Abstract
The hydroxyproline-containing proteins (hyproproteins) synthesized by cultured human fibroblasts have been partially characterized. The hyproprotein extracted from the cell layer was found to be similar to the collagen extracted from skin in the ratio of hydroxyproline to proline, chain composition, solubility, and resistance to proteolytic digestion.
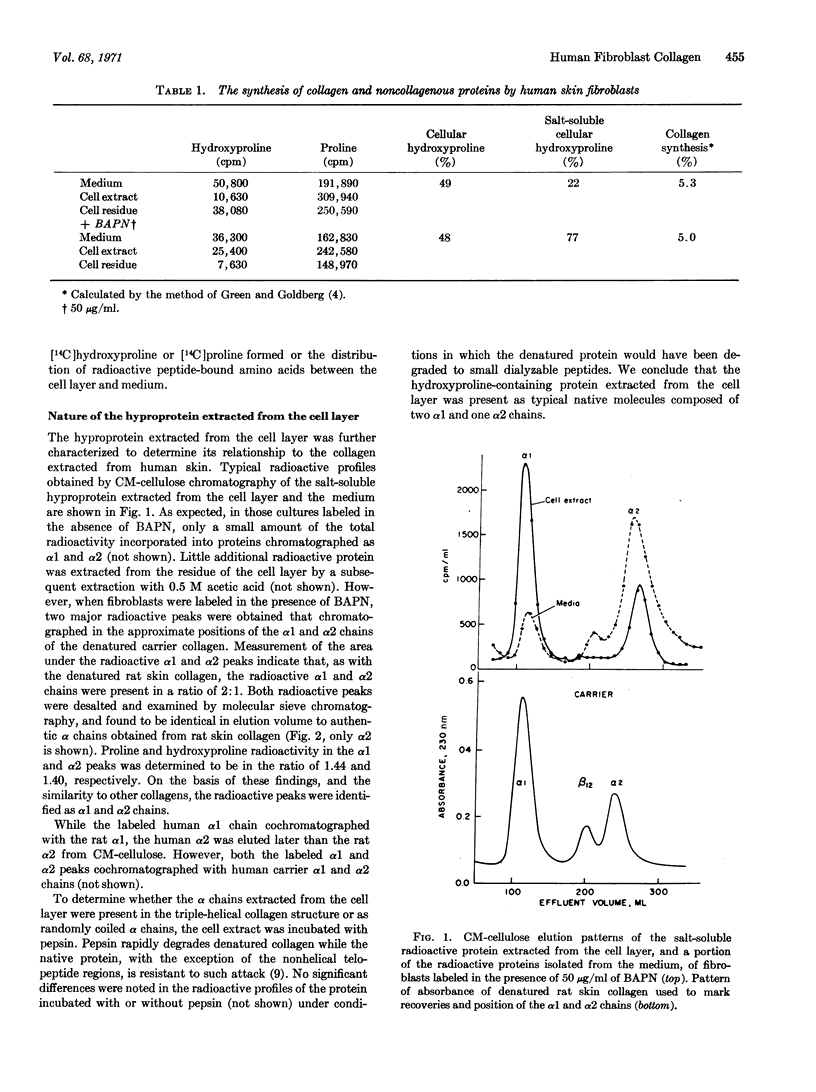
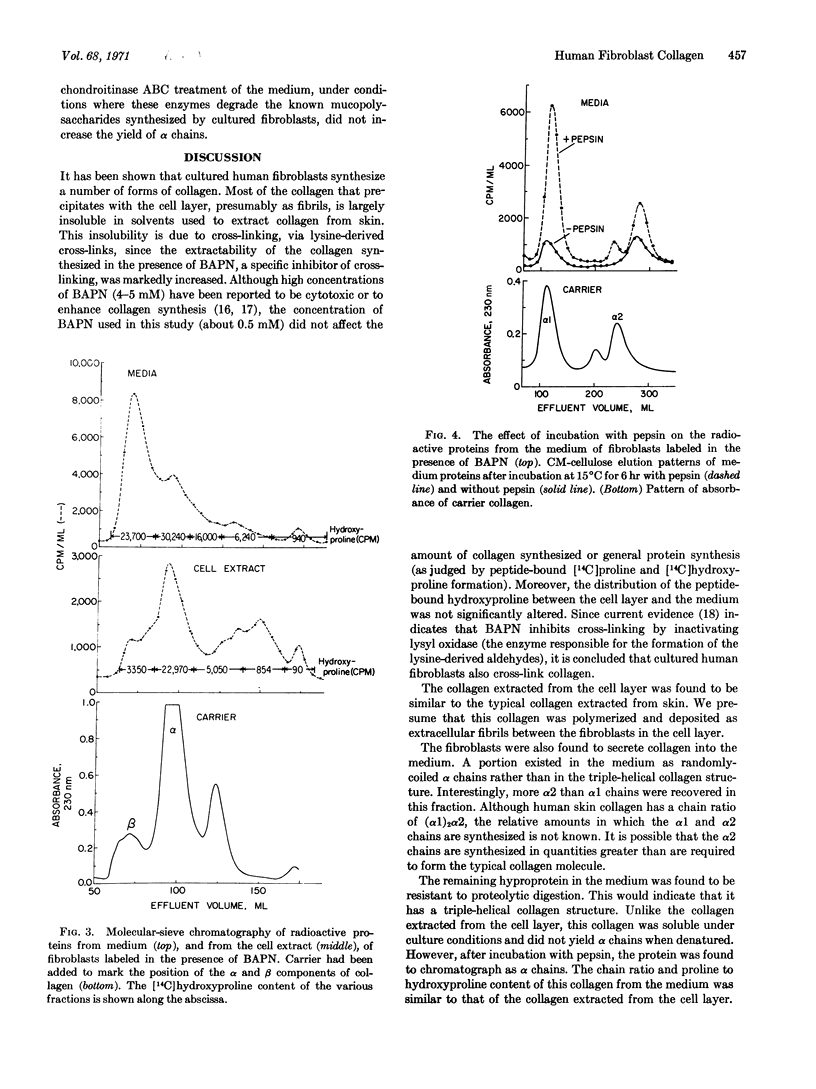
The hyproproteins isolated from the medium were different. About 20% of the peptide-bound hydroxyproline was found in randomly coiled chains. The α2 chains were present in considerable excess over the α1 chains, suggesting that the α2 chain may be synthesized in quantities greater than required to form a collagen molecule with a chain composition (α1)2α2. The remaining medium hyproprotein appeared to be an unusual form of native collagen which, unlike typical native collagen, was soluble under physiological conditions. This hyproprotein did not yield α chains when denatured and contained material that had a molecular weight greater than α chains. A similar size distribution was observed in the protein synthesized in the presence of β-aminopropionitrile, a specific inhibitor of collagen cross-linking. After treatment with pepsin, typical α1 and α2 chains were obtained from the protein in a 2:1 ratio. Since the medium protein is soluble and has properties different from the typical collagen molecule, it may represent a modified form that functions in the transport of collagen from the cell to the fiber.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleo J. J. Collagen synthesis in cultured cells: the influence of beta-aminopropionitrile. Proc Soc Exp Biol Med. 1969 Feb;130(2):451–454. doi: 10.3181/00379727-130-33577. [DOI] [PubMed] [Google Scholar]
- FESSLER J. H. Some properties of neutral-salt-soluble collagen. 1. Biochem J. 1960 Sep;76:452–463. doi: 10.1042/bj0760452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLOP P. M. Particle size and shape in a citrate extract of ichthyocol. Arch Biochem Biophys. 1955 Feb;54(2):486–500. doi: 10.1016/0003-9861(55)90061-8. [DOI] [PubMed] [Google Scholar]
- GOLDBERG B., GREEN H. AN ANALYSIS OF COLLAGEN SECRETION BY ESTABLISHED MOUSE FIBROBLAST LINES. J Cell Biol. 1964 Jul;22:227–258. doi: 10.1083/jcb.22.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN H., GOLDBERG B. COLLAGEN SYNTHESIS BY HUMAN FIBROBLAST STRAINS. Proc Soc Exp Biol Med. 1964 Oct;117:258–261. doi: 10.3181/00379727-117-29551. [DOI] [PubMed] [Google Scholar]
- Green H., Goldberg B. Synthesis of collagen by mammalian cell lines of fibroblastic and nonfibroblastic origin. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1360–1365. doi: 10.1073/pnas.53.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON S. F., SMITH R. H. Studies on the biosynthesis of collagen. I. The growth of fowl osteoblasts and the formation of collagen in tissue culture. J Biophys Biochem Cytol. 1957 Nov 25;3(6):897–912. doi: 10.1083/jcb.3.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEROY E. C., KAPLAN A., UDENFRIEND S., SJOERDSMA A. A HYDROXYPROLINE-CONTAINING, COLLAGEN-LIKE PROTEIN IN PLASMA AND A PROCEDURE FOR ITS ASSAY. J Biol Chem. 1964 Oct;239:3350–3356. [PubMed] [Google Scholar]
- LEVENE C. I., GROSS J. Alterations in state of molecular aggregation of collagen induced in chick embryos by beta-aminopropionitrile (lathyrus factor). J Exp Med. 1959 Nov 1;110:771–790. doi: 10.1084/jem.110.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene C. I. The effect of lathyrogenic compounds on morphology and growth of cultured cells. Lab Invest. 1968 Jul;19(1):25–28. [PubMed] [Google Scholar]
- MERCHANT D. J., KAHN R. H. Fiber formation in suspension cultures of L strain fibroblasts. Proc Soc Exp Biol Med. 1958 Feb;97(2):359–362. doi: 10.3181/00379727-97-23743. [DOI] [PubMed] [Google Scholar]
- PETERKOFSKY B., PROCKOP D. J. A method for the simultaneous measurement of the radioactivity of proline-C14 and hydroxyproline-C14 in biological materials. Anal Biochem. 1962 Nov;4:400–406. doi: 10.1016/0003-2697(62)90141-0. [DOI] [PubMed] [Google Scholar]
- Piez K. A. Molecular weight determination of random coil polypeptides from collagen by molecular sieve chromatography. Anal Biochem. 1968 Nov;26(2):305–312. doi: 10.1016/0003-2697(68)90342-4. [DOI] [PubMed] [Google Scholar]
- Pinnell S. R., Martin G. R. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc Natl Acad Sci U S A. 1968 Oct;61(2):708–716. doi: 10.1073/pnas.61.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest R. E., Davies L. M. Cellular proliferation and synthesis of collagen. Lab Invest. 1969 Aug;21(2):138–142. [PubMed] [Google Scholar]
- RUBIN A. L., PFAHL D., SPEAKMAN P. T., DAVISON P. F., SCHMITT F. O. Tropocollagen: significance of protease-induced alterations. Science. 1963 Jan 4;139(3549):37–39. doi: 10.1126/science.139.3549.37. [DOI] [PubMed] [Google Scholar]
- SCHMITT F. O. Contributions of molecular biology to medicine. Bull N Y Acad Med. 1960 Nov;36:725–749. [PMC free article] [PubMed] [Google Scholar]
- Schiffmann E., Lavender D. R., Miller E. J., Corcoran B. A. Amino acids at the nucleating site in mineralizing elastic tissue. Calcif Tissue Res. 1969;3(2):125–135. doi: 10.1007/BF02058655. [DOI] [PubMed] [Google Scholar]


