Abstract
This study investigated long-term cellular and humoral immunity against pertussis after booster vaccination of 4-year-old children who had been vaccinated at 2, 3, 4, and 11 months of age with either whole-cell pertussis (wP) or acellular pertussis (aP) vaccine. Immune responses were evaluated until 2 years after the preschool booster aP vaccination. In a cross-sectional study (registered trial no. ISRCTN65428640), blood samples were taken from wP- and aP-primed children prebooster and 1 month and 2 years postbooster. Pertussis vaccine antigen-specific IgG levels, antibody avidities, and IgG subclasses, as well as T-cell cytokine levels, were measured by fluorescent bead-based multiplex immunoassays. The numbers of pertussis-specific memory B cells and gamma interferon (IFN-γ)-producing T cells were quantified by enzyme-linked immunosorbent spot assays. Even 2 years after booster vaccination, memory B cells were still present and higher levels of pertussis-specific antibodies than prebooster were found in aP-primed children and, to a lesser degree, also in wP-primed children. The antibodies consisted mainly of the IgG1 subclass but also showed an increased IgG4 portion, primarily in the aP-primed children. The antibody avidity indices for pertussis toxin and pertactin in aP-primed children were already high prebooster and remained stable at 2 years, whereas those in wP-primed children increased. All measured prebooster T-cell responses in aP-primed children were already high and remained at similar levels or even decreased during the 2 years after booster vaccination, whereas those in wP-primed children increased. Since the Dutch wP vaccine has been replaced by aP vaccines, the induction of B-cell and T-cell memory immune responses has been enhanced, but antibody levels still wane after five aP vaccinations. Based on these long-term immune responses, the Dutch pertussis vaccination schedule can be optimized, and we discuss here several options.
INTRODUCTION
Despite high rates of vaccination coverage in young children since the 1940s and 1950s, whooping cough is reemerging in high-income countries. In the Netherlands, this reemergence was noticed from 1996 onward. Since then, peak incidences were observed every 2 to 3 years, which were most evident in children 4 to 5 years of age who had been vaccinated with whole-cell pertussis (wP) vaccine at 2, 3, 4, and 11 months of age (1). However, the vaccine efficacy of the Dutch wP vaccine was not optimal, due to low concentrations of and low antibody responses to pertussis toxin (PT), filamentous hemagglutinin (FHA), and pertactin (Prn) (2–4). Therefore, in 2001 an acellular pertussis (aP) preschool booster vaccination at 4 years of age was implemented, which shifted the age of the highest pertussis incidence toward ≥9 years of age (5). From 2005 onward, all primary wP vaccinations have been replaced by aP vaccinations. However, despite the implementation of aP vaccinations in the industrialized world since the 1990s, the pertussis reemergence has not been halted. In 2012, a new pertussis incidence peak in the Netherlands was observed in adolescents and adults, who can infect newborns who have not been fully vaccinated, with high risks of severe disease and even death.
The immune mechanisms important for protection against pertussis in humans remain elusive. Protection against pertussis is probably multifactorial (6) and is suggested to be mediated by both humoral (7, 8) and cell-mediated (9–13) immunity. High levels of antibodies against pertussis indicate previous infection or recent vaccination and probably are associated with protection against pertussis (8, 14). In general, higher antibody levels have been observed after switching from wP to aP vaccinations (3, 15). These antibody responses consist of different subclasses with IgG1 as the dominant subtype, followed by IgG2, IgG3, and IgG4 (16). The induction of IgG subclasses is regulated by T-cell cytokine production and is influenced by the nature and dose of the vaccine antigens as well as the age of the vaccinees (17).
Previously higher memory B-cell responses and greater avidity of pertussis-specific antibodies in aP-primed children than in wP-primed children at 4 years of age were reported (2, 18), indicating a more robust humoral immune response over time after infant vaccination with aP vaccines. Additionally, aP vaccination induced higher Th1 and Th2 T-cell responses 3 years after the primary vaccination series than did wP vaccination (19).
For optimal vaccination strategies, it is important to evaluate the longevity of the pertussis-specific immune response. The aim of this study is to evaluate the long-term antibody production and memory B-cell and T-cell immune responses in children 6 years of age, 2 years after aP preschool booster vaccination. The children had previously been vaccinated during infancy with either the Dutch wP vaccine or an aP vaccine.
MATERIALS AND METHODS
Study population.
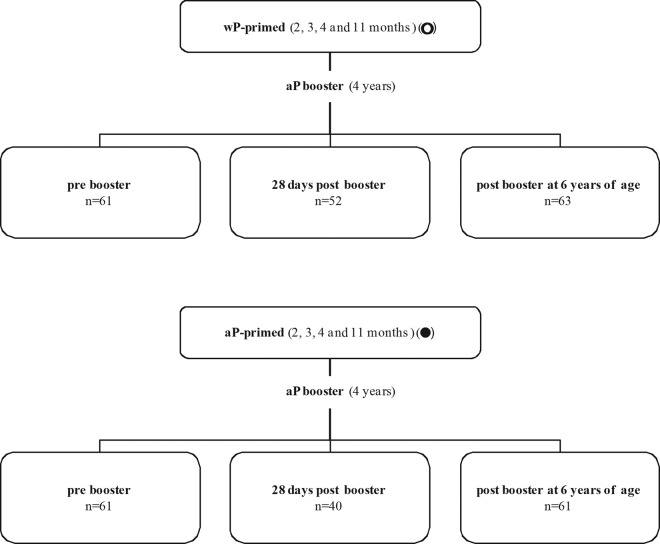
The children described in this study represent a subset from a larger cross-sectional study (registered trial no. ISRCTN65428640), performed between 2007 and 2009 in the Netherlands, that investigated the immunity to Bordetella pertussis in children 3 to 9 years of age. In this study, single blood samples (8 to 15 ml) were collected by venipuncture in two groups of wP-primed children 4 years of age (prebooster, n = 61; 28 days postbooster, n = 52), one group of wP-primed children 6 years of age (2 years postbooster, n = 63), and three corresponding groups of aP-primed children 4 and 6 years of age (prebooster, n = 61; 28 days postbooster, n = 40; 2 years postbooster, n = 61) for antibody determination (Fig. 1). For a randomly selected subset of these groups of children, memory B-cell responses (n = 11 to 19 per group) and T-cell responses (n = 5 to 15 per group) also were determined, by using samples varying in peripheral blood mononuclear cell (PBMC) numbers from high to low. In all groups, male and female subjects were included equally. For each child, both parents provided informed consent. This study was conducted according to the Declaration of Helsinki and good clinical practice guidelines, with the approval of the ethics review committee (STEG-METC, Almere, Netherlands).
Fig 1.
Groups of children used in the cross-sectional study. wP-primed and aP-primed children received Infanrix-IPV as a booster aP vaccination at 4 years of age. The children were studied before the booster and 28 days and 2 years after the booster. The total numbers of children recruited in the different study groups are shown. For the various assays, variable numbers of children were included as indicated. In all groups, male and female subjects were included equally.
Vaccines.
At the time of this study, all children had received either the Dutch whole-cell pertussis vaccine (diphtheria and tetanus toxoids [DT]-wP-inactivated poliovirus vaccine [IPV]-Haemophilus influenzae type b [Hib] vaccine; NVI, Bilthoven, the Netherlands) or the acellular pertussis vaccine (DT-aP-IPV-Hib vaccine, Infanrix-IPV-Hib; GlaxoSmithKline Biologicals S.A., Rixensart, Belgium) at 2, 3, 4, and 11 months of age. Children who had received the Pediacel vaccine (Sanofi Pasteur) at 11 months of age were excluded from this study. At 4 years of age, all children had received an additional preschool booster vaccination with Infanrix-IPV (GlaxoSmithKline Biologicals). Infanrix-IPV contains 25 μg PT, 25 μg FHA, and 8 μg Prn.
Serological assays.
Plasma levels of IgG directed against PT, FHA, and Prn were detected in all samples by using a fluorescent bead-based multiplex immunoassay against PT, FHA (both from Kaketsuken, Kuramoto, Japan), and recombinant Prn (20), as described previously (2, 21). The in-house reference was calibrated against FDA human pertussis antiserum lot 3 (for PT and FHA) and lot 4 (for Prn). Although a new international WHO reference is available, results were expressed in enzyme-linked immunosorbent assay units (EU)/ml to keep this study in line with our earlier published data. An arbitrary level of >20 EU/ml for the PT antigen was defined as protective (22, 23).
For pertussis-specific IgG subclasses, a modified multiplex immunoassay was used, as described previously (24). The contribution of the mean fluorescence intensity (MFI) for each IgG subclass was assessed as a percentage from the sum of MFI values for the 4 IgG subclasses together.
The avidity of PT- and Prn-specific antibodies was measured in plasma with the multiplex immunoassay, as described previously (3). For FHA, the avidity assay with thiocyanate leads to unacceptably high background levels. The avidity index was expressed as a percentage of the IgG levels remaining in the presence of ammonium thiocyanate in comparison with those in phosphate-buffered saline, for which the avidity was set at 100%.
B-cell ELISPOT assays.
PBMCs were isolated from 4-ml BD Vacutainer cell-preparation tubes, washed, and stored at −135°C, and plasma samples were frozen at −20°C until further testing; subsequently, B cells were stimulated and antigen-specific enzyme-linked immunosorbent spot (ELISPOT) assays for PT, FHA, and Prn were performed as described, using the same antigens as for the serological assays (25). The numbers of antigen-specific memory B cells were determined per 105 B cells. Mean spot values for noncoated wells served as negative-control values and were subtracted. To determine the geometric mean number per group, the lower limit of quantification was set at a value of 0.1 memory B cells per 105 B cells. Fresh and frozen cells were compared previously and showed no differences in memory B-cell numbers (25).
IFN-γ ELISPOT assays and cytokines in T-cell culture supernatants.
Gamma interferon (IFN-γ) ELISPOT assays were performed as described previously (19). In short, 3 × 105 PBMCs depleted of B cells were stimulated with the pertussis antigens as mentioned earlier, i.e., PT (inactivated for 10 min at 80°C), native FHA, and recombinant Prn for 5 days at 37°C in 5% CO2. Pokeweed mitogen (PWM) was used as a positive-control stimulus for all samples, and individual data were included in the data analysis when more than 200 IFN-γ-producing cells per 10,000 PBMCs were found upon PWM stimulation. Spots were developed using biotin-labeled anti-IFN-γ, streptavidin, and 5-bromo-4-chloro-3-indolylphosphate (BCIP)/nitroblue tetrazolium (NBT) and were counted using Immunospot analyzer software (CTL-Europe GmbH, Bonn, Germany).
Mean spot values for nonstimulated cells served as negative-control values and were subtracted from the antigen-stimulated cell values for each sample. Fresh and frozen cells were compared previously and showed no differences in IFN-γ-producing cell numbers (26).
Cell culture supernatants were stored at −80°C until further use. By using Bio-Rad cytokine assay kits (Bio-Rad Laboratories, Hercules, CA), IFN-γ, interleukin-10 (IL-10), IL-5, and IL-17 concentrations were determined according to the manufacturer's procedure. Samples were excluded from the data analysis if the number of counted beads was too low, making the measurements unreliable (27, 28).
Statistical methods.
Results are expressed as geometric mean concentrations (GMCs) with 95% confidence intervals (CIs) unless otherwise indicated. The Mann-Whitney test was used to determine significant differences between groups differing in either age or priming. P < 0.05 was considered significant.
RESULTS
Total IgG and IgG subclass levels in wP- and aP-primed children.
Before the booster and 28 days after the booster at 4 years of age and 2 years after the booster at 6 years of age, the GMCs of pertussis-specific total IgG, IgG1, and IgG4 for all three antigens were significantly higher in aP-primed children than in wP-primed children (Table 1). Additionally, PT- and Prn-specific IgG3 levels were significantly higher prebooster and FHA- and Prn-specific IgG2 levels were higher at both 28 days and 2 years after preschool booster vaccination.
Table 1.
Levels of total IgG and IgG subclasses before and after aP preschool booster vaccination
| Time of assessment and antigen | IgG and IgG subclass levels (EU/ml)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total IgG |
IgG1 |
IgG2 |
IgG3 |
IgG4 |
||||||
| wP | aP | wP | aP | wP | aP | wP | aP | wP | aP | |
| Prebooster (wP, n = 61; aP, n = 61) | ||||||||||
| PT | 4.5 | 7.7b | 1.3 | 3.2b | 1.7 | 1.7 | 0.02 | 0.05b | 0.02 | 0.07b |
| FHA | 8.8 | 16b | 6.2 | 14b | 0.46 | 0.36 | 0.08 | 0.10 | 0.09 | 0.60b |
| Prn | 3.1 | 24b | 1.8 | 20b | 0.28 | 0.36 | 0.07 | 0.18b | 0.02 | 0.67b |
| 28 days postbooster (wP, n = 52; aP, n = 40) | ||||||||||
| PT | 61 | 187b | 38 | 140b | 5.5 | 4.6 | 0.65 | 0.56 | 0.40 | 14b |
| FHA | 195 | 521b | 181 | 450b | 2.0 | 8.2b | 1.6 | 2.2 | 3.2 | 36b |
| Prn | 187 | 1253b | 178 | 1215b | 1.2 | 3.4b | 1.3 | 1.2 | 1.3 | 18b |
| 2 yr postbooster (wP, n = 63; aP, n = 61) | ||||||||||
| PT | 11c | 26b,c | 5.1c | 16b,c | 1.7 | 2.7 | 0.03 | 0.04 | 0.05c | 0.38b,c |
| FHA | 45c | 81b,c | 42c | 75b,c | 0.16d | 0.38b | 0.10 | 0.08 | 0.97c | 2.7b,c |
| Prn | 19c | 110b,c | 17c | 103b,c | 0.19 | 0.44b | 0.11 | 0.14 | 0.27c | 2.9b,c |
Data are expressed as geometric mean values specific for PT, FHA, or Prn in children who were primed with either wP or aP. All values for 28 days postbooster were significantly increased (P < 0.05) compared with prebooster levels, and those for 2 years postbooster were significantly decreased (P > 0.05) compared with 28-day postbooster values.
Significantly increased levels in aP-primed children compared to wP-primed children (P < 0.05).
Significantly increased values in children 2 years after booster vaccination compared to prebooster levels (P < 0.05).
Significantly decreased levels in children 2 years after booster vaccination compared to prebooster levels (P < 0.05).
Following aP booster vaccination, all pertussis-specific total IgG and subclass levels increased after 28 days, compared with prebooster levels, and subsequently decreased over the next 2 years in both wP- and aP-primed children. However, total IgG as well as IgG1 and IgG4 levels for all 3 antigens still significantly exceeded the prebooster levels after 2 years. At age 6 years, significantly more (P = 0.0024) aP-primed children (35/61 children) still had PT-specific antibody levels above the arbitrary protective level of 20 EU/ml, compared to 19/63 wP-primed children. IgG1 was the most prevalent subclass for all three pertussis antigens in all children, especially after booster vaccination. IgG4 levels for all three pertussis antigens also increased prominently 28 days postbooster in aP-primed children and remained higher than in wP-primed children at 6 years of age.
Memory B-cell responses.
We found significantly higher numbers of Prn-specific memory B cells in aP-primed children than in wP-primed children already at 4 years prebooster, as well as 28 days and 2 years after booster vaccination, while PT-specific cell levels were significantly higher only 28 days postbooster (Fig. 2). For wP-primed children, pertussis-specific B-cell numbers for all antigens increased at both 28 days and 2 years postbooster, compared to prebooster numbers, except for PT at 28 days postbooster. The numbers for FHA-specific memory B-cell responses in wP-primed children dropped significantly between 28 days and 2 years after booster vaccination. In aP-primed children, the numbers of memory B cells for all three antigens were enhanced at 28 days and subsequently showed a significant decline at 2 years after booster vaccination. At that time point, only the PT-specific numbers of memory B cells still exceeded prebooster numbers. The geometric mean percentages of total IgG-producing cells showed no differences between groups; values for wP-vaccinated children were 13.9%, 17.5%, and 14.7% and those for aP-vaccinated children were 17.4%, 16.3%, and 15.7% prebooster, 28 days postbooster, and 2 years postbooster, respectively.
Fig 2.
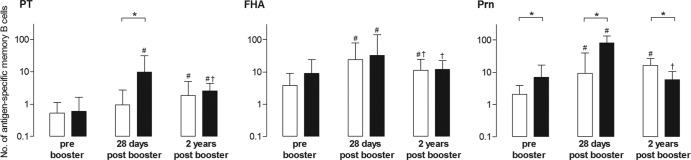
Numbers of memory B cells specific for PT, FHA, and Prn per 105 B cells in wP-primed children (white bars) and aP-primed children (black bars) prebooster and 28 days and 2 years after booster vaccination (n = 11 to 19 per group). Bars indicate geometric mean values with 95% CIs. ∗, significantly different numbers in aP-primed children compared to wP-primed children, P < 0.05; #, significantly increased numbers postbooster compared to prebooster, P < 0.05; †, significantly decreased numbers 2 years postbooster compared to 28 days postbooster, P < 0.05.
Avidity indices of PT- and Prn-specific IgG antibodies.
The avidity indices of antibodies specific for PT and Prn were higher in aP-primed children than in wP-primed children at the 3 time points, but values for PT had become similar in children 6 years of age (Fig. 3). The aP preschool booster significantly increased the avidity indices in wP-primed children at 28 days postbooster, and values remained significantly higher until 2 years postbooster. In aP-primed children, with already higher prebooster indices, the avidity pattern for PT resembled the memory B-cell response, showing a significant increase at 28 days, but the increase was followed by a significant decrease to prebooster values after 2 years. In contrast, the Prn-specific avidity indices were significantly increased at 28 days postbooster but remained high up to 2 years postbooster in aP-primed children.
Fig 3.
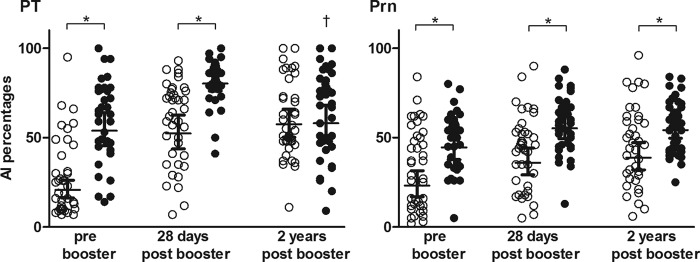
Affinity indices (AIs) of IgG antibodies to PT or Prn prebooster and 28 days and 2 years postbooster in wP-primed (○) or aP-primed (●) children, expressed as percentages. All groups consisted of 40 samples except for the aP-primed prebooster group (n = 35). Horizontal bars, geometric mean values with 95% CIs. ∗, significantly increased values in aP-primed children compared to wP-primed children, P < 0.05; †, significantly decreased values 2 years postbooster compared to 28 days postbooster, P < 0.05.
Numbers of pertussis-specific IFN-γ-producing cells.
Already before the preschool booster, higher numbers of IFN-γ-producing cells were found in aP-primed children than in wP-primed children (Table 2) (19). In wP-primed children, the number of IFN-γ-producing cells increased upon booster vaccination and still exceeded prebooster values for all three pertussis antigens after 2 years. In contrast, in aP-primed children, the number of IFN-γ-producing cells remained high during the first 28 days postbooster. At 2 years postbooster, however, cell numbers had declined to lower numbers than prebooster values for all three pertussis antigens and even tended to be lower than in wP-primed children, although this was not significant.
Table 2.
Numbers of IFN-γ-producing cells per 105 PBMCs 5 days after stimulation with PT, FHA, or Prn
| Vaccine and time of assessment | No. of cells and GMC after stimulation with: |
|||||
|---|---|---|---|---|---|---|
| PT |
FHA |
Prn |
||||
| n | GMC (95% CI) | n | GMC (95% CI) | n | GMC (95% CI) | |
| wP | ||||||
| Prebooster | 15 | 0.5 (0.2–1.6) | 14 | 4.4 (1.2–16) | 12 | 1.0 (0.2–3.8) |
| 28 days postbooster | 12 | 5.3 (1.1–27)a | 10 | 15.1 (2.9–79) | 11 | 7.3 (1.4–39) |
| 2 yr postbooster | 15 | 9.9 (2.2–45)a | 15 | 51 (16–163)a | 13 | 13 (2.4–74)a |
| aP | ||||||
| Prebooster | 13 | 18 (3.6–91) | 13 | 92 (22–374) | 13 | 27 (6.9–108) |
| 28 days postbooster | 11 | 7.0 (1.2–41) | 11 | 46 (10–215) | 11 | 6.0 (1.2–31) |
| 2 yr postbooster | 15 | 3.6 (1.1–12)b | 15 | 44 (21–94)b | 13 | 5.7 (2.1–15)b |
Significant increase postbooster compared to prebooster vaccination (P < 0.05).
Significant decrease postbooster compared to prebooster vaccination (P < 0.05).
Cytokine responses in cell culture supernatants.
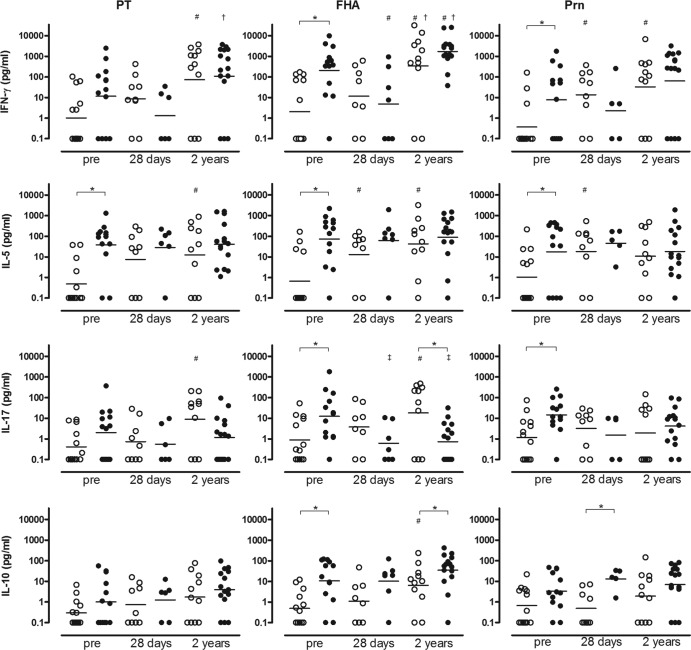
PBMCs depleted of B cells from children 4 and 6 years of age contained high percentages of CD3+ T cells (72 to 85% of total) after stimulation for 5 days. The cells from aP-primed children 4 years of age showed significantly higher prebooster IFN-γ, IL-5, and IL-17 responses for all three antigens except for PT-specific IFN-γ and IL-17 levels and higher FHA-specific IL-10 levels than in wP-primed children (Fig. 4). In aP-primed children, only the Prn-specific IL-10 response was still significantly higher 28 days after booster vaccination; at 2 years postbooster, the FHA-specific IL-10 response was higher, whereas the FHA-specific 1L-17 response was lower, than in wP-primed children.
Fig 4.
IFN-γ, IL-5, IL-17, and IL-10 T-cell cytokine responses in culture supernatants of PBMCs stimulated with PT, FHA, or Prn, from wP-primed (○) or aP-primed (●) children before booster (n = 12 to 15) and 28 days (n = 5 to 9) and 2 years (n = 10 to 15) after booster vaccination. Horizontal bars, geometric mean concentrations with 95% CIs. ∗, significantly different values in aP-primed children compared to wP-primed children, P < 0.05; #, significantly increased values postbooster compared to prebooster, P < 0.05; ‡, significantly decreased values postbooster compared to prebooster, P < 0.05; †, significantly increased values 2 years postbooster compared to 28 days postbooster, P > 0.05.
In wP-primed children, the booster aP vaccination resulted in increased IFN-γ, IL-5, IL-17, and IL-10 responses for all three pertussis antigens at 28 days postbooster, which were significant only for Prn-specific IFN-γ and IL-5 and FHA-specific IL-5 responses. Two years postbooster in wP-primed children, all cytokine responses still exceeded the prebooster values and showed significantly increased IFN-γ, IL-5, and IL-17 responses specific for all three pertussis antigens except for Prn-specific IL-5 and IL-17 responses. Additionally, the FHA-specific IL-10 values 2 years after booster vaccination were significantly higher than prebooster values.
Remarkably, in aP-primed children, almost all pertussis-specific cytokine responses remained similar at 28 days postbooster, compared to prebooster values, and the FHA-specific IFN-γ and IL-17 responses even were decreased. At 2 years postbooster, the IFN-γ responses increased, which was significant for PT and FHA. In contrast, IL-5 and IL-10 responses did not change and the FHA-specific IL-17 response still was significantly decreased, compared to prebooster values. Although FHA is not strictly Bordetella specific, T-cell cytokine responses after stimulation with FHA purified from Bordetella pertussis showed about the same pattern as found for the strictly pertussis-specific PT and Bordetella-specific Prn, emphasizing the general pattern of these specific T-cell results.
DISCUSSION
Although acellular pertussis vaccines have been used in infants for some decades and a considerable number of countries have introduced preschool booster aP vaccination, long-term postbooster immunological data are limited. This study investigated the long-term humoral and cellular immune responses in children 6 years of age who had received the preschool aP booster 2 years earlier and had been vaccinated with either wP or aP vaccines during infancy.
In this study, we showed that the preschool aP booster at 4 years of age resulted in significantly higher pertussis-specific IgG antibody levels in aP-primed children than those in wP-primed children, which remained higher for at least 2 years postbooster, particularly for pertactin. Antibodies consisted mainly of the IgG1 subclass, but primarily aP-primed children also showed elevated IgG4 antibody levels. The avidity of antibody responses also was higher in aP-primed children, both before and after the booster. In wP-primed children, the numbers of pertussis-specific memory B cells were increased, in line with higher IgG antibody levels and avidity indices at 2 years after booster vaccination. It is noteworthy that the avidities of PT-specific antibodies were similar at 6 years of age in aP- and wP-primed children. Importantly, all T-cell responses in aP-primed children were already high prebooster and did not really change 2 years postbooster, whereas the T-cell responses in wP-primed children increased over that period. Together, these data indicated that the preschool aP booster improved pertussis immunity until 2 years after the booster immunization and that aP-primed children might have an advantage over wP-primed children at 6 years of age. However, recent studies in the United States have shown a high incidence of pertussis in aP-vaccinated children from 9 years onward, indicating that protection against pertussis with aP vaccines is relatively short-lived and antibody levels wane substantially within 4 to 5 years, even after a fifth aP vaccination (29, 30). It is conceivable that the pertussis-specific immune responses of aP-primed Dutch children also will wane quickly within 5 years after the preschool booster vaccination and that these children will become more vulnerable to pertussis as well (29, 30).
We reported earlier that 3 years after wP or aP primary immunizations in the first year of life, only low levels of antibodies to the pertussis vaccine antigens were found (3), suggesting a fast decay of these levels with both vaccine types, as previous studies have already shown (2, 31, 32). Although we cannot exclude the possibility that the findings for wP-primed children at 4 to 6 years of age are influenced by the use of the nonoptimal Dutch wP vaccine, the vaccination schedule and vaccines used for the aP-primed children in our study were comparable to those in other studies. The increased antibody levels that gradually decline over 2 years after the preschool booster in our study are in line with the observations of Meyer et al., who also showed sharp decreases in antibody levels 3.5 years after a fifth aP vaccination at preschool age (33). Overall, their data on FHA- and Prn-specific IgG levels at 3.5 years postbooster are rather comparable to our data at 2 years postbooster, and the difference in PT-specific antibody levels is likely due to the difference in time after booster vaccination. Our IgG values shortly after the fifth preschool booster aP vaccination were at least 3-fold higher than the data of Sänger et al., but a reduced-antigen-dose booster vaccine was used in that study (34). After an adolescent booster, antibody levels show the same decay as found after a preschool booster (35). Although antibody levels rapidly wane, the avidity indices of Prn-specific antibodies in aP-vaccinated versus wP-vaccinated children contributed to greater strength of antibody binding, which might indicate better protection. However, this does not apply to PT-specific antibodies, since their avidity indices in aP-primed children seemed to decline shortly after booster vaccination. The avidity of FHA-specific antibodies has not been measured, since FHA is not specific for B. pertussis alone and FHA-specific antibodies show cross-reactivity with FHA proteins present in other bacteria (36).
Although IgG1 was the predominant subclass for all pertussis antigens in both groups of children, higher IgG4 levels were present postbooster, especially in children who had been aP-primed (24). The pertussis vaccine used for priming seems to determine the IgG subclass composition elicited in a secondary antibody response upon booster vaccination. In other studies, IgG1 was found to be the predominant subclass after pertussis vaccination and IgG4 contributed only marginally to the total IgG level (16, 24). The more pronounced antipertussis IgG4 response in aP-primed children, which also was observed by Giammanco et al. (16), might reflect more Th2 skewing of the immune response after aP vaccination (37). Previous studies have shown that high concentrations of PT, FHA, and Prn in most pediatric aP vaccines elicit a Th2 or mixed Th1/Th2 immune response (9, 12, 38). The wP vaccine contains lower concentrations of less purified antigens and more additional substances (like lipopolysaccharide) that are capable of inducing a more Th1-associated immune response (9, 12). Although the higher IgG4 responses correlated with PT- and Prn-specific IgE levels (24), we observed only slightly higher Th2 responses than Th1 responses 1 month after the fifth consecutive aP vaccination (19). Also, the T-cell data 2 years postbooster do not show more Th2 skewing. In this study, the samples were not obtained longitudinally from the children at the different time points and some group sample sizes in the T-cell assays were relatively small. Therefore, interpretation of the T-cell responses over time should be made with care, but surely follow-up studies are indicated based on these results.
We have indications that booster responses induced by vaccination in combination with natural boosting with pertussis have resulted in increased T-cell responses in wP-primed children 6 and 9 years of age (39, 40). In that study, however, the numbers of possible undiagnosed cases on the basis of elevated PT antibody levels were similar for wP-primed and aP-primed children.
We have shown that, in the currently available aP vaccines, pertactin is the most immunogenic antigen. Recently, a number of B. pertussis strains not expressing the vaccine antigen Prn have been isolated from pertussis patients in a number of countries where the aP vaccines have been implemented for some time now (41). We might speculate that the high antibody levels induced by the aP vaccines, especially against Prn, have triggered the bacteria to escape from this immune pressure. Until now, these escape mutants have not been more virulent than the wild-type pertussis strains; however, we must continue the surveillance of these strains carefully. Protection against these pertussis strains is now dependent on immune responses primarily against PT, with FHA not being specific, making the antibody spectrum of the current acellular vaccines even narrower.
To summarize, since the wP vaccine has been replaced by aP vaccines, induction of B-cell and T-cell memory responses has been improved, resulting in higher levels of circulating antibodies with better avidity. However, based on the high T-cell immune responses already present before the preschool booster, the dose of aP vaccine and the vaccination schedule may need to be reconsidered in order to improve immune responses. The still high T-cell responses at 4 years of age and the reported local hypersensitivity after the fifth aP vaccination suggest that the preschool booster vaccination might be shifted to a later age to induce better long-term immune responses (19). Also, since Meyer et al. (33) reported that a reduced-antigen-dose aP vaccine was as immunogenic as high-dose preschool booster aP vaccines and at least as well tolerated, the choice between low-dose and high-dose preschool boosters should be made carefully. Evaluation of long-term humoral and cellular immune responses after vaccination against pertussis will be important for future decisions concerning pertussis vaccination schedules for adolescents and adults. In the short term, improved vaccination schedules could better stimulate the immune systems of children and protect them longer against pertussis. Our data suggest that postponing the boosters at 11 months and 4 years to later times (15 to 18 months and 6 years, respectively) would be possible. In the long term, perhaps new-generation aP vaccines are needed to improve protection against pertussis for newborns, older age groups, and even the whole population.
ACKNOWLEDGMENTS
We thank all the children who participated in this study and all the research staff members from the Linnaeus Institute, Spaarne Hospital (Hoofddorp, the Netherlands), who were involved in this study.
We have no conflicts of interest to declare.
This study was funded by the Dutch government through the Ministry of Health, Welfare, and Sport. A small part of the study was funded by the European Research Network, supported by the European Commission under the Health Cooperation Work Programme of the 7th Framework Programme.
Footnotes
Published ahead of print 3 July 2013
REFERENCES
- 1.de Greeff SC, Schellekens JF, Mooi FR, de Melker HE. 2005. Effect of vaccination against pertussis on the incidence of pertussis in The Netherlands, 1996–2003. Ned. Tijdschr. Geneeskd. 149:937–943 (In Dutch.) [PubMed] [Google Scholar]
- 2.Guiso N, Njamkepo E, Vie le Sage F, Zepp F, Meyer CU, Abitbol V, Clyti N, Chevallier S. 2007. Long-term humoral and cell-mediated immunity after acellular pertussis vaccination compares favourably with whole-cell vaccines 6 years after booster vaccination in the second year of life. Vaccine 25:1390–1397 [DOI] [PubMed] [Google Scholar]
- 3.Hendrikx LH, Berbers GA, Veenhoven RH, Sanders EA, Buisman AM. 2009. IgG responses after booster vaccination with different pertussis vaccines in Dutch children 4 years of age: effect of vaccine antigen content. Vaccine 27:6530–6536 [DOI] [PubMed] [Google Scholar]
- 4.Berbers GA, van de Wetering MS, van Gageldonk PG, Schellekens JF, Versteegh FG, Teunis PF. 2013. A novel method for evaluating natural and vaccine induced serological responses to Bordetella pertussis antigens. Vaccine. [Epub ahead of print.] 10.1016/j.vaccine.2013.05.073 [DOI] [PubMed] [Google Scholar]
- 5.de Greeff SC, Mooi FR, Schellekens JF, de Melker HE. 2008. Impact of acellular pertussis preschool booster vaccination on disease burden of pertussis in The Netherlands. Pediatr. Infect. Dis. J. 27:218–223 [DOI] [PubMed] [Google Scholar]
- 6.Mills KH. 2001. Immunity to Bordetella pertussis. Microbes Infect. 3:655–677 [DOI] [PubMed] [Google Scholar]
- 7.Trollfors B, Taranger J, Lagergard T, Lind L, Sundh V, Zackrisson G, Lowe CU, Blackwelder W, Robbins JB. 1995. A placebo-controlled trial of a pertussis-toxoid vaccine. N. Engl. J. Med. 333:1045–1050 [DOI] [PubMed] [Google Scholar]
- 8.Storsaeter J, Hallander HO, Gustafsson L, Olin P. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907–1916 [DOI] [PubMed] [Google Scholar]
- 9.Ausiello CM, Urbani F, la Sala A, Lande R, Cassone A. 1997. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect. Immun. 65:2168–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leef M, Elkins KL, Barbic J, Shahin RD. 2000. Protective immunity to Bordetella pertussis requires both B cells and CD4+ T cells for key functions other than specific antibody production. J. Exp. Med. 191:1841–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rieber N, Graf A, Hartl D, Urschel S, Belohradsky BH, Liese J. 2011. Acellular pertussis booster in adolescents induces Th1 and memory CD8+ T cell immune response. PLoS One 6:e17271. 10.1371/journal.pone.0017271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan M, Murphy G, Ryan E, Nilsson L, Shackley F, Gothefors L, Oymar K, Miller E, Storsaeter J, Mills KH. 1998. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 93:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran Minh NN, He Q, Edelman K, Olander RM, Viljanen MK, Arvilommi H, Mertsola J. 1999. Cell-mediated immune responses to antigens of Bordetella pertussis and protection against pertussis in school children. Pediatr. Infect. Dis. J. 18:366–370 [DOI] [PubMed] [Google Scholar]
- 14.Taranger J, Trollfors B, Lagergard T, Sundh V, Bryla DA, Schneerson R, Robbins JB. 2000. Correlation between pertussis toxin IgG antibodies in postvaccination sera and subsequent protection against pertussis. J. Infect. Dis. 181:1010–1013 [DOI] [PubMed] [Google Scholar]
- 15.Ausiello CM, Lande R, Urbani F, Di Carlo B, Stefanelli P, Salmaso S, Mastrantonio P, Cassone A. 2000. Cell-mediated immunity and antibody responses to Bordetella pertussis antigens in children with a history of pertussis infection and in recipients of an acellular pertussis vaccine. J. Infect. Dis. 181:1989–1995 [DOI] [PubMed] [Google Scholar]
- 16.Giammanco A, Taormina S, Chiarini A, Dardanoni G, Stefanelli P, Salmaso S, Mastrantonio P. 2003. Analogous IgG subclass response to pertussis toxin in vaccinated children, healthy or affected by whooping cough. Vaccine 21:1924–1931 [DOI] [PubMed] [Google Scholar]
- 17.Coffman RL, Lebman DA, Rothman P. 1993. Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 54:229–270 [DOI] [PubMed] [Google Scholar]
- 18.Hendrikx LH, de Rond LG, Ozturk K, Veenhoven RH, Sanders EA, Berbers GA, Buisman AM. 2011. Impact of infant and preschool pertussis vaccinations on memory B-cell responses in children at 4 years of age. Vaccine 29:5725–5730 [DOI] [PubMed] [Google Scholar]
- 19.Schure RM, Hendrikx LH, de Rond LGH, Ozturk K, Sanders EAM, Berbers GAM, Buisman AM. 2012. T-cell responses before and after the fifth consecutive acellular pertussis vaccination in 4-year-old Dutch children. Clin. Vaccine Immunol. 19:1879–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loosmore SM, Yacoob RK, Zealey GR, Jackson GE, Yang YP, Chong PS, Shortreed JM, Coleman DC, Cunningham JD, Gisonni L, Klein MH. 1995. Hybrid genes over-express pertactin from Bordetella pertussis. Vaccine 13:571–580 [DOI] [PubMed] [Google Scholar]
- 21.van Gageldonk PG, van Schaijk FG, van der Klis FR, Berbers GA. 2008. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J. Immunol. Methods 335:79–89 [DOI] [PubMed] [Google Scholar]
- 22.Long SS, Welkon CJ, Clark JL. 1990. Widespread silent transmission of pertussis in families: antibody correlates of infection and symptomatology. J. Infect. Dis. 161:480–486 [DOI] [PubMed] [Google Scholar]
- 23.Versteegh FG, Mertens PL, de Melker HE, Roord JJ, Schellekens JF, Teunis PF. 2005. Age-specific long-term course of IgG antibodies to pertussis toxin after symptomatic infection with Bordetella pertussis. Epidemiol. Infect. 133:737–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrikx LH, Schure RM, Ozturk K, de Rond LG, de Greeff SC, Sanders EA, Berbers GA, Buisman AM. 2011. Different IgG-subclass distributions after whole-cell and acellular pertussis infant primary vaccinations in healthy and pertussis infected children. Vaccine 29:6874–6880 [DOI] [PubMed] [Google Scholar]
- 25.Buisman AM, de Rond CG, Ozturk K, Ten Hulscher HI, van Binnendijk RS. 2009. Long-term presence of memory B-cells specific for different vaccine components. Vaccine 28:179–186 [DOI] [PubMed] [Google Scholar]
- 26.Kreher CR, Dittrich MT, Guerkov R, Boehm BO, Tary-Lehmann M. 2003. CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J. Immunol. Methods 278:79–93 [DOI] [PubMed] [Google Scholar]
- 27.Jacobson JW, Oliver KG, Weiss C, Kettman J. 2006. Analysis of individual data from bead-based assays (“bead arrays”). Cytometry A 69:384–390 [DOI] [PubMed] [Google Scholar]
- 28.Hanley BP. 2008. Variance in multiplex suspension array assays: a distribution generation machine for multiplex counts. Theor. Biol. Med. Model. 5:3. 10.1186/1742-4682-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. 2012. Waning protection after fifth dose of acellular pertussis vaccine in children. N. Engl. J. Med. 367:1012–1019 [DOI] [PubMed] [Google Scholar]
- 30.Witt MA, Katz PH, Witt DJ. 2012. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin. Infect. Dis. 54:1730–1735 [DOI] [PubMed] [Google Scholar]
- 31.Giuliano M, Mastrantonio P, Giammanco A, Piscitelli A, Salmaso S, Wassilak SG. 1998. Antibody responses and persistence in the two years after immunization with two acellular vaccines and one whole-cell vaccine against pertussis. J. Pediatr. 132:983–988 [DOI] [PubMed] [Google Scholar]
- 32.Hallander HO, Gustafsson L, Ljungman M, Storsaeter J. 2005. Pertussis antitoxin decay after vaccination with DTPa: response to a first booster dose 3 1/2–6 1/2 years after the third vaccine dose. Vaccine 23:5359–5364 [DOI] [PubMed] [Google Scholar]
- 33.Meyer CU, Habermehl P, Knuf M, Hoet B, Wolter J, Zepp F. 2008. Immunogenicity and reactogenicity of acellular pertussis booster vaccines in children: standard pediatric versus a reduced-antigen content formulation. Hum. Vaccin. 4:203–209 [DOI] [PubMed] [Google Scholar]
- 34.Sänger R, Behre U, Krause KH, Loch HP, Soemantri P, Herrmann D, Schmitz-Hauss E, Wolter J, Hoet B. 2007. Booster vaccination and 1-year follow-up of 4-8-year-old children with a reduced-antigen-content dTpa-IPV vaccine. Eur. J. Pediatr. 166:1229–1236 [DOI] [PubMed] [Google Scholar]
- 35.Mertsola J, Van Der Meeren O, He Q, Linko-Parvinen A, Ramakrishnan G, Mannermaa L, Soila M, Pulkkinen M, Jacquet JM. 2010. Decennial administration of a reduced antigen content diphtheria and tetanus toxoids and acellular pertussis vaccine in young adults. Clin. Infect. Dis. 51:656–662 [DOI] [PubMed] [Google Scholar]
- 36.Isacson J, Trollfors B, Taranger J, Lagergard T. 1995. Acquisition of IgG serum antibodies against two Bordetella antigens (filamentous hemagglutinin and pertactin) in children with no symptoms of pertussis. Pediatr. Infect. Dis. J. 14:517–521 [DOI] [PubMed] [Google Scholar]
- 37.Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckmann MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE. 1990. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 8:303–333 [DOI] [PubMed] [Google Scholar]
- 38.Mascart F, Hainaut M, Peltier A, Verscheure V, Levy J, Locht C. 2007. Modulation of the infant immune responses by the first pertussis vaccine administrations. Vaccine 25:391–398 [DOI] [PubMed] [Google Scholar]
- 39.Schure RM, de Rond L, Ozturk K, Hendrikx L, Sanders E, Berbers G, Buisman AM. 2012. Pertussis circulation has increased T-cell immunity during childhood more than a second acellular booster vaccination in Dutch children 9 years of age. PLoS One 7:e41928. 10.1371/journal.pone.0041928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendrikx LH, Ozturk K, de Rond LG, de Greeff SC, Sanders EA, Berbers GA, Buisman AM. 2011. Serum IgA responses against pertussis proteins in infected and Dutch wP or aP vaccinated children: an additional role in pertussis diagnostics. PLoS One 6:e27681. 10.1371/journal.pone.0027681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Queenan AM, Cassiday PK, Evangelista A. 2013. Pertactin-negative variants of Bordetella pertussis in the United States. N. Engl. J. Med. 368:583–584 [DOI] [PMC free article] [PubMed] [Google Scholar]