Abstract
We have been investigating modulation strategies tailored around the selective stimulation of the host's immune system as an alternative to direct targeting of microbial pathogens by antibiotics. One such approach is the use of a group of small cationic peptides (BT) produced by a Gram-positive soil bacterium, Brevibacillus texasporus. These peptides have immune modulatory properties that enhance both leukocyte functional efficiency and leukocyte proinflammatory cytokine and chemokine mRNA transcription activities in vitro. In addition, when provided as a feed additive for just 4 days posthatch, BT peptides significantly induce a concentration-dependent protection against cecal and extraintestinal colonization by Salmonella enterica serovar Enteritidis. In the present studies, we assessed the effects of feeding BT peptides on transcriptional changes on proinflammatory cytokines, inflammatory chemokines, and Toll-like receptors (TLR) in the ceca of broiler chickens with and without S. Enteritidis infection. After feeding a BT peptide-supplemented diet for the first 4 days posthatch, chickens were then challenged with S. Enteritidis, and intestinal gene expression was measured at 1 or 7 days postinfection (p.i.) (5 or 11 days of age). Intestinal expression of innate immune mRNA transcripts was analyzed by quantitative real-time PCR (qRT-PCR). Analysis of relative mRNA expression showed that a BT peptide-supplemented diet did not directly induce the transcription of proinflammatory cytokine, inflammatory chemokine, type I/II interferon (IFN), or TLR mRNA in chicken cecum. However, feeding the BT peptide-supplemented diet primed cecal tissue for increased (P ≤ 0.05) transcription of TLR4, TLR15, and TLR21 upon infection with S. Enteritidis on days 1 and 7 p.i. Likewise, feeding the BT peptides primed the cecal tissue for increased transcription of proinflammatory cytokines (interleukin 1β [IL-1β], IL-6, IL-18, type I and II IFNs) and inflammatory chemokine (CxCLi2) in response to S. Enteritidis infection 1 and 7 days p.i. compared to the chickens fed the basal diet. These small cationic peptides may prove useful as alternatives to antibiotics as local immune modulators in neonatal poultry by providing prophylactic protection against Salmonella infections.
INTRODUCTION
Research efforts in our laboratory have focused on developing immunoprophylactic strategies (1–4) and selective genetics (5) that prevent or control intestinal Salmonella enterica organ and intestinal colonization in poultry. Specifically, our research has concentrated on upregulating the innate immune response in chickens during the immunologically inefficient first week posthatch (6).
Recently, a novel Gram-positive bacterium, Brevibacillus texasporus (ATCC PTA-5854), was isolated from the soil and found to produce BT, a group of structurally related cationic peptides (7). BT peptides were found to be highly efficacious against a natural outbreak of colibacillosis in broiler chickens based on improved performance and reduced mortality in comparison with unmedicated birds at a level (12 ppm) that was below the MIC for Escherichia coli (8). In vitro, BT displays efficient bactericidal activity against Gram-positive bacteria (MIC of 1 ppm) but a reduced efficacy against Gram-negative bacteria (MIC of >20 ppm). Interestingly, orally delivered BT seems to completely lack direct antibacterial activities (12 ppm) (8). In addition, chickens given BT as a feed additive for the first 4 days posthatch provided protection against both cecal colonization and extraintestinal Salmonella enterica serovar Enteritidis infections in a concentration-dependent manner and induced the upregulation of peripheral blood heterophil (the avian equivalent to the mammalian neutrophil) and monocyte functional activities (4, 9).
The exact mechanisms of interaction between BT peptides and the cecum in chickens have not been determined. Therefore, the objective of the present experiments was to assess the effects of feeding BT peptides for the first 4 days posthatch on transcriptional changes on proinflammatory cytokines, inflammatory chemokines, and Toll-like receptors (TLR) in the ceca of broiler chickens with or without infection with S. Enteritidis.
MATERIALS AND METHODS
Experimental animals.
Experiments were conducted according to the regulations established by the U.S. Department of Agriculture Animal Care and Use Committee. Broiler chickens used in this study were obtained from a commercial breeder and were all of the same genetic background. Chicks were placed in floor pens containing wood shavings, provided supplemental heat, water, and a balanced, unmedicated corn and soybean meal-based chick starter diet ad libitum that met or exceeded the levels of critical nutrients recommended by the National Research Council (10). Salmonella was not detected in the feed or from the paper tray liners.
Partial purification of BT.
B. texasporus E58 cells were grown in 1 liter of lysogeny broth (LB) in an air shaker at 37°C for 3 days. The culture was spun in a clinical centrifuge at 3,000 × g for 15 min. The supernatant was collected, and 500 g of ammonium sulfate was added and dissolved. The sample was spun in the clinical centrifuge at 3,000 × g for 15 min. The pellet was dissolved in 200 ml of distilled water. The solution was then boiled for 15 min and cooled on ice. The sample was filtered with a 0.2-mm-pore-size filter (Nalgene Inc., Rochester, NY). The filtrate was mixed with 0.2 liters of chloroform at room temperature for 20 min with a stir bar. The mixture was separated into two phases by centrifugation in the clinical centrifuge at 3,000 × g for 15 min. The organic phase was collected and dried in a vacuum evaporator as described previously (7).
Premix preparation.
The dried material was dissolved in ethanol, and the BT concentration was analyzed for anti-Staphylococcus aureus activity by standard microdilution methods as described previously (MIC = 0.8 μg/ml) (7). The solution was sprayed onto cornmeal and then dried. The resulting material was used as a premix for the chicken studies.
S. Enteritidis challenge.
An isolate of S. Enteritidis was obtained from NVSL (Ames, IA) (ID 9711771, part 24). The isolate was selected for resistance to novobiocin and carbenicillin (NO-CN) and was maintained in tryptic soy broth or tryptic soy agar at 40°C. Brilliant green agar (BGA), a selective culture medium for Salmonella, was used to culture the resistant isolate in experimental studies and contained 100 mg/ml CN and 25 mg/ml NO to inhibit growth of other bacteria (BGA–NO-CN). Inoculum for challenge was prepared from 18- to 24-h-grown tryptic soy broth, and NO-CN cultures were maintained at 39°C and diluted in sterile phosphate-buffered saline (PBS) (pH 7.2). A stock solution (1 × 109 CFU/ml) was prepared, and bacterial concentration was determined spectrophotometrically using a standard curve at a reference wavelength of 625 nm.
Experimental challenge design.
One-day-old broiler chickens were randomly distributed into four experimental groups (group 1, control diet, uninfected; group 2, control diet, infected with S. Enteritidis; group 3, BT-supplemented diet, uninfected; group 4, BT-supplemented diet, infected with S. Enteritidis). Each group contained 30 birds fed a balanced, unmedicated corn and soybean meal-based diet that contained either 0 (control) or 24 ppm BT for 4 days. On the fifth day after hatch, all BT feed was removed and replaced with control diet feed for the remainder of the experiment. In addition, on the fourth day after hatch, all chickens were orally challenged with either 5 × 106 CFU/ml S. Enteritidis or mock challenged with sterile PBS. One and 7 days after challenge (5 and 11 days after hatch), 15 chickens from each group were killed by cervical dislocation, cecal contents were analyzed for S. Enteritidis colonization, and cecal tonsils were collected for quantitative real-time PCR (qRT-PCR). All experiments were conducted three times. Therefore, the ceca from a total of 45 chickens for each of the 4 groups (15 chickens each in 3 experiments) were used to prepare the mRNA for the qRT-PCR assays described below. RNA from each bird (n = 45) was isolated and assayed separately and not pooled. Each RNA sample was replicated 3 times per immune gene per experiment).
Sample collection for bacterial counts.
The ceca from each chicken was removed aseptically, and the contents (0.25 g) were serially diluted to 1:100, 1:1,000, or 1:10,000 and spread onto BGA–NO-CN plates. The plates were incubated at 37°C for 24 h, and the number of NO-CN-resistant S. Enteritidis cells per gram of cecal contents was determined. The data from each experimental group were pooled from three separate trials for statistical analysis.
Sample collection for mRNA.
Chickens from each experimental group were euthanized at either 1 or 7 days postchallenge. A 25-mg piece of tissue was removed from the cecal tonsils. The tissue was washed in PBS and placed in a 2-ml microcentrifuge tube with 1 ml of RNAlater (Qiagen Inc., Valencia, CA) and stored at −20°C until processed.
RNA isolation.
Tissues were removed from RNAlater and processed using the RNeasy minikit (Qiagen Inc.) according to the manufacturer's protocol, where tissues were placed in 600 ml of buffer RLT and homogenized using a handheld TissueRuptor (Qiagen Inc.), and total RNA was eluted in 50 μl of DNase-free water and stored at −80°C. RNA was quantified using a spectrophotometer (NanoDrop Products, Wilmington, DE). The data from these three repeated experiments were pooled for presentation and statistical analysis. Total RNA (300 ng) from each sample was prepared.
Quantitative real-time PCR.
Primer and probe sets for the cytokines and 28S rRNA were designed using the Primer Express software program (PE Applied Biosystems, Foster City, CA).
Cytokine and chemokine mRNA expression was quantitated using a well-described method. Primers and probes for cytokines, chemokines, and 28S rRNA-specific amplification have been described (11, 12) and are provided in Table 1. The qRT-PCR was performed using the TaqMan fast universal PCR master mix and one-step RT-PCR master mix reagents. Amplification and detection of specific products were performed using the Applied Biosystems 7500 Fast real-time PCR system with the following cycle profile: one cycle of 48°C for 30 min and 95°C for 20 s and 40 cycles of 95°C for 3 s and 60°C for 30 s. Quantification was based on the increased fluorescence detected by the 7500 Fast sequence detection system due to hydrolysis of the target-specific probes by the 5′ nuclease activity of the rTth DNA polymerase during PCR amplification. Normalization was carried out against 28S rRNA, which was used as a housekeeping gene. To correct for differences in RNA levels between samples within the experiment, the correction factor for each sample was calculated by dividing the mean threshold cycle (CT) value for 28S rRNA-specific product for each sample by the overall mean CT value for the 28S rRNA-specific product from all samples. The corrected cytokine mean was calculated as follow: average of each replicate × cytokine slope/28S slope × 28S correction factor.
Table 1.
Real-time quantitative RT-PCR probes and primers for proinflammatory cytokines, inflammatory chemokines, and type I and II interferons
| RNA target | Sequence typeb | Probe/primer sequencec | Accession no.a |
|---|---|---|---|
| 28S | Probe | 5′-(FAM)-AGGACCGCTACGGACCTCCACCA-(TAMRA)-3′ | X59733 |
| F | 5′-GGCGAAGCCAGAGGAAACT-3′ | ||
| R | 5′-GACGACCGATTGCACGTC-3′ | ||
| IL-1β | Probe | 5′-(FAM)-CCACACTGCAGCTGGAGGAAGCC-(TAMRA)-3′ | AJ245728 |
| F | 5′-GCTCTACATGTCGTGTGTGATGAG-3′ | ||
| R | 5′-TGTCGATGTCCCGCATGA-3′ | ||
| IL-6 | Probe | 5′-(FAM)-AGGAGAAATGCCTGACGAAGCTCTCCA-TAMRA)-3′ | AJ250838 |
| F | 5′-GCTCGCCGGCTTCGA-3′ | ||
| R | 5′-GGTAGGTCTGAAAGGCGAACAG-3′ | ||
| IL-15 | Probe | 5′-(FAM)-CCACCCAATCCAGGAAATGTTAACCCA-(TAMRA)-3′ | NM_204571.1 |
| F | 5′-AGCTGAACTGCTGCCACATTT-3′ | ||
| R | 5′-TTTCCTCTGTTCTTCTTTGTCTGAATC-3′ | ||
| IL-18 | Probe | 5′-(FAM)-CCGCGCCTTCAGCAGGGATG-(TAMRA)-3′ | AJ416937 |
| F | 5′-AGGTGAAATCTGGCAGTGGAAT-3′ | ||
| R | 5′-ACCTGGACGCTGAATGCAA-3′ | ||
| IFN-γ | Probe | 5′-(FAM)-TGGCCAAGCTCCCGATGAACGA-(TAMRA)-3′ | YO7922 |
| F | 5′-GTGAAGAAGGTGAAAGATATATCATGGA-3′ | ||
| R | 5′-GCTTTGCGTGGATTCTCA-3′ | ||
| IFN-α | Probe | 5′-(FAM)-CTCAACCGGATCCACCGCTACACG-(TAMRA)-3′ | U07868 |
| F | 5′-GACAGCCAACGCCAAAGC-3′ | ||
| R | 5′-GTCGCTGCTGTCCAAGCATT-3′ | ||
| CXCLi1 | Probe | 5′-(FAM)-CCACATTCTTGCAGTGAGGTCCGCT-(TAMRA)-3′ | AF277660 |
| F | 5′-CCAGTGCATAGAGACTCATTCCAAA-3′ | ||
| R | 5′-TGCCCATCTTTCAGAGTAGCTATGAACT-3′ | ||
| CXCLi2 | Probe | 5′-(FAM)-CTTTACCAGCGCGTCCTACCTTGCGACA-(TAMRA)-3′ | AJ009800 |
| F | 5′-GCCCTCCTCCTGGTTTCAG-3′ | ||
| R | 5′-TGGCACCGCCAGCTCATT-3′ |
For the genomic DNA sequence.
F, forward; R, reverse.
FAM, 5-carboxyfluorescein; TAMRA, N,N,N,N′-tetramethyl-6-carboxyrhodamine.
Statistical analysis.
The mean and standard error of the mean were calculated for 40 CT values for each of the 4 treatment groups. Differences between group 1, group 2, group 3, and group 4 were determined by analysis of variance. Significant differences were further separated using Duncan's multiple range test. Fold changes in RNA levels were calculated from mean 40 CT values by the formula 2(40 CT in groups 2, 3, or 4 − 40 CT in group 1). A P value of <0.05 was considered statistically significant.
RESULTS
Proinflammatory cytokine mRNA expression.
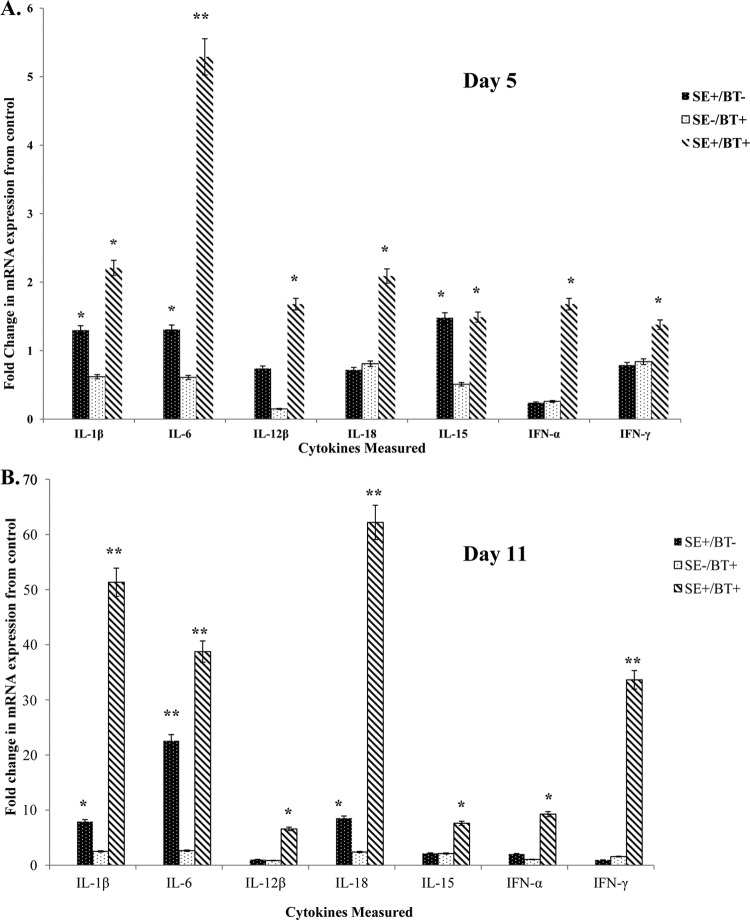
The effect of feeding BT peptides to young chickens on the expression of proinflammatory cytokine mRNA expression in the cecum on day 5 posthatch (1 day after removal of BT peptide-supplemented feed) is shown in Fig. 1A. S. Enteritidis infection in chickens on the control diet (SE+/BT−) induced a small but significant (P ≤ 0.05) upregulation of IL-1β, IL-6, and IL-15. BT-supplemented feed (SE−/BT+) had no direct effect on proinflammatory cytokine mRNA expression in the cecum. BT-supplemented diet appeared to “prime” the intestine for a significant synergistic upregulation of all proinflammatory cytokines except for IL-15 following infection with S. Enteritidis (SE+/BT+).
Fig 1.
Effect of feeding BT peptide-supplemented ration on the expression of proinflammatory cytokine mRNA (IL-1β, IL-6, IL-12β, IL-18, IL-15, IFN-α, IFN-γ) in the ceca from experimental chickens with or without infection with S. Enteritidis. One-day-old broiler chickens were randomly distributed into two experimental groups. Each group contained 25 birds fed a balanced, unmedicated corn and soybean meal-based diet that contained either 0 (control) or 24 ppm BT for 4 days. On the fourth day after hatch, all BT feed was removed and replaced with the control diet feed for the remainder of the experiment, and all chickens were orally challenged with 5 × 106 CFU/ml S. Enteritidis. (A) Ceca collected 5 days posthatch (1 day after removal of BT peptide-supplemented diet); (B) ceca collected 11 days posthatch (7 days after removal of BT peptide-supplemented diet). Data represent the means ± standard errors of the means (SEM) from three independent experiments. Data are presented as the fold change in mRNA expression relative to the noninfected, normal ration-fed control chickens (SE−/BT−). Columns with asterisks are significantly different at either P values of ≤0.05 (*) or P values of ≤0.01 (**) from SE−/BT− chickens.
The effect of feeding BT peptides to young chickens on the expression of proinflammatory cytokine mRNA expression in the cecum on day 11 posthatch (7 days after removal of BT peptide-supplemented feed) is shown in Fig. 1B. S. Enteritidis cecal colonization for 7 days in chickens on the control diet (SE+/BT−) stimulated a significant (P ≤ 0.05) upregulation of IL-1β, IL-6, and IL-18. BT-supplemented feed (SE−/BT+) had no direct effect on proinflammatory cytokine mRNA expression in the cecum. BT-supplemented diet maintained a priming effect in the intestine for at least a week after removal, as evidenced by the significant upregulation of all proinflammatory cytokines during a persistent infection with S. Enteritidis (SE+/BT+).
Inflammatory chemokine mRNA expression.
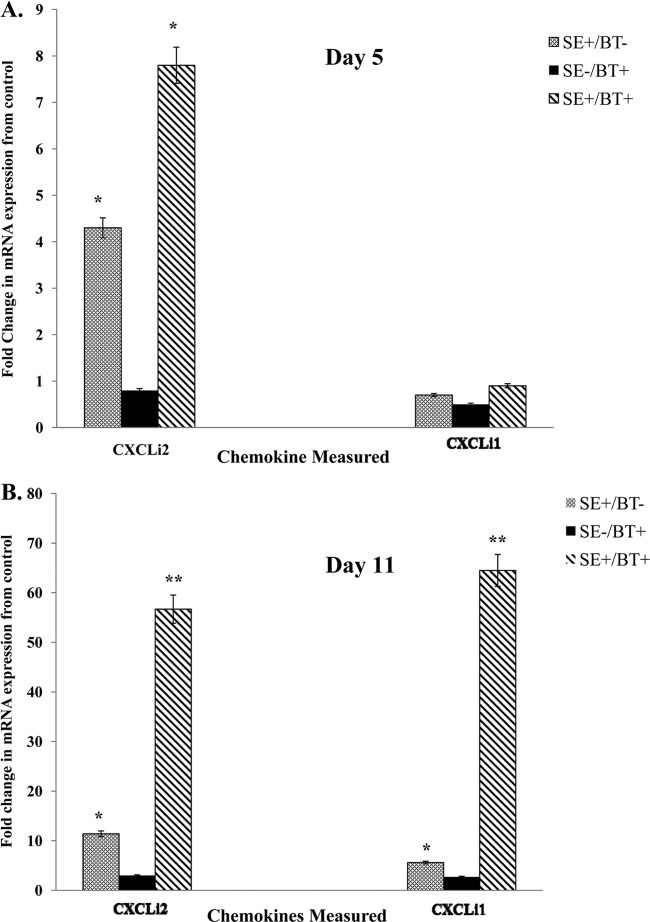
The effect of feeding BT peptides to young chickens on inflammatory chemokine mRNA expression in the cecum on days 5 or 11 posthatch (1 or 7 days after removal of BT peptide-supplemented feed, respectively) is shown in Fig. 2A and B. BT-supplemented feed (SE−/BT+) had no direct effect on chemokine mRNA expression in the cecum. S. Enteritidis infection in chickens on the control diet (SE+/BT−) induced a significant (P ≤ 0.05) upregulation of CXCLi2 at 1 and 7 days after infection (days 5 and 11 posthatch). However, in S. Enteritidis infection in chickens on the control diet (SE+/BT−), no effect was observed on the expression of CXCLi1 (Fig. 2A), but there was a small but significant increase in CXCLi1 by 7 days after infection (Fig. 2B). Likewise, BT-supplemented diet had a priming effect on the cecum, resulting in a significant upregulation of CXCLI2 1 and 7 days after infection with S. Enteritidis (SE+/BT+) (Fig. 2B). BT-supplemented diet had a priming effect on CXCLi1 only on day 7 postinfection (Fig. 2B).
Fig 2.
Effect of feeding BT peptide-supplemented ration on the expression of inflammatory chemokine mRNA (CxCLi2, CXCLi1) in the ceca from experimental chickens with or without infection with S. Enteritidis. One-day-old broiler chickens were randomly distributed into two experimental groups. Each group contained 25 birds fed a balanced, unmedicated corn and soybean meal-based diet that contained either 0 (control) or 24 ppm BT for 4 days. On the fourth day after hatch, all BT feed was removed and replaced with the control diet feed for the remainder of the experiment, and all chickens were orally challenged with 5 × 106 CFU/ml S. Enteritidis. (A) Ceca collected 5 days posthatch (1 day after removal of BT peptide-supplemented diet); (B) ceca collected 11 days posthatch (7 days after removal of BT peptide-supplemented diet). Data represent the means ± SEM from three independent experiments. Data are presented as the fold change in mRNA expression relative to the noninfected, normal ration-fed control chickens (SE−/BT−). Columns with asterisks are significantly different at either P values of ≤0.05 (*) or P values of ≤0.01 (**) from SE−/BT− chickens.
Toll-like receptor mRNA expression.
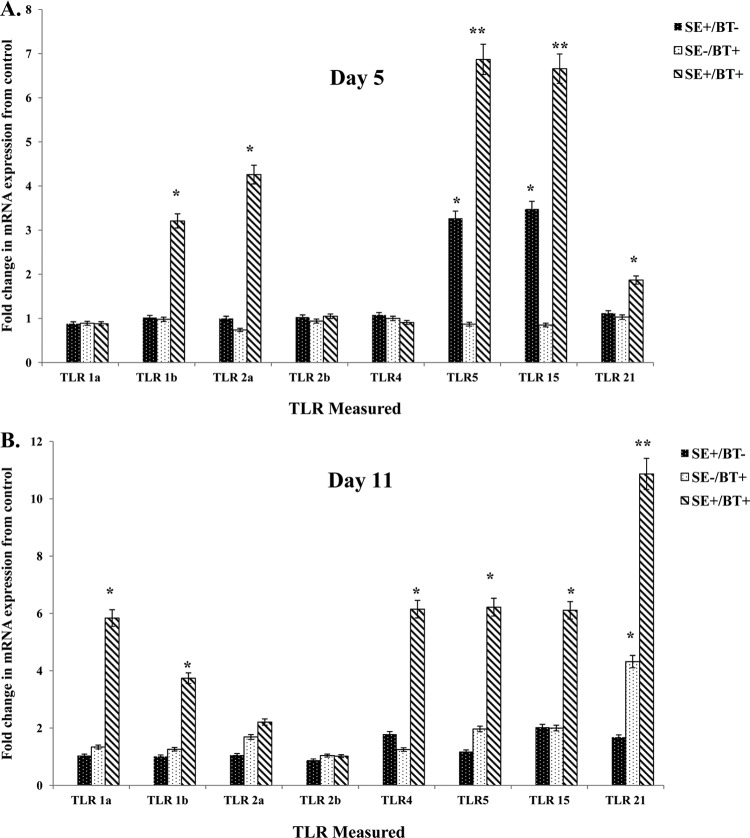
The effect of BT peptides on TLR expression in cecum 5 days posthatch (1 day after removal of BT peptide-supplemented feed) is shown in Fig. 3A. S. Enteritidis infection in chickens on the control diet (SE+/BT−) induced a significant upregulation in expression of TLR5 and TLR15. BT-supplemented feed (SE−/BT+) had no significant, direct effect on TLR mRNA expression in the cecum. BT-supplemented diet appeared to “prime” the intestine for a significant (P ≤ 0.05) synergistic upregulation of TLR1b, TLR2a, TLR5, TLR15, and TLR21 following infection with S. Enteritidis (SE+/BT+).
Fig 3.
Effect of feeding BT peptide-supplemented ration on the expression of TLR mRNA (TLR1a, TLR1b, TLR2a, TLR2b, TLR4, TLR5, TLR15, TLR21) in the ceca from experimental chickens with or without infection with S. Enteritidis. One-day-old broiler chickens were randomly distributed into two experimental groups. Each group contained 25 birds fed a balanced, unmedicated corn and soybean meal-based diet that contained either 0 (control) or 24 ppm BT for 4 days. On the fourth day after hatch, all BT feed was removed and replaced with the control diet feed for the remainder of the experiment, and all chickens were orally challenged with 5 × 106 CFU/ml S. Enteritidis. (A) Ceca collected 5 days posthatch (1 day after removal of BT peptide-supplemented diet); (B) ceca collected 11 days posthatch (7 days after removal of BT peptide-supplemented diet). Data represent the means ± SEM from three independent experiments. Data are presented as the fold change in mRNA expression relative to the noninfected, normal ration-fed control chickens (SE−/BT−). Columns with asterisks are significantly different at either P values of ≤0.05 (*) or P values of ≤0.01 (**) from SE−/BT− chickens.
The effect of feeding BT peptides to young chickens on the expression of TLR mRNA in the cecum on day 11 posthatch (7 days after removal of BT peptide-supplemented feed) is shown in Fig. 3B. S. Enteritidis cecal colonization for 7 days in chickens on the control diet (SE+/BT−) did not upregulate the expression of TLR mRNA. BT-supplemented feed (SE−/BT+) had no direct effect on TLR mRNA expression in the cecum except for TLR21. BT-supplemented diet induced a priming effect in the cecum for at least a week after removal, as evidenced by the significant upregulation of all TLR mRNA (except TLR2a and TLR2b) during a persistent infection with S. Enteritidis (SE+/BT+).
S. Enteritidis cecal colonization.
The effect of BT peptide on S. Enteritidis cecal colonization of chickens 1 and 7 days postchallenge is shown in Fig. 4. The number of S. Enteritidis CFU/ml in the cecal contents of chickens in BT-fed groups was significantly less (P ≤ 0.01) than in the control diet-fed group at both 1 and 7 days postchallenge. Similarly, the percentage of S. Enteritidis cecal culture-positive chickens among the BT-fed birds was significantly less than that among the control diet-fed chickens (10% versus 59%, respectively). All nonchallenged chicks were S. Enteritidis negative regardless of the diet administered (data not shown).
Fig 4.
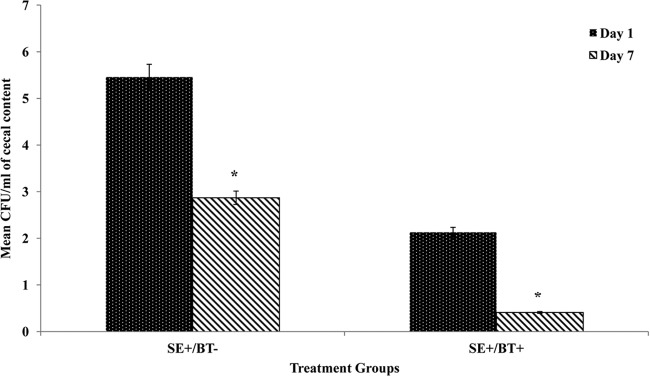
Effect of feeding BT peptide-supplemented ration on cecal colonization by S. Enteritidis. One-day-old broiler chickens were randomly distributed into two experimental groups. Each group contained 25 birds fed a balanced, unmedicated corn and soybean meal-based diet that contained either 0 (control) or 24 ppm BT for 4 days. On the fourth day after hatch, all BT feed was removed and replaced with the control diet feed for the remainder of the experiment, and all chickens were orally challenged with 5 × 106 CFU/ml S. Enteritidis. One or 7 days after challenge (5 or 10 days after hatch), all chickens were killed and cecal contents were analyzed for S. Enteritidis colonization. Data represent means and standard deviations from three independent experiments. An asterisk indicates differences among treatments are statistically significant (P ≤ 0.05).
DISCUSSION
Both viral and bacterial diseases remain a threat to the poultry industry, and countermeasures to prevent and control them are needed due to food safety issues and production losses. The design of new immunological interventions or therapeutic antimicrobials to reduce microbial pathogens in poultry is now, more than ever, required. Based on the data provided herein and in previous experiments (4, 8, 9), the use of BT peptides as a feed additive for poultry may be an important component of an on-the-farm program for the control of food safety pathogens, including Salmonella.
We have targeted the innate immune responses for the development of immunomodulatory and/or antimicrobial compounds for prevention or treatment of bacterial infections in neonatal poultry (2, 3, 13–17). Since the innate immune response is not pathogen specific, the ability to stimulate the response in birds is a promising approach of increasing resistance to a variety of pathogens. The one characteristic of the avian innate response that we have exploited is the ability of the response to be modulated during the first week after hatch (3, 15, 18, 19). We have shown that the stimulated response results in an increased resistance to Salmonella infections with concomitant increases in heterophil functional activity (2, 3, 13–17). Further, we found that this stimulation was self-limiting, lasting 3 to 5 days after administration of the immune modulator (4, 16). These results indicate that the innate immune responses can be augmented and that natural products can potentially be used as antimicrobial compounds.
Cationic antimicrobial peptides encompass a large group of small peptides that are widely distributed in most forms of life, from microbes to invertebrates to plants to humans (20–22). Functionally, these peptides modulate the immune response and/or direct antimicrobial activity (23–28). In mammals, most of these cationic peptides have little direct antimicrobial activity but do enhance the innate immune responses without the harmful inflammatory responses (24, 26–29). The BT peptides used in the present studies have similar characteristics to these “host defense peptides” in contrast with the “antimicrobial peptides” that have direct antibiotic-like activity on pathogens (25). In addition, preliminary experiments have shown a growth-promoting effect (improved weight gains and feed conversion) in broiler chickens fed BT peptides continuously for a 42-day grow out (8). To date, we have observed no obvious toxic effects normally associated with an uncontrolled inflammatory response in chickens provided BT peptides as a feed additive. Thus, because of its low MIC but strong innate immune modulatory activities, we suggest that BT peptides may be considered host defense peptides when provided as a feed additive to chickens.
Activation of the innate immune response is initiated following a series of rigorous, rapid, and precise discriminatory steps that differentiate between “self” and “nonself” based on the recognition of broadly conserved molecular patterns by germ line-encoded pattern recognition receptors (PRRs) (30), which include the families of Toll-like receptors (TLRs) and NOD-like receptors (NLRs). PRRs are crucial regulators of intestinal innate immunity and are thus essential to the control of host defense by maintaining mucosal homeostasis. They play key roles in recognition and sensing of nonpathogen danger signals, inhibition of invasion of facultative/obligate pathogens and other threats, induction of antimicrobial effector pathways, and control of adaptive immune responses, by acting through a series of interdependent signaling events (31, 32). Environmental factors, including dietary components, may alter PRR function (30).
Our findings that increased expression of TLR mRNA following S. Enteritidis infection of BT peptide-fed birds suggest that BT peptides and S. Enteritidis pathogen-associated molecular patterns (PAMPs) may interact at the level of TLR recognition and signaling (33). The priming effect of the BT peptide-supplemented diet resembles “endogenous TLR ligands” that are recognized as PAMP-sensitizing molecules (34). These PAMP-sensitizing molecules have been categorized as released intracellular proteins, modified lipids, and other soluble mediators. Like the BT peptides used here, these molecules may serve a beneficial purpose by enhancing (priming) the sensitivity of host tissues to potential microbial challenge. Alternatively, BT peptides may play a specialized role in local intestinal innate responses. For example, it is possible that the BT peptides may act as TLR accessory molecules (35, 36). TLR accessory molecules, such as the human host defense peptide LL-37, are used by TLR for microbial recognition, signaling, and regulation of innate immune responses (36, 37). LL-37 converts nonstimulatory self-DNA into a potent stimulator of dendritic cells (37). Further experiments are in progress to fully characterize this potential functional mechanism of the BT peptides.
The present results demonstrate that BT peptides play a specialized regulatory role in the local intestinal innate responses. Contact between the Salmonella enterica serovars Enteritidis or Typhimurium and intestinal epithelium in 1- to 4-day-old chickens resulted in the upregulated expression of proinflammatory cytokines (IL-1β and IL-6) (38–43) and the inflammatory chemokines CXCLi1 and CXCLi2 (38–45). Feeding the BT peptide-supplemented feed primes the local mucosal to respond transcriptionally in a qualitative manner to a stimulus. The differential expression of CXCLi2 and CXCLi1 during acute response to S. Enteritidis (1 day postinfection) illustrates the direct recruitment of heterophils to the intestine early during a S. Enteritidis infection, thus increasing the intestinal mucosa's ability to limit infection. Moreover, the increased expression of the chemokines, CXCLi1 and CXCLi2, 7 days after a S. Enteritidis infection is indicative of a functional switch of the intestinal mucosa from a defensive nature to one that is more regulatory/homeostatic (46). It is important to note that we have observed no pathological effects in the intestine due to this increased expression of the inflammatory chemokines.
Multiple inflammatory mediators are involved in modulating the cellular response to an infection. Inflammatory mediators that function in this modulating role are known as priming agents. Priming has been defined as the potentiation of the phagocyte activation process by previous exposure to priming (47). Priming has a direct effect on cell shape, integrin/selectin expression, and longevity of the phagocyte and thus has a profound effect on the chemotactic, adhesive, and survival properties of the host innate cells (47, 48). Characteristically, the priming agent does not induce a direct functional response (47). The priming activity of BT peptides on the transcription of chicken intestinal proinflammatory cytokines, inflammatory chemokines, type I/II IFNs, and TLRs was verified in the present experiments. Although BT peptide priming modulated the expression of multiple innate immune response genes in the cecum during an infection with S. Enteritidis, the BT peptide-supplemented diet, by itself, did not directly induce gene expression of any of the immune response genes. By definition, this represents true priming of the local cecal innate immune response.
In summary, when challenged with Salmonella enterica serovar Enteritidis, chickens fed a BT peptide-supplemented diet for the first 4 days posthatch had a significant increase in mRNA transcripts for TLR4, TLR15, TLR21, proinflammatory cytokines, and inflammatory chemokines (CxCLi1, CxCLi2) 1 and 7 days p.i. The BT peptide-supplemented diet alone did not directly induce an increase in expression of any of the innate immune response in chicken cecum. Practically speaking, the ability to orally deliver the cationic peptides in the diet to chickens at a time of immunologic inefficiency and increased susceptibility to bacterial infections (first week posthatch) is of utmost importance to the poultry industry. Our findings describe a new strategy to induce a protective immune response with TLR/proinflammatory activity.
Footnotes
Published ahead of print 17 July 2013
REFERENCES
- 1.Kogut MH. 2002. Dynamics of a protective avian inflammatory response: the role of an IL-8-like cytokine in the recruitment of heterophils to the site of organ invasion by Salmonella enteritidis. Comp. Immunol. Microbiol. Infect. Dis. 25:159–172 [DOI] [PubMed] [Google Scholar]
- 2.Lowry VK, Farnell MB, Ferro PJ, Swaggerty CL, Bahl A, Kogut MH. 2005. Purified β-glucan as an abiotic feed additive upregulates the innate immune response in immature chickens against Salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 98:309–318 [DOI] [PubMed] [Google Scholar]
- 3.He H, Lowry VK, Swaggerty CL, Ferro P, Kogut MH. 2005. In vitro activation of chicken leukocytes and in vivo protection against Salmonella enteritidis organ invasion and peritoneal S. enteritidis infection-induced mortality in neonatal chickens by immunomodulatory CpG oligodeoxynucleotide. FEMS Immunol. Med. Microbiol. 43:81–89 [DOI] [PubMed] [Google Scholar]
- 4.Kogut MH, Genovese KJ, He H, Li MA, Jiang YW. 2007. The effects of the BT/TAMUs 2032 cationic peptides on innate immunity and susceptibility of young chickens to extraintestinal Salmonella enterica serovar Enteritidis infection. Int. Immunopharmacol. 7:912–919 [DOI] [PubMed] [Google Scholar]
- 5.Swaggerty CL, Pevzner IY, He H, Genovese KJ, Nisbet DJ, Kaiser P, Kogut MH. 2009. Selection of broilers with improved innate immune responsiveness to reduce on-farm infection by food-borne pathogens: a review. Foodborne Path. Dis. 6:772–783 [DOI] [PubMed] [Google Scholar]
- 6.Wells LL, Lowry VK, DeLoach JR, Kogut MH. 1998. Age dependent phagocytosis and bactericidal activities of the chicken heterophil. Dev. Comp. Immunol. 22:103–109 [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Ballard J, Jiang YW. 2005. Structure and biosynthesis of the BT peptide antibiotic from Brevibacillus texasporus. Appl. Environ. Microbiol. 71:8519–8530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang YW, Sims MD, Conway DP. 2005. The efficacy of TAMUS 2032 in preventing a natural outbreak of colibacillosis in broiler chickens in floor pens. Poult. Sci. 84:1857–1859 [DOI] [PubMed] [Google Scholar]
- 9.Kogut MH, He H, Genovese KJ, Jiang Y. 2010. Feeding the BT cationic peptides to chickens at hatch reduces cecal colonization by Salmonella enterica serovar Enteritidis and primes innate immune cell functional activity. Foodborne Path. Dis. 7:23–30 [DOI] [PubMed] [Google Scholar]
- 10.National Research Council 1994. Nutritional requirements of poultry, 9th edition. National Academy Press, Washington, DC [Google Scholar]
- 11.Kaiser P, Rothwell L, Galyov EE, Barrow PA, Burnside J, Wigley P. 2000. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 146(Part 12):3217–3226 [DOI] [PubMed] [Google Scholar]
- 12.Kogut MH, Rothwell L, Kaiser P. 2003. Differential regulation of cytokine gene expression by avian heterophils during receptor-mediated phagocytosis of opsonized and nonopsonized Salmonella enteritidis. J. Interferon Cytokine Res. 23:319–327 [DOI] [PubMed] [Google Scholar]
- 13.Crippen TL, Bischoff KM, Lowry VK, Kogut MH. 2003. P33 activates antimicrobial functions of heterophils from chicken peripheral blood. J. Food Prot. 66:787–792 [DOI] [PubMed] [Google Scholar]
- 14.He H, Genovese KJ, Swaggerty CJ, Nisbet DJ, Kogut MH. 2007. In vivo priming heterophil innate immune functions and increasing resistance to Salmonella enteritidis infection in neonatal chickens by immune stimulatory CpG oligodeoxynucleotides. Vet. Immunol. Immunopathol. 117:275–283 [DOI] [PubMed] [Google Scholar]
- 15.Genovese KG, He H, Lowry VK, Nisbet DJ, Kogut MH. 2007. Dynamics of the avian inflammatory response to Salmonella following administration of the Toll-like receptor 5 agonist, flagellin. FEMS Immunol. Med. Microbiol. 51:112–117 [DOI] [PubMed] [Google Scholar]
- 16.MacKinnon KM, He H, Swaggerty CL, McReynolds JL, Genovese KJ, Duke SE, Nerren JR, Kogut MH. 2009. In ovo treatment with CpG oligodeoxynucleotides decreases colonization of Salmonella enteriditis in broiler chickens. Vet. Immunol. Immunopathol. 127:371–375 [DOI] [PubMed] [Google Scholar]
- 17.Swaggerty CL, He H, Genovese KJ, Duke SE, Kogut MH. 2012. Loxoribine pretreatment reduces Salmonella Enteritidis organ invasion in 1-day-old chickens. Poult. Sci. 91:1038–1042 [DOI] [PubMed] [Google Scholar]
- 18.McGruder ED, Kogut MH, Corrier DE, DeLoach JR, Hargis BM. 1995. Comparison of prophylactic and therapeutic efficacy of Salmonella enteritidis-immune lymphokines against Salmonella enteritidis organ invasion in neonatal Leghorn chicks. Avian Dis. 39:21–27 [PubMed] [Google Scholar]
- 19.Kogut MH, McGruder ED, Hargis EM, Corrier DE, DeLoach JR. 1995. In vivo activation of heterophil function in chickens following injection with Salmonella enteritidis-immune lymphokines. J. Leukoc. Biol. 57:56–62 [DOI] [PubMed] [Google Scholar]
- 20.Brogden KA, Ackerman M, McCray PB, Jr, Tuck BF. 2003. Antimicrobial peptides in animals and their role in host defenses. Int. J. Antimicrob. Agents 22:465–478 [DOI] [PubMed] [Google Scholar]
- 21.Vizioli J, Salzet M. 2002. Antimicrobial peptides from animals: focus on invertebrates. Trends Pharmacol. Sci. 23:494–496 [DOI] [PubMed] [Google Scholar]
- 22.Linde A, Ross CR, Davis EG, Dib L, Blecha F, Melgarejo T. 2008. Innate immunity and host defense peptides in veterinary medicine. J. Vet. Intern. Med. 22:247–265 [DOI] [PubMed] [Google Scholar]
- 23.Ganz T. 2003. Defensins: antimicrobial peptides of innate immunity. Rev. Immunol. 3:710–720 [DOI] [PubMed] [Google Scholar]
- 24.Bowdish DME, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock REW. 2005. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 77:451–459 [DOI] [PubMed] [Google Scholar]
- 25.Hancock REW, Sahl H-G. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557 [DOI] [PubMed] [Google Scholar]
- 26.Mookherjee N, Hancock REW. 2007. Cationic host defense peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell. Mol. Life Sci. 64:922–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott MG, Dullaghan E, Mookherjee N, Glavas N, Waldbrook M, Thompson A, Wang A, Lee K, Doria S, Hamill P, Yu JJ, Li Y, Donin O, Guarns MM, Finlay BB, North JR, Hancock REW. 2007. An anti-infective peptide that selectively modulates the innate immune response. Nat. Biotechnol. 25:465–472 [DOI] [PubMed] [Google Scholar]
- 28.Hancock REW, Nijnik A, Philpott DJ. 2012. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 10:243–254 [DOI] [PubMed] [Google Scholar]
- 29.Finlay BB, Hancock REW. 2004. Can innate immunity be enhanced to treat microbial infections? Nat. Rev. Microbiol. 2:497–504 [DOI] [PubMed] [Google Scholar]
- 30.Creagh EM, O'Neill LA. 2006. TLRs, NLRs, and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 27:352–357 [DOI] [PubMed] [Google Scholar]
- 31.Kawai T, Akira S. 2007. TLR signaling. Sem. Immunol. 19:24–32 [DOI] [PubMed] [Google Scholar]
- 32.Henao-Mejia J, Elinav E, Strowig T, Flavell RA. 2012. Inflammasomes: far beyond inflammation. Nat. Immunol. 13:321–324 [DOI] [PubMed] [Google Scholar]
- 33.Kogut MH, Chiang HS, Swaggerty CL, Pevzner IY, Zhou H. 2012. Gene expression analysis of Toll-like receptor pathways in heterophils from genetic lines that differ in their susceptibility to Salmonella enteritidis. Front. Genetics 3:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erridge C. 2010. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J. Leukoc. Biol. 87:989–999 [DOI] [PubMed] [Google Scholar]
- 35.Lee CC, Avalos AM, Ploegh HL. 2012. Accessory molecules for Toll-like receptors and their function. Nat. Rev. Immunol. 12:168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akashi-Takamura S, Miyake K. 2008. TLR accessory molecules. Curr. Opin. Immunol. 20:420–425 [DOI] [PubMed] [Google Scholar]
- 37.Lande R, Gregorio Facchinetti JV, Chatterjee B, Wang Y-H, Homey B, Cao W, Wang Y-H, Su B, Nestle FO, Zal T, Mellman I, Schroder J-M, Liu Y-J, Gilliet M. 2007. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449:564–569 [DOI] [PubMed] [Google Scholar]
- 38.Setta A, Barrow PA, Kaiser P, Jones MA. 2012. Immune dynamics following infection of avian macrophages and epithelial cells with typhoidal and non-typhoidal Salmonella enterica serovars: bacterial invasion and persistence, nitric oxide and oxygen production, differential host gene expression, NF-κB signaling and cell cytotoxicity. Vet. Immunol. Immunopathol. 146:212–224 [DOI] [PubMed] [Google Scholar]
- 39.Beal RK, Powers C, Wigley P, Barrow PA, Smith AL. 2004. Temporal dynamics of the cellular, humoral, and cytokine responses in chickens during primary and secondary infection with Salmonella enterica serovar Typhimurium. Avian Pathol. 17:571–588 [DOI] [PubMed] [Google Scholar]
- 40.Berndt A, Wilhelm A, Jugert C, Pieper J, Sachse K, Methner U. 2007. Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect. Immun. 75:5993–6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haghighi HR, Abdul-Careem MF, Dara RA, Chambers JR, Sharif S. 2008. Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection. Vet. Microbiol. 126:225–233 [DOI] [PubMed] [Google Scholar]
- 42.Withanage GS, Kaiser P, Wigley P, Powers C, Mastroeni P, Brooks H, Barrow P, Smith A, Maskell D. 2004. Rapid expression of chemokines and proinflammatory cytokines in newly hatched chickens infected with Salmonella enterica serovar Typhimurium. Infect. Immun. 72:2152–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaughnessy RG, Meade KG, Cahalane S, Allan B, Reiman C, Callahan JJ, O'Farrelly C. 2009. Innate immune gene expression differentiates the early intestinal responses between Salmonella and Campylobacter. Vet. Immunol. Immunopathol. 132:191–198 [DOI] [PubMed] [Google Scholar]
- 44.Fasina YO, Holt PS, Moran ET, Moore RW, Conner DE, McKee SR. 2008. Intestinal cytokine response of commercial source broiler chicks to Salmonella typhimurium infection. Poult. Sci. 87:1335–1346 [DOI] [PubMed] [Google Scholar]
- 45.Cheeseman JH, Levy NA, Kaiser P, Lillehoj HS, Lamont SJ. 2009. Salmonella enteritidis-induced alteration of inflammatory CXCL chemokine messenger-RNA expression and histologic changes on the ceca of infected chicks. Avian Dis. 52:229–234 [DOI] [PubMed] [Google Scholar]
- 46.Kaiser P, Hardt W. 2011. Salmonella typhimurium diarrhea: switching the mucosal epithelium from homeostasis to defense. Curr. Opin. Immunol. 23:456–463 [DOI] [PubMed] [Google Scholar]
- 47.Rohn TT, Nelson LK, Sipes KM, Swain SD, Jutila KL, Quinn MT. 1999. Priming of human neutrophils by peroxynitrite: potential role in enhancement of the local inflammatory response. J. Leukoc. Biol. 65:59–70 [DOI] [PubMed] [Google Scholar]
- 48.Condliff AM, Kitchen E, Chilvers ER. 1998. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin. Sci. 94:461–471 [DOI] [PubMed] [Google Scholar]