Abstract
Haemophilus parasuis causes Glässer's disease, a syndrome of polyserositis, meningitis, and arthritis in swine. Previous studies with H. parasuis have revealed virulence disparity among isolates and inconsistent heterologous protection. In this study, virulence, direct transmission, and heterologous protection of 4 isolates of H. parasuis (SW114, 12939, MN-H, and 29755) were evaluated using a highly susceptible pig model. In an initial experiment, isolates 12939, MN-H, and 29755 caused Glässer's disease, while strain SW114 failed to cause any clinical signs of disease. One pig from each group challenged with MN-H or 29755 failed to develop clinical disease but was able to transmit H. parasuis to noninfected pigs, which subsequently developed Glässer's disease. Pigs colonized with SW114, 29755, or MN-H that were free of clinical disease were protected from a subsequent challenge with isolate 12939. In a following experiment, pigs vaccinated with strain SW114 given as either a bacterin intramuscularly or a live intranasal vaccine were protected from subsequent challenge with isolate 12939; however, some pigs given live SW114 developed arthritis. Overall these studies demonstrated that pigs infected with virulent isolates of H. parasuis can remain healthy and serve as reservoirs for transmission to naive pigs and that heterologous protection among H. parasuis isolates is possible. In addition, further attenuation of strain SW114 is necessary if it is to be used as a live vaccine.
INTRODUCTION
Haemophilus parasuis is a Gram-negative, NAD-dependent bacterium of the family Pasteurellaceae that causes Glässer's disease in swine, which is characterized by systemic invasion and bacteremia, resulting in a syndrome characterized by polyserositis (peritonitis, pleuritis, pericarditis), meningitis, and arthritis. Clinical signs of disease include fever, depression, anorexia, swollen joints with lameness, dyspnea, and central nervous system signs. Development of disease is sporadic but can be devastating when it occurs (1). H. parasuis can also cause pneumonia in swine without signs of systemic disease (2–4). Disease patterns suggest that maternal antibody may provide early protection, allowing piglets to be colonized without resulting disease while developing an active immune response (5). This is supported by experimental studies demonstrating that exposure of neonatal piglets to the predominant virulent strain on the farm can be used as a method of prophylaxis (6). Also, it is difficult to experimentally reproduce systemic disease in conventionally reared pigs, and colostrum-deprived pigs are often used as an experimental model (7, 8). Various practices, such as early weaning, may result in intermittent colonization of piglets, resulting in exposure to the bacterium when pigs are mixed in the nursery or later after maternal antibody levels have waned. Elevated health status may result in the lack of specific maternal antibody to a diverse number of strains, leaving piglets vulnerable, while coinfection with other bacteria and viruses may also increase the susceptibility to infection and disease.
Fifteen serovars of H. parasuis have been identified, but many isolates are nontypeable (9). Serovars 4 and 5 are isolated most frequently in conjunction with clinical disease in the United States (10). Experimental evidence indicates that H. parasuis may colonize the nasal cavity and trachea initially, resulting in loss of cilia and damage to the ciliated epithelium even though the organism does not appear to be closely associated with these cells (11, 12). Damage to the mucosal epithelial cells may facilitate invasion (11, 12). H. parasuis has also been shown to invade epithelial and endothelial cells in vitro (13–15). After initial colonization, there is bacterial invasion resulting in bacteremia and systemic spread of H. parasuis. Virulence has been associated with serum and phagocytosis resistance and the ability to invade endothelial cells (14, 16, 17). Specific virulence factors are largely unknown, but capsule and certain variants of trimeric autotransporters (VtaA) have been found to be associated with pathogenic strains and phagocytosis resistance, and the outer membrane protein P2 has been associated with serum resistance (17–20). It is not unusual for a number of different strains to be found in a herd or even in the same animal, and it is believed that nonpathogenic strains commonly colonize the nasal cavities of swine (21–23). Reports have been mixed as to the degree of heterologous protection among strains (24). This may be due to relatedness of the strains, exposure to live bacteria on a mucosal surface versus bacterins given parenterally, and prior colonization status of the animal.
In the current study, we examined the virulence, direct transmission, and heterologous protection of 4 isolates of H. parasuis in a highly susceptible population of pigs. In an initial experiment, 3 of the 4 isolates were found to be pathogenic (12939, 29755, and MN-H), while one caused no signs of disease despite colonizing the nasal cavity (SW114). We also demonstrated direct transmission of the virulent isolates from clinically healthy animals to naive pigs, resulting in systemic disease. In a second experiment, heterologous protection was demonstrated using the SW114 isolate as either a bacterin or live vaccine. However, arthritis occurred in some of the pigs vaccinated with the live SW114, indicating that further attenuation of this strain would be necessary if it was to be used as a live vaccine.
MATERIALS AND METHODS
H. parasuis isolates and inocula.
H. parasuis 29755 is a serovar 5 isolate cultured from the lung of a pig with Glässer's disease and has previously been shown to be virulent in colostrum-deprived pigs (7, 8). H. parasuis 12939 was isolated from the lung of a pig with Glässer's disease that was also coinfected with porcine reproductive and respiratory syndrome virus. It has reacted to both serovar 1 and 4 typing serum, as well as having been reported as nontypeable. H. parasuis MN-H is a serovar 13 isolate from a severe epidemic of mortality in pigs that had signs consistent with Glässer's disease. Porcine circovirus type 2, bovine viral diarrhea virus, Pasteurella multocida, and Arcanobacterium pyogenes were also isolated from the outbreak when MN-H was isolated. H. parasuis SW114 is the serovar 3 reference strain and was isolated from the nasal cavity of a healthy pig (10). To prepare the inocula, all H. parasuis isolates were cultured on Casman's agar supplemented with 5% horse serum and 1% NAD at 37°C in 5% CO2 for 24 h. A culture suspension with an A600 of 0.42 was prepared in phosphate-buffered saline (PBS), which typically yields approximately 2 × 108 CFU/ml, and pigs were inoculated intranasally with 1 ml (0.5 ml/nostril) of this suspension. PBS was used as a sham inoculum.
Bacterin preparation.
The SW114 strain of H. parasuis was cultured on Casman's agar at 37°C in 5% CO2 for 24 h, and a culture suspension with an A600 of 0.42 was prepared in PBS. Subsequently, 10% buffered formalin was added to a final concentration of 0.25%, and the preparation was allowed to sit at room temperature overnight with mixing and then stored at 4°C. A Casman plate was streaked with 100 μl of the undiluted preparation. No growth was observed after 48 h of incubation at 37°C in 5% CO2. The suspension was centrifuged for 15 min at 6,000 × g, and the pellet was washed once in PBS and then resuspended in a combination of PBS and Emulsigen D adjuvant sufficient to yield the equivalent of 109 CFU/ml in 20% adjuvant.
Source of pigs.
Pigs for both animal experiments were obtained from an experimental herd. The original pigs from this facility were derived by caesarian-derived, colostrum-deprived (CDCD) methodology and maintained in a HEPA-filtered barrier with irradiated pig feed and filtered, chlorinated, UV-treated water. All pigs derived from this population were raised in this facility by normal production methods. Sentinel pigs were euthanized monthly, and full diagnostic workups were conducted to ensure freedom of swine pathogens. In addition, if there were any unexpected clinical signs or mortality, these pigs were also euthanized and given a full diagnostic workup. Diagnostic monitoring included gross pathology, histopathology, serology, virus isolation, fecal float, electron microscopy, bacterial culture, select immunofluorescence testing and immunohistochemistry procedures, and select molecular diagnostic testing, including PCR. Many of the agents were screened by multiple procedures. Pathogens that the herd of origin was determined to be free of by diagnostic testing were as follows: Actinobacillus spp., Giardia spp., pseudorabies virus, Bordetella bronchiseptica, hemagglutinating encephalomyelitis virus, porcine respiratory coronavirus, bovine viral diarrhea virus, Haemophilus spp., rotavirus, Chlamydia spp., hepatitis E virus, Salmonella spp., circovirus, influenza virus, Streptococcus suis, Cryptosporidium spp., Leptospira spp. (5 serovars), Toxoplasma gondii, cytomegalovirus, Mycoplasma spp., transmissible gastroenteritis virus, encephalomyocarditis virus, Pasteurella spp., Trichinella spiralis, enterovirus G1 to G8, porcine parvovirus, vesicular stomatitis virus, Erysipelothrix spp., porcine reproductive and respiratory syndrome virus, Yersinia spp.
Original pigs were derived from Large White × Landrace females and boars that were F2s from Large White × Landrace females and synthetic sire lines (PIC L24 and L26). Mating programs were designed to minimize inbreeding. Sows were bred by fresh artificial insemination, gestated in individual pens, and farrowed in traditional farrowing crates. At 24- to 28-day weaning, all pigs were moved to growth pens. Appropriate feed for pig ages was given. No antibiotics (feed, water, or injection) or vaccines were ever used in the 10-year period from facility derivation until the start of this study.
The animals were moved from the source herd to the National Animal Disease Center, where they were housed in biosafety level 2 (BSL-2) animal isolation facilities and cared for in compliance within the guidelines of the National Animal Disease Center Institutional Animal Care and Use Committee. Nasal swabs were taken from each pig the day that both animal experiments began and were negative for H. parasuis by culture.
Experiment 1 design.
Twenty-five pigs from 4 litters were distributed into 5 groups of 5 pigs such that each group contained 1 pig each from 3 of the litters (one each that was 36, 53, or 68 days old) and 2 pigs each from the fourth litter (48 days old). One group each was challenged intranasally with 1 of the 4 isolates of H. parasuis (29755, 12939, MN-H, and SW114) or sham inoculated with PBS. Cultured dilutions indicated that the inocula contained 108.31 CFU/ml (SW114), 108.68 CFU/ml (12939), 108.32 CFU/ml (29755), or 107.23 CFU/ml (MN-H). One week after challenge, nasal swabs were taken from all surviving pigs and one sham-inoculated pig was cohoused with each of the healthy survivors of the 29755 and MN-H groups. Three weeks after challenge, nasal swabs were taken from all surviving pigs and they were rechallenged intranasally with 108.83 CFU of H. parasuis isolate 12939. During the course of the experiment, pigs were examined for clinical signs approximately every 4 h, except for an 8-h overnight period, and any pig showing signs of Glässer's disease, such as joint swelling and lameness, incoordination, tremors, severe depression, or reluctance to move was immediately euthanized. Remaining pigs were euthanized 10 days after secondary challenge. At the time of euthanasia, swabs of nasal cavity, lung, joint, abdominal cavity, thoracic cavity, and meninges were collected and agitated in 1 ml of PBS, and 100 μl of this was cultured on Casman's plates.
Experiment 2 design.
Eighteen pigs from 8 different litters that were 5 to 6 weeks old were randomly divided into 4 groups. Group 1 consisted of 4 pigs that were intramuscularly (i.m.) vaccinated with a bacterin made from H. parasuis strain SW114. One milliliter of the adjuvanted bacterin was given twice, once on day 0 and again 3 weeks later. Group 2 consisted of 8 pigs that were given intranasal live SW114 as a vaccine on day 0 of the experiment. Groups 3 (n = 4) and 4 (n = 2) consisted of nonvaccinated pigs. On day 85 of the experiment, pigs in groups 1 to 3 were challenged intranasally with 108.48 CFU of H. parasuis isolate 12939. Nasal swabs were taken on days 0, 7, 14, 21, and 35 of the experiment, and blood was collected for sera on days 0 and 85. After challenge with H. parasuis isolate 12939, pigs were examined for clinical signs approximately every 4 h, except for an 8-h overnight period, and any pigs showing signs of Glässer's disease were euthanized immediately; surviving pigs were euthanized 10 days after challenge. At the time of euthanasia, swabs were collected and cultured as described for experiment 1.
Serology.
Sera collected from experiment 2 were submitted to the University of Minnesota Veterinary Diagnostic Laboratory to run their H. parasuis OppA enzyme-linked immunosorbent assay (ELISA).
An assay was also performed using sera from vaccinated pigs to evaluate antibody-mediated complement killing of homologous (SW114) and heterologous (12939) strains of H. parasuis and to determine if this correlated with protection. Heat-inactivated (HI) pre- and postvaccination sera from each of the pigs in experiment 2 were used as the antibody source individually, and non-HI serum from a CDCD pig was used as a complement source. For each sample tested, 20 μl of HI serum (heated to 56°C for 1 h) from the pigs was added to 70 μl of non-HI CDCD serum in one well of a 96-well plate, followed by the addition of 10 μl (∼106 CFU) of H. parasuis prepared by the same method as the inoculum for the pig studies. The samples were incubated at 37°C for 1 h, after which 10-fold serial dilutions of each sample were cultured on duplicate Casman's plates. The plates were incubated at 37°C for 48 h, and the colonies were counted. As controls, the number of CFU in the inoculum, the number of CFU after incubation with 90 μl of HI CDCD serum, and the number of CFU after incubation with 20 μl HI CDCD serum plus 70 μl of non-HI CDCD serum were also determined.
Statistics.
Prism software (version 5.04; GraphPad Software, San Diego, CA) was used for all statistical analyses. Two-way analysis of variance (ANOVA) with Bonferroni multiple-comparison posttest was used for analyzing serology data.
RESULTS
Experiment 1.
Pigs were challenged with 1 of 4 isolates of H. parasuis to determine isolate pathogenicity and virulence in a naive pig model of infection. Pigs challenged with 3 of the 4 H. parasuis isolates (12939, 29755, and MN-H) developed signs of Glässer's disease, including joint swelling and lameness, tremors, depression, and reluctance to move. Of the pigs challenged with isolate 12939, one died without premonitory signs on day 3 postinfection and the rest were euthanized due to signs of Glässer's disease between 1 and 3 days postinfection. Four of the 5 pigs inoculated with isolate 29755 were euthanized due to signs of Glässer's disease between 3 and 4 days postinfection, and 4 of the 5 pigs inoculated with isolate MN-H were euthanized due to signs of Glässer's disease between 2 and 5 days postinfection. Gross lesions observed in these pigs typically consisted of serosal effusions of amber-colored fluid and mild pneumonia; significant fibrin deposition on the serosal surfaces was not observed. One pig each from the 29755- and MN-H-challenged groups and all pigs challenged with strain SW114 remained healthy and showed no signs of disease throughout the experiment. H. parasuis was isolated from nasal swabs of all surviving infected pigs at 1 week and 3 weeks postinfection, indicating that all pigs were colonized. None of the sham-inoculated pigs developed disease, nor was H. parasuis isolated from nasal swabs taken from these pigs. Table 1 summarizes the ages of pigs, when they were euthanized, and sites from which H. parasuis was cultured.
Table 1.
Summary of age at time of challenge, day postinfection that pig died or was euthanized, and culture results for the 3 H. parasuis strains that caused disease in experiment 1
| Pig | Group | Age (days) | Day p.i.a | Culture result for: |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Nasal cavity | Lung | Thorax | Abdomen | Joint | Meninges | ||||
| 974 | MN-H | 68 | 5 | + | − | − | − | + | NCb |
| 979 | MN-H | 53 | 5 | + | + | − | − | − | + |
| 4263 | MN-H | 48 | 4 | + | − | − | + | + | − |
| 4265 | MN-H | 48 | 2 | + | − | − | − | + | − |
| 984 | MN-H | 36 | Survived | + | NC | NC | NC | NC | NC |
| 973 | 29755 | 68 | 4 | + | − | + | + | + | NC |
| 978 | 29755 | 53 | 4 | + | + | + | + | + | + |
| 4262 | 29755 | 48 | 4 | + | − | + | + | + | + |
| 4264 | 29755 | 48 | 3 | + | − | + | + | + | + |
| 983 | 29755 | 36 | Survived | + | NC | NC | NC | NC | NC |
| 972 | 12939 | 68 | 2 | + | − | + | + | + | NC |
| 977 | 12939 | 53 | 3 | + | + | + | + | + | + |
| 4260 | 12939 | 48 | 3 | + | + | + | − | + | + |
| 4261 | 12939 | 48 | 1 | + | + | + | + | + | + |
| 982 | 12939 | 36 | 2 | + | + | − | − | − | − |
Day postinfection (p.i.) that pig died or was euthanized.
NC, not cultured.
One week after the initial challenge, one sham-inoculated pig each was placed in with the survivors that had been challenged with isolate 29755 or MN-H to determine if the colonized but clinically healthy pig would transmit H. parasuis to the naive pig and cause disease. Five days after the naive pig was placed in with the MN-H survivor, the contact pig showed signs of Glässer's disease and was euthanized. This pig had gross lesions of polyserositis, with large amounts of fibrin deposited on the serosal surfaces, and H. parasuis was isolated from all sites sampled except the meninges. Seven days after the naive pig was placed in with the 29755 survivor, the contact pig became lame with a swollen hock and was euthanized. H. parasuis was isolated from the nasal cavity and the joint.
Three weeks after primary challenge, the 10 surviving pigs (3 sham-inoculated pigs [naive], 5 SW114-challenged pigs, 1 29755-challenged pig, and 1 MN-H-challenged pig) were challenged intranasally with H. parasuis isolate 12939 to determine whether they would be protected from heterologous challenge. One naive pig died without premonitory signs 2 days postchallenge, and the other 2 naive pigs were euthanized between 1 and 3 days postchallenge due to signs of Glässer's disease. Gross findings and culture results were similar to those from the pigs originally challenged with this isolate. The 2 pigs previously inoculated with isolate 29755 or MN-H and all 5 pigs previously inoculated with SW114 remained healthy. These pigs were euthanized 10 days after secondary challenge, and no gross lesions were noted. H. parasuis was isolated from the nasal cavity and lungs of the pig originally inoculated with isolate 29755, from the nasal cavity of the pig originally inoculated with isolate MN-H, and from the nasal cavity of 1 of the 5 pigs originally inoculated with isolate SW114. No H. parasuis was isolated from systemic sites (joint, abdominal cavity, thoracic cavity, or meninges) in any of these pigs.
Experiment 2.
A second experiment was conducted to further explore whether strain SW114 could induce heterologous protection given as either a bacterin i.m. or a live intranasal vaccine. Of the 8 pigs in group 2 that were intranasally inoculated with live SW114 culture, 4 developed swollen hocks and lameness on days 2 to 5 postinoculation. H. parasuis was cultured from a joint tap performed on one of the pigs. DNA sequence analysis of the ompP5 gene of this isolate revealed that its sequence was identical to the sequence of the SW114 ompP5 gene, which is otherwise unique in our culture collection (25) and is also unique among ∼140 H. parasuis complete ompP5 sequences currently available in GenBank. Pigs that developed lameness were treated with one injection of ceftiofur (Excede; Pfizer) in accordance with the drug label and recovered uneventfully. H. parasuis was isolated from the nasal swabs of all pigs inoculated with live SW114 on days 7, 14, and 21 postinoculation (regardless of treatment with ceftiofur) and from about one-half of these pigs on day 35 postinoculation. H. parasuis was not isolated from the nasal swabs of the pigs in any of the other groups at any time.
After challenge with isolate 12939 of H. parasuis, the 4 nonvaccinated pigs in group 3 developed signs of Glässer's disease, including lethargy, swollen joints, lameness, and neurologic signs. All 4 pigs were euthanized 2 days postchallenge, and H. parasuis was isolated from the nasal cavity, trachea, and at least 1 systemic site (2/4 thoracic cavities, 1/4 abdominal cavities, 3/4 joints, and/or meninges) in all 4 pigs and from the lungs of 2 of the pigs. No clinical signs were seen in any of the pigs in groups 1 and 2 that were vaccinated i.m. with the SW114 bacterin or intranasally with the live culture of SW114 or in the 2 nonvaccinated, nonchallenged pigs of group 4. Ten days after challenge, pigs in groups 1, 2, and 4 were euthanized and necropsied. No gross lesions were observed, and H. parasuis was isolated from the nasal cavity of 1 pig each in group 1 and group 2, but from no other sites.
Serology.
Three of the 4 pigs vaccinated i.m. with the SW114 bacterin, but none of the pigs from the other groups, were positive for H. parasuis antibody as measured by the OppA ELISA using the sera taken just prior to challenge with isolate 12939 (experimental day 85) (data not shown). Sera from pigs in experiment 1 were not evaluated.
To evaluate antibody-mediated complement killing of homologous and heterologous strains of H. parasuis and determine if this in vitro phenotype correlated with in vivo protection, we performed a serum sensitivity assay using H. parasuis strains SW114 and 12939 (Fig. 1). There was a small but statistically significant reduction in numbers of viable SW114 and 12939 cells after exposure to non-HI CDCD serum but not HI CDCD serum (Fig. 1A). No additional reduction in viable bacteria was seen with either H. parasuis strain when prevaccination sera were used as the antibody source in the assay (Fig. 1B and C). Conversely, there was a statistically significant reduction in SW114 and 12939 viability when sera collected on day 85 postvaccination were used as the antibody source (Fig. 1B and C). While sera from nonvaccinated pigs at day 85 did result in a moderate, though significant, reduction in bacteria as well, the reduction was less, and statistically different, from that observed with postvaccination sera (Fig. 1B and C). There was no difference in killing of SW114 between sera collected from pigs vaccinated with bacterin and the live SW114 (Fig. 1B). Ceftiofur treatment did have a significant impact on the development of the antibody response following live SW114 immunization, as the abilities to kill 12939 were found to be significantly different when postvaccination sera collected from treated and nontreated pigs were compared (Fig. 1C). Specifically, sera collected from live-SW114-immunized pigs that were not treated with ceftiofur were able to completely kill 12939; however, the killing capacity of sera from pigs treated with ceftiofur was identical to that of sera from bacterin-immunized pigs. This difference in sensitivity was not seen with strain SW114, as SW114 was killed when postvaccination sera from ceftiofur-treated pigs was used as an antibody source (data not shown).
Fig 1.
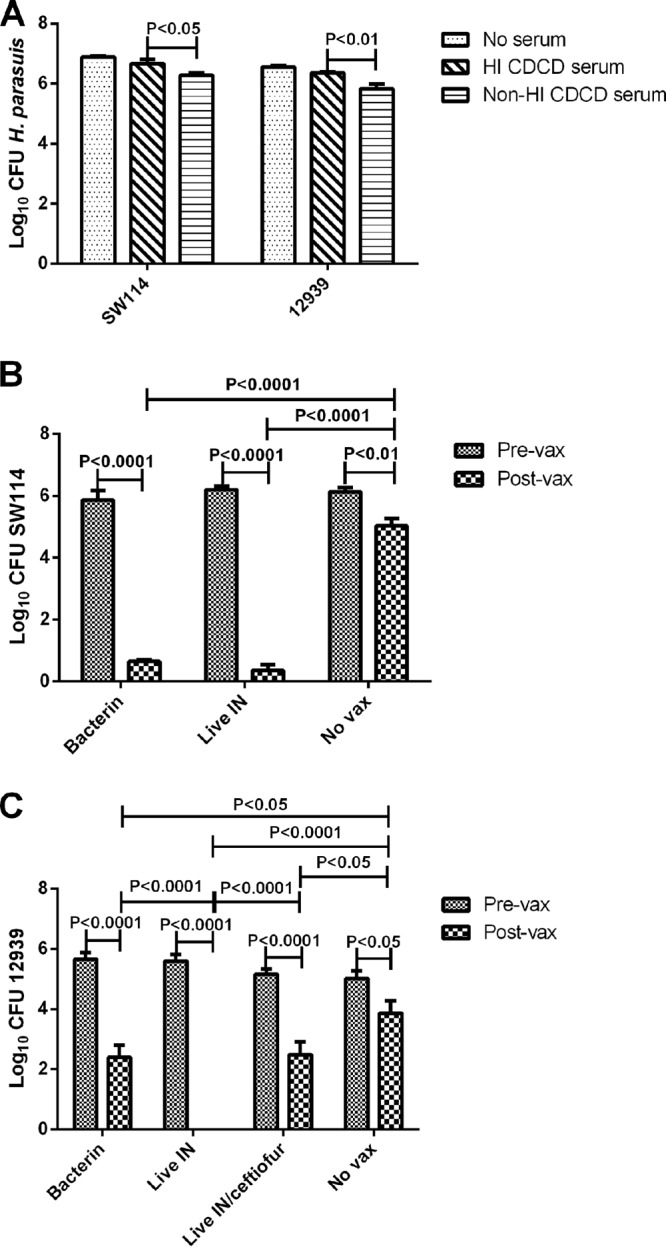
Complement-mediated killing of H. parasuis strains SW114 and 12939. (A) Log10 CFU of H. parasuis SW114 and 12939 after incubation for 1 h in PBS without added serum, with heat-inactivated (HI) caesarian-derived, colostrum-deprived (CDCD) pig serum, or with non-HI CDCD pig serum (bars represent the means ± standard errors of 5 replicates). (B and C) Log10 CFU of H. parasuis SW114 (B) and 12939 (C) after a 1-h incubation with non-HI CDCD pig serum as a complement source and HI prevaccination (Pre-vax) or postvaccination (Post-vax) sera collected from pigs vaccinated with SW114 bacterin intramuscularly (bacterin) or live SW114 intranasally (Live IN) or not vaccinated (No vax) in experiment 2 (bars represent the means ± standard errors). For data in panel C, the group receiving SW114 intranasally was separated into 2 groups depending on ceftiofur treatment as described in Results. Capped lines indicate statistical significance.
DISCUSSION
To date, little is known about genetic differences among H. parasuis isolates and genetic factors that contribute to H. parasuis virulence. One of the goals of this study was to directly compare the virulences of a set of H. parasuis isolates so as to identify strains with divergent virulences for future genome sequence comparisons. Based on previous experimental work and/or diagnostic information, it was expected that isolates 29755, 12939, and MN-H would be pathogenic with various virulences and strain SW114 would be nonpathogenic (7–9). Results from experiment 1 indicate that 3 of the 4 isolates are capable of causing systemic disease. Disease developed most rapidly in the pigs challenged with isolate 12939, and H. parasuis was isolated from numerous systemic sites. Disease signs developed slightly later in the groups challenged with 29755 and MN-H, and one pig remained healthy in both groups. H. parasuis was more broadly distributed in pigs challenged with 29755 than in those challenged with MN-H. Interestingly, the survivors from both the 29755 and the MN-H groups were from the same litter, which were the youngest pigs to be challenged. While this may be a coincidence, it is possible that there was an age or genetic effect on systemic disease progression.
The fourth strain, SW114, caused no observable disease in experiment 1. In the second experiment, however, H. parasuis with genetic evidence confirming that it was strain SW114 was isolated from arthritic joints. These pigs recovered quickly and without complications after treatment with ceftiofur, but it is unknown whether disease would have progressed had treatment not been initiated. This strain was believed to be nonpathogenic based on previous reports where it was given either intranasally or intraperitoneally to specific-pathogen-free pigs with no resulting clinical signs of disease and no gross lesions seen at necropsy (9, 26, 27). It is a strain that was originally isolated from the nasal cavity of a healthy pig (28). Strain SW114, along with all type strains for serovars 1 to 7, was virulent when given intraperitoneally to guinea pigs. However, when SW114 was given by the intratracheal route to guinea pigs, only transient clinical signs and minimal evidence for infection were present at necropsy, in contrast to results for some of the other type strains that are considered more virulent and caused disease by this route (29). Thus, although this strain appears to be less virulent than the others, it retains the ability to cause systemic disease under certain conditions that have yet to be fully identified.
Direct transmission of H. parasuis from intranasally challenged pigs with clinical signs of disease to naive pigs has been shown to occur previously (30). In the current study, naive pigs placed in direct contact with healthy pigs colonized with 29755 or MN-H displayed clinical signs of disease within a week, while the 2 source pigs continued to remain healthy. These results indicate that subclinical infections with virulent strains occur in some pigs and that these animals can then serve as reservoirs for transmission. In addition, our results provide evidence that an H. parasuis isolate cultured from the upper respiratory tract of a healthy pig is not necessarily avirulent.
In view of the results from the first experiment, a second experiment was designed to further test whether strain SW114 could be used as a vaccine and determine whether administering it as a live intranasal vaccine would offer better protection than a bacterin. In general, it has been shown that piglets vaccinated with a bacterin are protected against challenge with the homologous strain of H. parasuis. However, reports have been inconsistent as to the degree of protection afforded bacterin-vaccinated piglets subsequently challenged with heterologous strains (31–33). Furthermore, there is evidence that the virulence of the vaccine strain may make a difference in the degree of protection seen with bacterins (34). Conversely, in a small experiment examining the virulence of H. parasuis strains, pigs exposed to an aerosol of nonpathogenic serotypes later resisted challenge with a virulent isolate, indicating that mucosal exposure to live bacteria may induce cross-protective immunity (27).
In our study, SW114 protected against heterologous challenge when given as a bacterin or a live vaccine. Given prior reports, we expected the live vaccine to be superior to the bacterin in inducing heterologous protection. Live bacterial vaccines have been shown in many cases to be more effective at eliciting broad immune responses involving both antibody- and cell-mediated immunity. Also, live vaccines are often superior at stimulating mucosal immunity, which may be crucial for controlling H. parasuis colonization and disease. The variability in results of previous studies examining cross-protection with bacterins may be explained by examining the relatedness of the isolates used in the studies and/or the prior colonization/immune status of the piglets used. For instance, in young pigs maternally derived immunity often interferes with the development of an active immune response to a vaccine, especially with bacterins that largely induce a systemic antibody response. Since we used naive pigs, no such interference would have occurred. Thus, whether both vaccines would be equally efficacious in the face of maternal immunity still needs to be investigated. Unfortunately, the fact that SW114 demonstrated some virulence makes it a less attractive candidate as a live vaccine. However, further rational attenuation may make it a more suitable candidate, or identification of other nonpathogenic strains may be undertaken. We are currently carrying out further sequence and virulence comparisons of these strains and other H. parasuis isolates that may identify virulence factors and possible attenuation targets.
To develop better vaccines for H. parasuis, factors that correlate with protection need to be identified; in particular, antemortem serological antibody assays are an ideal target. Thus, we used sera from the pigs in our experiment to evaluate vaccine-induced antibodies in an antibody-mediated, complement killing assay, which revealed some interesting results. First, there was little difference between strain SW114 and 12939 in sensitivity to killing with nonimmune sera obtained from CDCD pigs. This is somewhat contradictory to a previous report indicating that nasal isolates, considered to be of low virulence, were more sensitive to serum killing (16). SW114 and 12939 were not tested in the previous report. Although all the nasal isolates tested were sensitive to complement killing in the previous report, there was a range of susceptibility seen with these isolates, and the fact that we observed that SW114 has some pathogenic capability may explanation this inconsistency. Also, the sources of sera used were different in our study and the previous one. In our study we used swine sera from a CDCD pig as the complement source and heat-inactivated sera from the experimental animals as an antibody source, while in the previous study sera from conventional animals was used; thus, the levels of specific and nonspecific antibodies may have been different for the sera used in the two studies. Differences in culture conditions, which may alter characteristics such as capsule production, could account for discrepancies as well.
Postvaccination sera were much more effective at killing H. parasuis than were prevaccination sera, and this is also in contrast to the same prior report wherein use of immune serum did not increase the sensitivity of H. parasuis to complement killing (16). Again this may be due to the individual strains and experimental specificities of the different experiments. In the previous report antibodies raised to a highly virulent strain, Nagasaki, did not appear to enhance serum sensitivity to the homologous strain or 2 heterologous strains (nasal and systemic isolates). Certain highly virulent strains, like Nagasaki, may be resistant to killing whether homologous antibodies are present or not, potentially due to inherent traits such as capsule type or production. The nature of the antibody response induced by the Nagasaki strain may be different and in part the reason for its high virulence potential. Cross-reactivity of induced antibodies may be specific to the particular strains used in each study as well, and this may explain the lack of antibody-enhanced killing in the previous study. In general, postvaccination sera in our study were not as effective at killing strain 12939, possibly due to the heterologous specificities of the antibodies. There was a slight increase in sensitivity of both strains SW114 and 12939 to sera from nonvaccinated pigs taken on day 85 of the experiment compared to sera from day 0. A possible explanation for this phenomenon is exposure to commensal bacterial flora over time, which may have induced some nonspecific, cross-reacting antibody. The outer membrane protein P2 has been implicated in conferring serum resistance to H. parasuis, and mutants lacking P2 have been shown to induce complement activation through the classical pathway by the binding of IgG to other outer membrane proteins (20, 35).
Another interesting finding in the antibody-mediated complement killing assay was the difference between postvaccination sera from the ceftiofur-treated and nontreated pigs given the live SW114 as a vaccine with regard to their ability to kill isolate 12939. Sera from pigs not treated with the antibiotic were more effective at mediating killing of 12939 than were sera from ceftiofur-treated pigs. It is important to note that treatment with ceftiofur was not effective in clearing H. parasuis from the nasal cavity. Systemic invasion by H. parasuis in the antibiotic-treated pigs failed to induce an enhanced systemic antibody response sufficient to achieve an increase in complement-mediated, antibody-dependent killing. The ELISA detects serum IgG specific for the OppA protein, and development of OppA-specific antibody appears to occur selectively in survivors of systemic disease but not in healthy, colonized pigs (S. Oliveira, personal communication). However, only 3 of 4 pigs given bacterin developed an OppA-specific serum antibody response, and none of the pigs given live SW114 as a vaccine developed an OppA-specific serum antibody response, regardless of ceftiofur treatment. It is possible that the live SW114-vaccinated, non-ceftiofur-treated pigs had a clinically inapparent systemic infection and/or established infection lower in the respiratory tract that did not result in obvious clinical disease but did result in the development of an antibody response that significantly aided in the ability to kill 12939. These data provide evidence that intranasal delivery of a live strain of H. parasuis induces a broader antibody response; however, rational attenuation is likely necessary to prevent vaccine-induced disease.
Pigs used in this experiment were unique in that they were obtained from an experimental herd kept in isolation in an ultraclean barrier environment for transgenic research purposes. The original stock was derived by caesarian section, but subsequent generations were allowed to farrow and suckle normally. They consistently tested negative for all common swine pathogens. Previous challenge studies that we have undertaken in conventional pigs with some of the same H. parasuis strains used in this study have provided results not entirely consistent with those reported here. While we have been able to sporadically reproduce systemic disease with some of these strains in conventional pigs, more often only the pigs' respiratory tracts become colonized and, at most, mild pneumonia results (our unpublished data). The results obtained with the pigs used in this study also support the concept that Glässer's disease can occur in the absence of coinfections with common pig viruses such as porcine reproductive and respiratory syndrome virus and porcine circovirus. Thus, the pigs used in the current study appeared to be exquisitely susceptible to challenge with H. parasuis and of great use for virulence studies because it would not be necessary to use colostrum-deprived pigs.
The combination of animal challenge studies and genomic comparisons under way in our laboratory will help identify differences among strains with disparate virulence profiles, hopefully leading to discoveries of virulence factors and potential immunological targets for vaccine development. Bioinformatics approaches will be used to discover genes that are conserved among strains as well as those that are unique. These results will provide a basis for testing the role of potential virulence genes in swine infection studies and may also allow development of detection assays that discriminate between strains of high and low virulence.
ACKNOWLEDGMENTS
We thank Fios Therapeutics, Inc., for the generous gift of pigs for this study. We also thank Trenton Ewing, Steven Kellner, Kim Driftmier, Gwen Nordholm, Sarah Shore, and Jessica Frerichs for their excellent technical assistance and Steve Adolphson, Dalene Whitney, and John Kent for their excellent animal care.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Published ahead of print 24 July 2013
REFERENCES
- 1.MacInnes JI, Desrosiers R. 1999. Agents of the “suis-ide diseases” of swine: Actinobacillus suis, Haemophilus parasuis, and Streptococcus suis. Can. J. Vet. Res. 63:83–89 [PMC free article] [PubMed] [Google Scholar]
- 2.Riley MG, Russell EG, Callinan RB. 1977. Haemophilus parasuis infection in swine. J. Am. Vet. Med. Assoc. 171:649–651 [PubMed] [Google Scholar]
- 3.Little TW. 1970. Haemophilus infection in pigs. Vet. Rec. 87:399–402 [DOI] [PubMed] [Google Scholar]
- 4.Brockmeier SL. 2004. Prior infection with Bordetella bronchiseptica increases nasal colonization by Haemophilus parasuis in swine. Vet. Microbiol. 99:75–78 [DOI] [PubMed] [Google Scholar]
- 5.Solano-Aguilar GI, Pijoan C, Rapp-Gabrielson V, Collins J, Carvalho LF, Winkelman N. 1999. Protective role of maternal antibodies against Haemophilus parasuis infection. Am. J. Vet. Res. 60:81–87 [PubMed] [Google Scholar]
- 6.Oliveira S, Batista L, Torremorell M, Pijoan C. 2001. Experimental colonization of piglets and gilts with systemic strains of Haemophilus parasuis and Streptococcus suis to prevent disease. Can. J. Vet. Res. 65:161–167 [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira S, Galina L, Blanco I, Canals A, Pijoan C. 2003. Naturally-farrowed, artificially reared pigs as an alternative model for experimental infection by Haemophilus parasuis. Can. J. Vet. Res. 67:146–150 [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco I, Galina-Pantoja L, Oliveira S, Pijoan C, Sanchez C, Canals A. 2004. Comparison between Haemophilus parasuis infection in colostrum-deprived and sow-reared piglets. Vet. Microbiol. 103:21–27 [DOI] [PubMed] [Google Scholar]
- 9.Kielstein P, Rapp-Gabrielson VJ. 1992. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J. Clin. Microbiol. 30:862–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapp-Gabrielson VJ, Gabrielson DA. 1992. Prevalence of Haemophilus parasuis serovars among isolates from swine. Am. J. Vet. Res. 53:659–664 [PubMed] [Google Scholar]
- 11.Vahle JL, Haynes JS, Andrews JJ. 1995. Experimental reproduction of Haemophilus parasuis infection in swine: clinical, bacteriological, and morphologic findings. J. Vet. Diagn. Invest. 7:476–480 [DOI] [PubMed] [Google Scholar]
- 12.Vahle JL, Haynes JS, Andrews JJ. 1997. Interaction of Haemophilus parasuis with nasal and tracheal mucosa following intranasal inoculation of cesarean derived colostrum deprived (CDCD) swine. Can. J. Vet. Res. 61:200–206 [PMC free article] [PubMed] [Google Scholar]
- 13.Vanier G, Szczotka A, Friedl P, Lacouture S, Jacques M, Gottschalk M. 2006. Haemophilus parasuis invades porcine brain microvascular endothelial cells. Microbiology 152:135–142 [DOI] [PubMed] [Google Scholar]
- 14.Aragon V, Bouchet B, Gottschalk M. 2010. Invasion of endothelial cells by systemic and nasal strains of Haemophilus parasuis. Vet. J. 186:264–267 [DOI] [PubMed] [Google Scholar]
- 15.Frandoloso R, Martinez-Martinez S, Gutierrez-Martin CB, Rodriguez-Ferri EF. 2012. Haemophilus parasuis serovar 5 Nagasaki strain adheres and invades PK-15 cells. Vet. Microbiol. 154:347–352 [DOI] [PubMed] [Google Scholar]
- 16.Cerda-Cuellar M, Aragon V. 2008. Serum-resistance in Haemophilus parasuis is associated with systemic disease in swine. Vet. J. 175:384–389 [DOI] [PubMed] [Google Scholar]
- 17.Olvera A, Ballester M, Nofrarias M, Sibila M, Aragon V. 2009. Differences in phagocytosis susceptibility in Haemophilus parasuis strains. Vet. Res. 40:24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pina S, Olvera A, Barcelo A, Bensaid A. 2009. Trimeric autotransporters of Haemophilus parasuis: generation of an extensive passenger domain repertoire specific for pathogenic strains. J. Bacteriol. 191:576–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa-Hurlado M, Ballester M, Galofre-Mila N, Darji A, Aragon V. 2012. VtaA8 and VtaA9 from Haemophilus parasuis delay phagocytosis by alveolar macrophages. Vet. Res. 43:57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B, Feng S, Xu C, Zhou S, He Y, Zhang J, Guo L, Liao M. 2012. Serum resistance in Haemophilus parasuis SC096 strain requires outer membrane protein P2 expression. FEMS Microbiol. Lett. 326:109–115 [DOI] [PubMed] [Google Scholar]
- 21.Oliveira S, Blackall PJ, Pijoan C. 2003. Characterization of the diversity of Haemophilus parasuis field isolates by use of serotyping and genotyping. Am. J. Vet. Res. 64:435–442 [DOI] [PubMed] [Google Scholar]
- 22.Olvera A, Cerda-Cuellar M, Nofrarias M, Revilla E, Segales J, Aragon V. 2007. Dynamics of Haemophilus parasuis genotypes in a farm recovered from an outbreak of Glasser's disease. Vet. Microbiol. 123:230–237 [DOI] [PubMed] [Google Scholar]
- 23.Cerda-Cuellar M, Naranjo JF, Verge A, Nofrarias M, Cortey M, Olvera A, Segales J, Aragon V. 2010. Sow vaccination modulates the colonization of piglets by Haemophilus parasuis. Vet. Microbiol. 145:315–320 [DOI] [PubMed] [Google Scholar]
- 24.Oliveira S, Pijoan C. 2004. Haemophilus parasuis: new trends on diagnosis, epidemiology and control. Vet. Microbiol. 99:1–12 [DOI] [PubMed] [Google Scholar]
- 25.Mullins MA, Register KB, Bayles DO, Nicholson TL, Loving CL, Brockmeier SL, Dyer DW, Phillips GJ. 2009. Characterization and comparative analysis of the genes encoding Haemophilus parasuis outer membrane proteins P2 and P5. J. Bacteriol. 191:5988–6002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kielstein P, Rosner H, Muller W. 1991. Typing of heat-stable soluble Haemophilus parasuis antigen by means of agar gel precipitation and the dot-blot procedure. J. Vet. Med. B Infect. Dis. Vet. Public Health 38:315–320 [DOI] [PubMed] [Google Scholar]
- 27.Nielsen R. 1993. Pathogenicity and immunity studies of Haemophilus parasuis serotypes. Acta Vet. Scand. 34:193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morozumi T, Nicolet J. 1986. Some antigenic properties of Haemophilus parasuis and a proposal for serological classification. J. Clin. Microbiol. 23:1022–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapp-Gabrielson VJ, Gabrielson DA, Schamber GJ. 1992. Comparative virulence of Haemophilus parasuis serovars 1 to 7 in guinea pigs. Am. J. Vet. Res. 53:987–994 [PubMed] [Google Scholar]
- 30.Amano H, Shibata M, Kajio N, Morozumi T. 1996. Pathogenicity of Haemophilus parasuis serovars 4 and 5 in contact-exposed pigs. J. Vet. Med. Sci. 58:559–561 [DOI] [PubMed] [Google Scholar]
- 31.Miniats OP, Smart NL, Ewert E. 1991. Vaccination of gnotobiotic primary specific pathogen-free pigs against Haemophilus parasuis. Can. J. Vet. Res. 55:33–36 [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi K, Naga S, Yagihashi T, Ikehata T, Nakano Y, Senna K, Maruyama T, Murofushi J. 2001. A cross-protection experiment in pigs vaccinated with Haemophilus parasuis serovars 2 and 5 bacterins, and evaluation of a bivalent vaccine under laboratory and field conditions. J. Vet. Med. Sci. 63:487–491 [DOI] [PubMed] [Google Scholar]
- 33.Bak H, Riising HJ. 2002. Protection of vaccinated pigs against experimental infections with homologous and heterologous Haemophilus parasuis. Vet. Rec. 151:502–505 [DOI] [PubMed] [Google Scholar]
- 34.Miniats OP, Smart NL, Rosendal S. 1991. Cross protection among Haemophilus parasuis strains in immunized gnotobiotic pigs. Can. J. Vet. Res. 55:37–41 [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou SM, Xu CG, Zhang B, Feng SX, Zhang LY, Zou Y, Liao M. 2013. Natural IgG antibodies in normal rabbit serum are involved in killing of the ompP2 mutant of Haemophilus parasuis SC096 strain via the classical complement pathway. Vet. J. 196:111–113 [DOI] [PubMed] [Google Scholar]


