B, Luzzi E, Masignani V, Serruto D, Veggi D, Contorni M, Morandi M, Bartalesi A, Cinotti V, Mannucci D, Titta F, Ovidi E, Welsch JA, Granoff D, Rappuoli R, Pizza M. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. U. S. A. 103:10834–10839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, Serruto D, Litt D, Kroll S, Welsch JA, Granoff DM, Rappuoli R, Pizza M. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giuliani MM, Biolchi A, Serruto D, Ferlicca F, Vienken K, Oster P, Rappuoli R, Pizza M, Donnelly J. 2010. Measuring antigen-specific bactericidal responses to a multicomponent vaccine against serogroup B meningococcus. Vaccine 28:5023–5030 [DOI] [PubMed] [Google Scholar]
- 17.Comanducci M, Bambini S, Brunelli B, Adu-Bobie J, Aricó B, Capecchi B, Giuliani MM, Masignani V, Santini L, Savino S, Granoff DM, Caugant DA, Pizza M, Rappuoli R, Mora M. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 195:1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comanducci M, Bambini S, Caugant DA, Mora M, Brunelli B, Capecchi B, Ciucchi L, Rappuoli R, Pizza M. 2004. NadA diversity and carriage in Neisseria meningitidis. Infect. Immun. 72:4217–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucidarme J, Comanducci M, Findlow J, Gray SJ, Kaczmarski EB, Guiver M, Vallely PJ, Oster P, Pizza M, Bambini S, Muzzi A, Borrow R. 2010. Characterization of fHbp, nhba (gna2132), nadA, porA, and sequence type in group B meningococcal case isolates collected in England and Wales during January 2008 and potential coverage of an investigational group B meningococcal vaccine. Clin. Vaccine Immunol. 17:919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bambini S, Muzzi A, Olcen P, Rappuoli R, Pizza M, Comanducci M. 2009. Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus. Vaccine 27:2794–2803 [DOI] [PubMed] [Google Scholar]
- 21.Holst J, Martin D, Arnold R, Huergo CC, Oster P, O'Hallahan J, Rosenqvist E. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27(Suppl 2):B3–B12. 10.1016/j.vaccine.2009.04.071 [DOI] [PubMed] [Google Scholar]
- 22.Vipond C, Wheeler JX, Jones C, Feavers IM, Suker J. 2005. Characterization of the protein content of a meningococcal outer membrane vesicle vaccine by polyacrylamide gel electrophoresis and mass spectrometry. Hum. Vaccin. 1:80–84 [DOI] [PubMed] [Google Scholar]
- 23.Jolley KA, Brehony C, Maiden MC. 2007. Molecular typing of meningococci: recommendations for target choice and nomenclature. FEMS Microbiol. Rev. 31:89–96 [DOI] [PubMed] [Google Scholar]
- 24.Urwin R, Russell JE, Thompson EA, Holmes EC, Feavers IM, Maiden MC. 2004. Distribution of surface protein variants among hyperinvasive meningococci: implications for vaccine design. Infect. Immun. 72:5955–5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadad R, Jacobsson S, Pizza M, Rappuoli R, Fredlund H, Olcén P, Unemo M. 2012. Novel meningococcal 4CMenB vaccine antigens - prevalence and polymorphisms of the encoding genes in Neisseria gonorrhoeae. APMIS 120:750–760 [DOI] [PubMed] [Google Scholar]
- 26.Klugman KP, Gotschlich EC, Blake MS. 1989. Sequence of the structural gene (rmpM) for the class 4 outer membrane protein of Neisseria meningitidis, homology of the protein to gonococcal protein III and Escherichia coli OmpA, and construction of meningococcal strains that lack class 4 protein. Infect. Immun. 57:2066–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice PA, Vayo HE, Tam MR, Blake MS. 1986. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J. Exp. Med. 164:1735–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derrick JP, Urwin R, Suker J, Feavers IM, Maiden MC. 1999. Structural and evolutionary inference from molecular variation in Neisseria porins. Infect. Immun. 67:2406–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, Ooi P, Smith RP, Weise P, Wetherell M, Xie X, Zagursky R, Zhang Y, Zlotnick GW. 2004. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect. Immun. 72:2088–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pizza M, Scarlato V, Masignani V, Giuliani MM, Aricò B, Comanducci M, Jennings GT, Baldi L, Bartolini E, Capecchi B, Galeotti CL, Luzzi E, Manetti R, Marchetti E, Mora M, Nuti S, Ratti G, Santini L, Savino S, Scarselli M, Storni E, Zuo P, Broeker M, Hundt E, Knapp B, Blair E, Mason T, Tettelin H, Hood DW, Jeffries AC, Saunders NJ, Granoff DM, Venter JC, Moxon ER, Grandi G, Rappuoli R. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816–1820 [DOI] [PubMed] [Google Scholar]
- 31.Bennett JS, Bentley SD, Vernikos GS, Quail MA, Cherevach I, White B, Parkhill J, Maiden MC. 2010. Independent evolution of the core and accessory gene sets in the genus Neisseria: insights gained from the genome of Neisseria lactamica isolate 020-06. BMC Genomics 11:652. 10.1186/1471-2164-11-652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett JS, Jolley KA, Earle SG, Corton C, Bentley SD, Parkhill J, Maiden MC. 2012. A genomic approach to bacterial taxonomy: an examination and proposed reclassification of species within the genus Neisseria. Microbiology 158:1570–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litt DJ, Savino S, Beddek A, Comanducci M, Sandiford C, Stevens J, Levin M, Ison C, Pizza M, Rappuoli R, Kroll JS. 2004. Putative vaccine antigens from Neisseria meningitidis recognized by serum antibodies of young children convalescing after meningococcal disease. J. Infect. Dis. 190:1488–1497 [DOI] [PubMed] [Google Scholar]
- 34.Gray SJ, Trotter CL, Ramsay ME, Guiver M, Fox AJ, Borrow R, Mallard RH, Kaczmarski EB. 2006. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J. Med. Microbiol. 55:887–896 [DOI] [PubMed] [Google Scholar]
- 35.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson EA, Feavers IM, Maiden MC. 2003. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology 149:1849–1858 [DOI] [PubMed] [Google Scholar]
- 37.Bennett JS, Griffiths DT, McCarthy ND, Sleeman KL, Jolley KA, Crook DW, Maiden MC. 2005. Genetic diversity and carriage dynamics of Neisseria lactamica in infants. Infect. Immun. 73:2424–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucidarme J, Comanducci M, Findlow J, Gray SJ, Kaczmarski EB, Guiver M, Kugelberg E, Vallely PJ, Oster P, Pizza M, Bambini S, Muzzi A, Tang CM, Borrow R. 2009. Characterization of fHbp, nhba (gna2132), nadA, porA, sequence type (ST), and genomic presence of IS1301 in group B meningococcal ST269 clonal complex isolates from England and Wales. J. Clin. Microbiol. 47:3577–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucidarme J, Tan L, Exley RM, Findlow J, Borrow R, Tang CM. 2011. Characterization of Neisseria meningitidis isolates that do not express the virulence factor and vaccine antigen factor H binding protein. Clin. Vaccine Immunol. 18:1002–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]
- 41.Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23:254–267 [DOI] [PubMed] [Google Scholar]
- 42.Lucidarme J. 2012. Potential coverage of an investigational, multicomponent, meningococcal vaccine with a focus on the ST-269 clonal complex. Ph.D. thesis. University of Manchester, Manchester, United Kingdom [Google Scholar]
- 43.Buckee CO, Gupta S, Kriz P, Maiden MC, Jolley KA. 2010. Long-term evolution of antigen repertoires among carried meningococci. Proc. Biol. Sci. 277:1635–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett JS, Thompson EA, Kriz P, Jolley KA, Maiden MC. 2009. A common gene pool for the Neisseria FetA antigen. Int. J. Med. Microbiol. 299:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta S, Maiden MC, Feavers IM, Nee S, May RM, Anderson RM. 1996. The maintenance of strain structure in populations of recombining infectious agents. Nat. Med. 2:437–442 [DOI] [PubMed] [Google Scholar]
- 46.Claus H, Elias J, Meinhardt C, Frosch M, Vogel U. 2007. Deletion of the meningococcal fetA gene used for antigen sequence typing of invasive and commensal isolates from Germany: frequencies and mechanisms. J. Clin. Microbiol. 45:2960–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsh JW, O'Leary MM, Shutt KA, Harrison LH. 2007. Deletion of fetA gene sequences in serogroup B and C Neisseria meningitidis isolates. J. Clin. Microbiol. 45:1333–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elias J, Schouls LM, van de Pol I, Keijzers WC, Martin DR, Glennie A, Oster P, Frosch M, Vogel U, van der Ende A. 2010. Vaccine preventability of meningococcal clone, Greater Aachen Region, Germany. Emerg. Infect. Dis. 16:465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wedege E, Bolstad K, Aase A, Herstad TK, McCallum L, Rosenqvist E, Oster P, Martin D. 2007. Functional and specific antibody responses in adult volunteers in New Zealand who were given one of two different meningococcal serogroup B outer membrane vesicle vaccines. Clin. Vaccine Immunol. 14:830–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, Moxon ER, Stella M, Comanducci M, Bambini S, Muzzi A, Andrews W, Chen J, Santos G, Santini L, Boucher P, Serruto D, Pizza M, Rappuoli R, Giuliani MM. 2010. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc. Natl. Acad. Sci. U. S. A. 107:19490–19495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marshall HS, Richmond PC, Nissen MD, Jiang Q, Anderson AS, Jansen KU, Reynolds G, Ziegler JB, Harris SL, Jones TR, Perez JL. 2012. Safety and immunogenicity of a meningococcal B bivalent rLP2086 vaccine in healthy toddlers aged 18–36 months: a phase 1 randomized-controlled clinical trial. Pediatr. Infect. Dis. J. 31:1061–1068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.