Abstract
Lactococcus lactis subsp. lactis BGMN1-5 produces a leaderless class II bacteriocin called LsbB. To identify the receptor for LsbB, a cosmid library of the LsbB-sensitive strain BGMN1-596 was constructed. About 150 cosmid clones were individually isolated and transferred to LsbB-resistant mutants of BGMN1-596. Cosmid pAZILcos/MN2, carrying a 40-kb insert, was found to restore LsbB sensitivity in LsbB-resistant mutants. Further subcloning revealed that a 1.9-kb fragment, containing only one open reading frame, was sufficient to restore sensitivity. The fragment contains the gene yvjB coding for a Zn-dependent membrane-bound metallopeptidase, suggesting that this gene may serve as the receptor for LsbB. Further support for this notion derives from several independent experiments: (i) whole-genome sequencing confirmed that all LsbB-resistant mutants contain mutations in yvjB; (ii) disruption of yvjB by direct gene knockout rendered sensitive strains BGMN1-596 and IL1403 resistant to LsbB; and (iii) most compellingly, heterologous expression of yvjB in naturally resistant strains of other species, such as Lactobacillus paracasei and Enterococcus faecalis, also rendered them sensitive to the bacteriocin. To our knowledge, this is the first time a membrane-bound peptidase gene has been shown to be involved in bacteriocin sensitivity in target cells. We also demonstrated a novel successful approach for identifying bacteriocin receptors.
INTRODUCTION
Microorganisms inhabit a wide range of habitats, ranging from the depth of the ocean to most surfaces and spaces on Earth, and they are capable of growing on both inorganic and organic matter. In order to defend their own habitats or to invade new territories, they have developed diverse antagonistic strategies to kill or weaken their competitors, one of them being the production of bacteriocins. Bacteriocins comprise a large group of antimicrobial peptides (AMPs) produced by many bacteria (1). They are especially numerous in lactic acid bacteria (LAB), which are common bacteria in diverse food and feed products. LAB are also common inhabitants of the gastrointestinal tracts of both invertebrates and vertebrates. Due to their abundance in our food and in our body, many LAB are generally regarded as safe (GRAS) for use in food applications, and a few bacteriocins are also approved for use as food preservatives (http://www.fda.gov/ohrms/dockets/dockets/95s0316/95s-0316-rpt0334-02-Ref-01-PLMMDI-vol262.pdf). There is an increasing interest in developing these AMPs into useful drugs for medical use because of the pressing need for novel sources of antimicrobials, especially important with regard to the alarming emergence of antibiotic-resistant bacteria.
Most bacteriocins have relatively narrow inhibitory spectra, being active mostly against closely related bacteria. However, some have wider inhibitory spectra that include important food spoilage bacteria like Listeria and Bacillus and pathogens such as some species of Staphylococcus and Enterococcus. One of the remarkable features of bacteriocins is that they are very potent, being active in nanomolar concentrations (2), thereby surpassing by about 1,000-fold the activity of antimicrobial peptides (e.g., defensins) that humans and other animals produce. One of the major reasons for this extreme potency is that bacteriocins apparently recognize specific receptors on target cells, while the interactions between eukaryotic peptides and microorganisms are mostly nonspecific.
Some bacteriocin receptors have already been identified. Among bacteriocins from Gram-positive bacteria, nisin and several other lantibiotics (class I) specifically bind to the cell wall precursor, lipid II, and kill target cells by inhibiting cell wall synthesis and/or forming lethal pores in the membrane (3). The nonlantibiotic bacteriocin lactococcin 972 also employs lipid II as a binding molecule, but the killing mechanism has not been elucidated (4). The mannose-phosphotransferase system (man-PTS) has been found to serve as a receptor for some nonlantibiotics (class II) (5). This is true for most pediocin-like bacteriocins (subclass IIa), which are related in both sequence and structure (6), and for the unrelated bacteriocins lactococcin A and B (subclass IId non-pediocin-like and linear bacteriocins) (7). Recently, another sugar transporter, a maltose-ABC transporter, was found to be required in target cells for sensitivity to garvicin ML, a circular bacteriocin (subclass IIc) (8). Although these bacteriocins target different receptors, their receptors all have in common that they are components of the bacterial membrane. This is in line with the general view that bacteriocins target the cell membrane, where lethal pores are formed, causing cellular leakage, disruption of membrane integrity, and cell death as the final outcome.
Previously we have reported that Lactococcus lactis subsp. lactis BGMN1-5 produces three bacteriocins: lactococcin B, LsbA, and LsbB (9, 10). The genes coding for the biosynthesis of bacteriocins LsbA and LsbB are located on a plasmid (pMN5), while the location of the genes involved in the biosynthesis of lactococcin B are not known (10). Bacteriocin LsbB is a small nonlantibiotic bacteriocin of 30 amino acid (aa) residues. It belongs to the same subclass (IId) as lactococcin A and B but is not related to those, based on the amino acid sequence. Further, it is synthesized without a leader peptide, whereas lactococcin A and B and most class II bacteriocins are synthesized with a leader required for export. The multidrug-resistance protein LmrB is responsible for the transport of LsbB out of the cell, as well as for immunity of the producer (9). In the present work we have developed a novel approach involving cosmid library construction to screen for bacteriocin receptor genes, and we also report the identification of the novel receptor for bacteriocin LsbB, namely, YvjB, a membrane-bound Zn-dependent metallopeptidase.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains, their derivatives, and plasmids used in this study are listed in Table 1. Lactococcus lactis and Enterococcus faecalis strains were grown in M17 medium (Merck GmbH, Darmstadt, Germany) supplemented with 0.5% (wt/vol) glucose (GM17) at 30°C, Lactobacillus paracasei was grown in deMan-Rogosa-Sharpe (MRS) medium (Merck), and Escherichia coli was grown in Luria broth (LB) at 37°C with aeration. Erythromycin was added to a final concentration of 10 μg ml−1 and 300 μg ml−1 for lactococci and E. coli, respectively. Ampicillin was added to a final concentration of 100 μg ml−1 for E. coli. When necessary, 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) (Fermentas, Vilnius, Lithuania) was added to LB medium plates at a final concentration of 40 μg ml−1 for blue/white color selection of colonies.
Table 1.
Bacterial strains and plasmids used in this study
| Strain, plasmid, or cosmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Strains or mutants | ||
| Lactococcus lactis subsp. lactis | ||
| IL1403 | Plasmid-free derivative of IL-596, LsbBs | 25 |
| B464 | ptn deletion mutant of IL1403, LsbBs | 5 |
| BGMN1-59WT | Plasmid-free derivative of L. lactis subsp. lactis BGMN1-5, LsbBs | 26 |
| BGMN1-596T | Derivative of BGMN1-596WT with pMN5 plasmid, LsbBr, LsbB producer | 26 |
| BGMN1-596R2/12, -16, -22, -23, -27 | MNNG-induced LsbBr mutants of strain BGMN1-596 | This study |
| BGMN1-596R3/11, -17, -19, -21, -25 | MNNG-induced LsbBr mutants of strain BGMN1-596 | This study |
| BGMN1-596R3/19-pAZILcos/MN2 | Complemented mutant with cosmid pAZILcos/MN2, LsbBs | This study |
| BGMN1-596R3/19-pAZIL/ZnMP | Complemented mutant with plasmid pAZIL/ZnMP, LsbBs | This study |
| BGMN1-596SR1 | Spontaneous LsbBr mutant | This study |
| BGMN1-596SR2 | Spontaneous LsbBr mutant | This study |
| Lactococcus lactis subsp. cremoris | ||
| MG7284 | Prt− Lac− Bacs Fusr Spcr LsbBr | |
| MG7284/pAZIL-lsbB | Derivative of MG7284 with pAZIL-lsbB, LsbBr, LsbB producer | This study |
| MG7284/pAZIL/ZnMP | Derivative of MG7284 with pAZIL/ZnMP, LsbBs | This study |
| Lactobacillus paracasei subsp. paracasei | ||
| BGHN14 | LsbBr | 27 |
| BGHN14/pAZILSJ/ZnMP | Derivative of BGHN14 with pAZILSJ/ZnMP, LsbBs | This study |
| Enterococcus faecalis | ||
| BGZLS10-27 | LsbBr | Laboratory collection |
| BGZLS10-27/pAZIL/ZnMP | Derivative of BGZLS10-27 with pAZIL/ZnMP, LsbBs | This study |
| Escherichia coli | ||
| DH5α | λ− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | 16 |
| HB101 | F− hsdS20 (rB− mB−) supE44 recA13 ara-14 proA2 rpsL20(Strr) xyl-5 mtl-1 galK2 lacY1 λ− | 28 |
| EC101 | JM101 containing repA gene of pWV01 in chromosome | 29 |
| Plasmids | ||
| pMN5 | 10 | |
| pAZIL | 7,109 bp; Emr, shuttle cloning vector | 18 |
| pAZIL/ZnMP | pAZIL carrying yvjB gene | This study |
| pAZILSJ | pAZIL with ori and repA sequence of pSJ2-8 | Laboratory collection |
| pAZILSJ/ZnMP | pAZILSJ carrying yvjB gene | This study |
| pGhost9 | Emr, thermosensitive vector | 19 |
| pGhost9/ES | pGhost9 carrying part of yvjB gene | This study |
| p-GEM-T-Easy | 3015 bp; Ampr, PCR cloning vector | Promega |
| Cosmids | ||
| pAZILcos | 8,194 bp; Emr, shuttle cosmid vector | 18 |
| pAZILcos/MN2 | Complemented cosmid pAZILcos carrying 40-kb chromosomal DNA fragment of BGMN1-596 | This study |
| pAZILcos/MN2-Sl2 | Cosmid pAZILcos/MN2 deleted with SalI restriction enzyme | This study |
| pAZILcos/MN2-Ps2 | Cosmid pAZILcos/MN2 deleted with PstI restriction enzyme | This study |
| pAZILcos/MN2-Nc2 | Cosmid pAZILcos/MN2 deleted with NcoII restriction enzyme | This study |
| pAZILcos/MN2-Sp9 | Cosmid pAZILcos/MN2 deleted with SpeI restriction enzyme | This study |
Ampr, resistance to ampicillin; Emr, resistance to erythromycin; Bac−, bacteriocin nonproducer; Bacs, sensitive to bacteriocin; Prt−, proteolytically inactive; Lac−, lactose-fermenting ability; LsbBs and LsbBr, sensitivity and resistance to LsbB bacteriocin, respectively.
Bacteriocin detection and activity assay.
For detection of bacteriocin activity, agar well diffusion assays were performed as described previously by Lozo et al. (11). A spot-on-lawn assay was used for semiquantitative measuring of sensitivity to synthetic LsbB (ChinaPeptides Co., Ltd., Shanghai, China). Precise measurements of resistance were performed using a microtiter plate assay (12).
DNA manipulations.
For clonal confirmation, pulse-field gel electrophoresis (PFGE) and DNA-DNA hybridization were performed, as described previously by Kojic et al. (10). Total and plasmid DNA from lactococci was isolated by the modified methods previously described (13, 14). For plasmid isolation from E. coli, a QIAprep Spin Miniprep Kit was used according to the manufacturer's recommendations (Qiagen, Hilden, Germany). Digestion with restriction enzymes was conducted according to the supplier's instructions (Fermentas). Plasmid constructs were introduced into lactococci, enterococci, and lactobacilli by electroporation using an Eporator (Eppendorf, Hamburg, Germany) (15). Standard heat shock transformation was used for plasmid transfer into E. coli (16). DNA fragments were purified from agarose gels using a QIAquick Gel extraction kit as described by the manufacturer (Qiagen). DNA was ligated with T4 DNA ligase (Agilent Technologies, USA) according to the manufacturer's recommendations. Sets of specific primers used in this study are listed in Table 2. KapaTaq DNA polymerase (Kapa Biosystems, Inc., Boston, MA, USA) was used to amplify DNA fragments by PCR using a GeneAmp PCR system 2700 thermal cycler (Applied Biosystems, Foster City, CA, USA). PCR products were purified with a QiaQuick PCR purification kit (Qiagen) according to the protocol of the supplier and sequenced by the Macrogen Sequencing Service (Macrogen, Netherlands). Sequences were compared to the NCBI database using BLAST. The DNA Strider program was used for open reading frame (ORF) prediction. Commercial p-GEM-T-Easy (Promega) vector was used for cloning of PCR products.
Table 2.
Sequence of specific primers used in this study
| Primer name | Sequence of primera | Template(s) |
|---|---|---|
| lsbBF | 5′-TAACGGATCCAATAGGGAAAATAG-3′ | pMN5 plasmid DNA |
| lsbBR | 5′-GCATAATAAAAACTGCAGCTATTG-3′ | |
| OFRF | 5′-GGCGTAAAAGATTCAGG-3′ | Chromosomal DNA of BGMN1-596, BGMN1-596R2, BGMN1-596R3, BGMN1-596SR, IL1403, and MG7284 |
| ORFR | 5′-GAAGGGTTGGTATAAGC-3′ |
Introduced restriction sites are indicated by underlined sequences.
N-Methyl-N′-nitro-N-nitrosoguanidine (MNNG) mutagenesis of LsbB-sensitive strain BGMN1-596.
L. lactis BGMN1-596 was grown overnight at 30°C in 10 ml of GM17 broth. The overnight culture was diluted 100-fold in 50 ml of GM17 broth and incubated at 30°C until the end of the exponential growth phase. Pelleted cells were washed with 10 ml of sodium phosphate buffer (100 mmol, pH 7), harvested by centrifugation, and finally resuspended in 1 ml of sodium phosphate buffer (100 mmol, pH 7). The cell suspension was divided into smaller volumes and mixed with 1 ml of MNNG (Sigma) dissolved in sodium phosphate buffer (pH 7) to obtain the final concentrations of 12.5, 25, 50, and 100 μg ml−1. The resulting mixtures were incubated for 1 h at 30°C. The cells were recovered by centrifugation, washed with 1 ml of sodium phosphate buffer (pH 7), resuspended in 1 ml of GM17 broth, and incubated for 1 h at 30°C. The mixtures were serially diluted (from 10−1 to 10−7), and cells were then grown on GM17 plates for determination of bacterial survival after mutagenesis (17). In addition, spontaneously LsbB-resistant mutants of BGMN1-596 were isolated as colonies growing in inhibition zones.
Construction of cosmid library of LsbB-sensitive strain BGMN1-596.
Total genomic DNA isolated from BGMN1-596 was partially digested with EcoRI at room temperature (ca. 23°C), and samples were collected at different time points. EDTA was immediately added to a final concentration of 10 mM (pH 8) to stop digestion. pAZILcos vector (18) was digested with EcoRI, dephosphorylated, and ligated with partially digested total DNA. Ligation mixtures were checked for the presence of high-molecular-weight concatemers by agarose gel electrophoresis before encapsulation into phage particles using a packaging kit (Agilent Technologies) and transduction of E. coli HB101 cells. Clones were selected on Luria-Bertani agar (LA) plates containing 300 μg ml−1 erythromycin. For long-term storage, the constructed cosmid library in E. coli was stored in LB medium containing 15% (vol/vol) glycerol at −80°C.
Construction of the yvjB gene knockout mutants.
The gene yvjB was insertionally inactivated in the bacterial genome by homologous integration using a previously described protocol (19) but with some minor modifications. A fragment containing part of the yvjB gene (EcoRI-ScaI; 946 bp) was cloned into pGhost9 vector, resulting in the construct pGhost9/ES. The LsbB-sensitive strains BGMN1-596 and IL1403 were transformed with pGhost9/ES and grown on GM17 plates at 28°C for 48 h. Obtained transformants were transferred to 28°C and 37°C and incubated for 48 h. In the next step, transformants grown at both temperatures were used as indicator strains in agar well diffusion assays. Strain MG7284/pAZIL-lsbB was used as a bacteriocin producer.
Global phenotypic testing of carbon source utilization.
Cellular respiration of L. lactis was measured in triplicate by the Phenotype MicroArrays system (Biolog, USA) according to the manufacturer's instructions. L. lactis strains (BGMN1-596WT and mutants resistant to LsbB) were streaked on GM17 agar plates. Colonies were scraped from the plates and resuspended in Biolog inoculating fluid IF-0a with growth supplements and Biolog redox dye mixture according to standard protocols recommended for Streptococcus species. Aliquots (100 μl) were added to each well of carbon source plates (PM1 and PM2). The plates were incubated at 30°C in an aerobic OmniLog incubator plate reader, and cellular respiration was measured kinetically by determining the colorimetric reduction of tetrazolium dye. Data were collected approximately every 10 min over a 72-h period and analyzed with Biolog kinetic and parametric software. The PM1 and PM2 Biolog assays assess the ability of a bacterium to utilize any of 190 carbon compounds as the sole carbon source.
Whole-genome sequencing.
DNA samples from wild-type and two mutant strains were sequenced at the Norwegian Sequencing Centre (sequencing.uio.no) using an Illumina MiSeq instrument, according to the manufacturer's recommendations. For each strain about 1.5 million paired-end reads of 2 by 250 bp were obtained. A preliminary assembly of the wild-type genome was obtained using ABySS (20), resulting in 157 contigs covering 2,465,230 bp. Genetic differences between the reference genome and the mutants were then detected using the polymorphism discovery algorithm VAAL from the Broad Institute (21), installed on the Abel Computing Cluster at the University of Oslo.
Nucleotide sequence accession number.
The nucleotide sequence of the cloned DNA fragment from cosmid pAZILcos/MN2 that carries the yvjB gene was submitted to EMBL and GenBank under accession number HG008906.
RESULTS
Heterologous expression of the lsbB gene in L. lactis MG7284.
As the original LsbB bacteriocin producer L. lactis BGMN1-5 is a multibacteriocin producer, the lsbB gene was cloned and expressed in the nonproducer L. lactis MG7284 to produce LsbB alone. The lsbB gene was amplified from plasmid pMN5 (isolated from BGMN1-596T) using primers lsbBF and lsbBR with incorporated restriction sites (BamHI and PstI, respectively) (Table 2). An amplicon of the expected size was purified, digested, and ligated into pAZIL vector predigested with the same restriction enzymes. A clone carrying the lsbB gene was first selected in E. coli DH5α and then transferred into L. lactis MG7284. The resulting LsbB-producing clone, L. lactis MG7284/pAZIL-lsbB, was used to screen for LsbB-resistant mutants.
Isolation of LsbB-resistant mutants from a random mutant bank of L. lactis BGMN1-596.
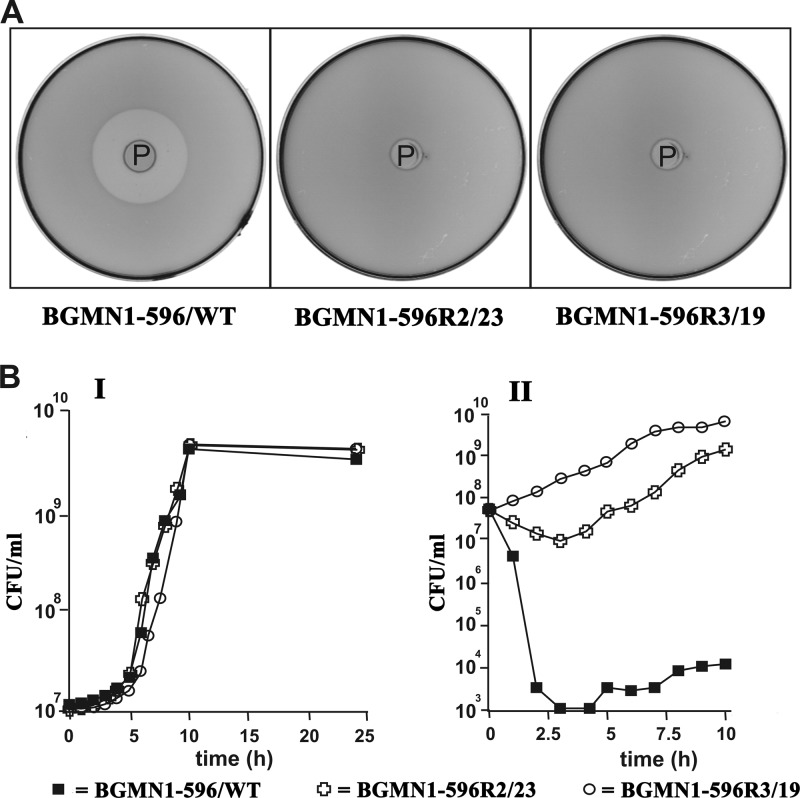
MNNG was used to generate random mutations in the wild-type L. lactis BGMN1-596. An appropriate survival rate of about 1% was achieved when MNNG was used at a concentration of 100 μg ml−1. LsbB-resistant mutants were selected on GM17 agar plates containing cell-free spent culture supernatant of the LsbB-producing clone L. lactis MG7284/pAZIL-lsbB. Thirty-nine colonies appeared on the selective plates after incubation for 48 h at 30°C. Cells from 5 of these 39 colonies were found to be sensitive to LsbB, while the remaining colonies still displayed a resistance phenotype, as shown for two such resistant mutants in Fig. 1A.
Fig 1.
(A) Inhibition plate assay comparing the wild-type strain BGMN1-596/WT with resistant mutants (BGMN1-596R2/23 and BGMN1-596R3/19) used as indicators. LsbB-producing (P) cells of L. lactis MG7284 pAZIL-lsbB were applied into the wells made in the indicator lawns. Plates were incubated overnight at 30°C for development of inhibition zones. (B) Growth rate of WT cells compared with that of resistant cells in the medium without (I) and with (II) 25 μg ml−1 LsbB.
Analysis of LsbB-resistant mutants.
Using synthetic LsbB in the bacteriocin test, we found that the resistant mutants could be divided into two groups, designated BGMN1-596R2 and BGMN1-596R3 (Table 3), based on their tolerance levels toward LsbB. Resistant mutants of the BGMN1-596R3 group had the ability to grow at LsbB concentrations above 1 mg ml−1, whereas mutants of the BGMN1-596R2 group could grow at LsbB concentrations of up to 62.5 μg ml−1. In contrast, the wild-type strain BGMN1-596 was capable of growth only in medium with less than 125 ng ml−1 of LsbB. Five mutants from each group were stored and used for further analysis.
Table 3.
Summary of mutations within the yvjB gene in LsbB-resistant mutants
| Designation of mutant | Location of mutations in the yvjB gene | Size (aa) |
|---|---|---|
| MNNG-induced mutants | ||
| Group BGMN1-596R2 | ||
| BGMN1-596R2/12 | Gly→Ser (188) | 428 |
| BGMN1-596R2/16 | Gly→Ser (188); Phe→Leu (414); Val→Gly (415); Asn→Lys (428) | 428 |
| BGMN1-596R2/22 | Gly→Ser (188); Phe→Leu (414); Val→Gly (415); Asn→Met (428) | 428 |
| BGMN1-596R2/23 | Gly→Ser (188) | 428 |
| BGMN1-596R2/27 | Gly→Ser (188); Phe(TTT)→STOP codon TAA(414) | 413 |
| Group BGMN1-596R3 | ||
| BGMN1-596R3/11 | Gln(CAA)→STOP codon TAA(172) | 171 |
| BGMN1-596R3/17 | Gln(CAA)→STOP codon TAA(172) | 171 |
| BGMN1-596R3/19 | Trp(TGG)→STOP codon TAG(248) | 247 |
| BGMN1-596R3/21 | Base C at position 558 deleted; STOP codon TGA(570) | 189 |
| BGMN1-596R3/25 | Gln(CAA)→STOP codon TAA(172) | 171 |
| Spontaneous mutants | ||
| Group BGMN1-596SR | ||
| BGMN1-596SR1 | Trp(TGG) →STOP codon TAG(26) | 25 |
| BGMN1-596SR2 | Trp(TGG) →STOP codon TGA(248) | 247 |
The growth patterns of the strain BGMN1-596 and mutants BGMN1-596R2/23 and BGMN1-596R3/19 were very similar in LsbB-free medium but differed greatly from each other when the strains were grown in a medium containing LsbB (25 mg ml−1), with BGMN1-596R3/19 having the best growth, followed by BGMN1-596R2/23, while the growth of the wild-type control strain BGMN1-596 as expected was severely affected (Fig. 1B).
In addition to the MNNG-generated resistant mutants, two spontaneously resistant mutants, named BGMN1-596SR1 and BGMN1-596SR2 (group BGMN1-596SR), were isolated from cultures exposed to high concentrations of LsbB (50 to 250 mg ml−1). In agar well diffusion assays these two mutants showed a resistance level similar to that of the BGMN1-596R3 group of mutants (Table 3).
LsbB bacteriocin does not use sugar transporters as a receptor.
It is known that pediocin-like bacteriocins (class IIa) and lactococcin A (a class IId bacteriocin from L. Lactis) employ man-PTS as a receptor. Resistant mutants to these bacteriocins normally harbor mutations that lead to downregulation of man-PTS genes (22) or to a nonfunctional man-PTS (mutations within the man-PTS genes themselves) (7). To determine whether LsbB employs man-PTS as a receptor, agar well diffusion assays with the wild-type L. lactis IL1403 and a man-PTS deletion mutant (strain B464) were performed. It was observed that LsbB killed bacterial cells of both strains with equal efficiency, confirming that man-PTS is not a receptor for LsbB. In addition, a metabolic assay (Biolog) with a panel of 190 different carbon sources was performed for two different LsbB-resistant mutants and the wild-type strain in order to examine whether another sugar transporter could be involved as a receptor. In this assay, we also included lactococcin A-resistant mutants and garvicin ML-resistant mutants as controls since the receptors for these bacteriocins (man-PTS for lactococcin A and maltose-ABC transporter for garvicin ML) are involved in sugar metabolism. As expected, the control experiment clearly demonstrated that lactococcin A-resistant mutants and garvicin ML-resistant mutants could not ferment glucose and maltose, respectively, substrates of the corresponding transporters. For the two LsbB-resistant mutants, some changes were found in the metabolic patterns compared with wild-type cells, but none of these changes were shared by both mutants.
Reversion of the resistant mutant BGMN1-596R3/19 to wild-type sensitivity by transformation with cosmids.
A cosmid library of the sensitive strain BGMN1-596 was constructed, and DNA samples from 148 cosmid clones with average insert sizes of 40 kb were individually isolated and transferred by electroporation into the resistant mutant BGMN1-596R3/19 for complementation. One cosmid clone, termed pAZILcos/MN2, was found to restore sensitivity to LsbB. This cosmid was also found to complement all other resistant mutants (BGMN1-596R2, BGMN1-596R3, and BGMN1-596SR, representing all three groups), indicating that they all contain mutations within the region that is contained in the cosmid pAZILcos/MN2. Using PFGE SmaI macrorestriction pattern analysis, it was confirmed that all isolated and complemented mutants were derivatives of the corresponding parental strain.
A gene encoding a membrane-bound metallopeptidase is required for LsbB sensitivity.
To identify the minimal genetic unit on the cosmid pAZILcos/MN2 that could transfer sensitivity to LsbB, pAZILcos/MN2 was digested with different restriction enzymes and subcloned into pAZIL before being transferred to the resistant mutant BGMN1-596R3/19 for functional analysis. Several constructs were able to restore sensitivity in BGMN1-596R3/19 (Fig. 2). These clones were used for mapping and sequencing the region of the pAZILcos/MN2 cosmid responsible for bacteriocin sensitivity (Fig. 2). The smallest DNA fragment that lad to regained LsbB sensitivity, construct Sp9, was completely sequenced by primer walking. The fragment was found to encode five open reading frames (ORFs), one of which is truncated (uppS, cdsA, yvjB, proS, and truncated noxD) (Fig. 2). To dissect further, the individual ORFs were subcloned and again transferred into BGMN1-596R/19. Only the clone (pAZIL/ZnMP) that contained the yvjB gene was able to restore sensitivity. This clone also rendered all other LsbB-resistant mutants sensitive to LsbB. Thus, the gene yvjB appears to be the minimal genetic unit required to restore bacteriocin sensitivity.
Fig 2.
Linear gene map of cosmid pAZILcos/MN2 and the scheme of constructed clones used for homologous and heterologous expression of the yvjB gene. Relevant restriction sites are indicated. The size and orientation of predicted ORFs are indicated by arrows.
yvjB knockout mutants confer resistance to LsbB.
To confirm that the gene yvjB is required for LsbB sensitivity in other L. lactis strains as well, yvjB knockout mutants of BGMN1-596 and IL1403 were constructed by insertional inactivation using the clone pGhost9/ES. pGhost9/ES contains a heat-labile replicon and a fragment of the yvjB gene and allows inactivation of yvjB by homologous recombination at elevated temperature (37°C). The results showed that transformants grown at 28°C (controls) were sensitive to LsbB bacteriocin, whereas transformants grown at 37°C became resistant to LsbB, indicating that inactivation of the yvjB gene confers resistance to LsbB.
Comparative DNA sequence analyses of the yvjB gene in LsbB-resistant mutants.
In parallel to the cloning approach described above, the genomes of two resistant mutants (BGMN1-596R2/23 and BGMN1-596R3/19) and the wild-type strain BGMN1-596 were sequenced to identify genes that may be involved in the resistance phenotype. It was found that both mutants indeed harbored mutations in the yvjB gene (Table 3), in addition to a number of other mutations dispersed in the genome. The yvjB mutation in strain BGMN1-596R2/19 generated a stop codon at position 248 of the encoded protein, leading to a truncated protein of 247 aa compared to the wild-type protein of 428 aa. The yvjB mutation in BGMN1-596R3/23 generated a frameshift mutation in codon 188. Sequencing of PCR-amplified yvjB from the 10 remaining resistant mutants (groups BGMN1-596R2, BGMN1-596R3, and BGMN1-596SR) gave similar results as all were shown to contain different mutations within this gene (Table 3). In all five mutants of the BGMN1-596R2 group, Gly-188 was replaced with Ser in the YvjB protein, giving a mutated protein of the same size (428 aa) as the wild type. Mutant BGMN1-596R2/27 in addition had a stop codon at position 414 producing a truncated form of the YvjB protein (413 aa). All mutants of group BGMN1-596R3 and the spontaneously resistant mutants (group BGMN1-596SR) carried stop codons at different positions, leading to various truncated forms of the YvjB protein (Table 3). Taken together, the sequencing results are in line with the results from the cloning approach, all of which indicates that gene yvjB is required for sensitivity to LsbB and likely serves as a receptor for the bacteriocin.
Heterologous expression of the yvjB gene confers sensitivity to LsbB.
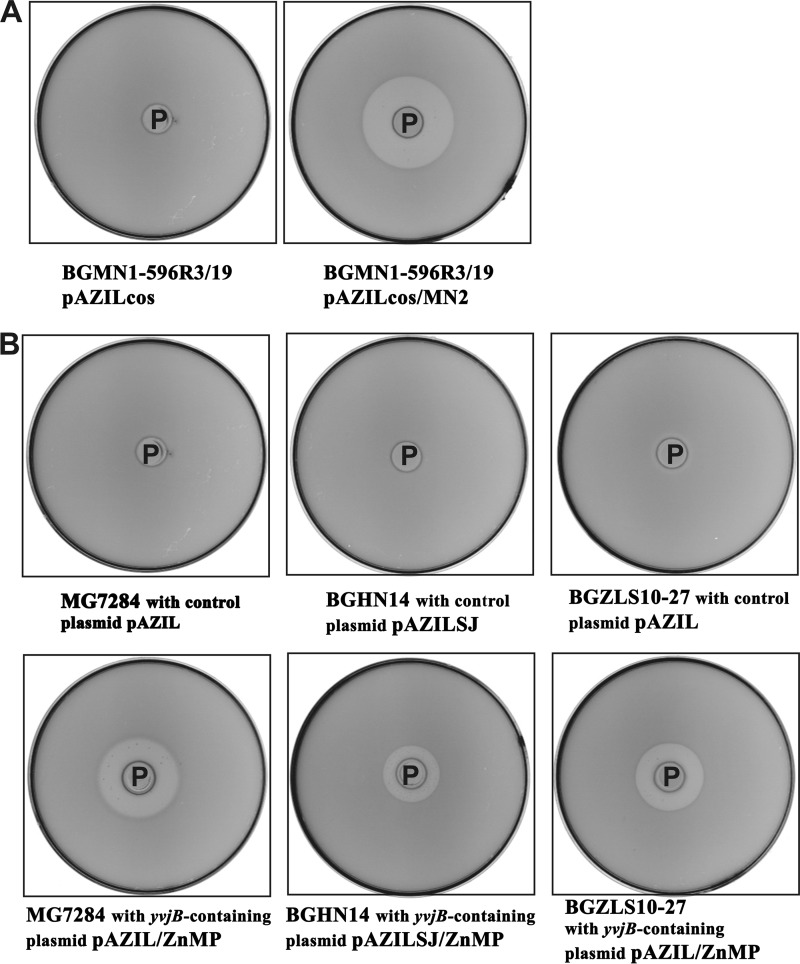
To examine whether the yvjB-dependent sensitivity to LsbB is strain or species specific, this gene was transferred into cells of various naturally resistant strains: L. lactis MG7284, E. faecalis BGZLS10-27, and L. paracasei BGHN14. For this purpose, the complete yvjB gene was cloned into pAZIL and pAZIL-SJ, giving rise to the constructs pAZIL/ZnMP and pAZILSJ/ZnMP, respectively. The construct pAZIL/ZnMP replicates in L. lactis and E. faecalis, whereas pAZILSJ/ZnMP replicates only in L. paracasei. Transformation of these bacteria with the cloned yvjB indeed rendered all of the strains sensitive to LsbB (Fig. 3), indicating that the observed yvjB-dependent sensitivity is not strain or species specific.
Fig 3.
Complementation assay. (A) Resistant mutant BGMN1-596R3/19 containing the empty plasmid (pAZILcos) or a plasmid carrying the yvjB gene (pAZIL-MN2). L. lactis MG7284/pAZIL-lsbB was used as an LsbB producer. (B) Heterologous expression of yvjB renders resistant cells of L. lactis subsp. lactis MG7284, Lactobacillus paracasei BGHN14, and Enterococcus faecalis BGZLS10-27 sensitive to the bacteriocin. Strains and plasmids are as indicated. See the legend of Fig. 1 for the experimental setup.
DISCUSSION
Most bacteriocins have relatively narrow inhibition spectra, often displaying activity toward species closely related to the bacteriocin producer. Due to this strong specificity, it is generally believed that most, if not all, bacteriocins recognize specific receptors on target cells. Although numerous bacteriocins from Gram-positive bacteria have been characterized over the years, only a few receptors so far have been reported; these are lipid II recognized by nisin and several other lantibiotics, as well as the class IId bacteriocin lactococcin 972 (3, 4, 23), man-PTS, targeted by class IIa pediocin-like bacteriocins and the class IId lactococcins A and B (5, 24), and maltose-ABC transporter, recognized by the circular bacteriocin garvicin ML (8). The possibility that LsbB employs sugar transporters as receptors was also explored. The results of a bacteriocin test (with strain B464 as an indicator) and Biolog assay strongly indicate that the resistance phenotype observed for the LsbB mutants did not result from a defective sugar metabolic pathway. In the present work, we report the discovery of a new receptor, namely, the membrane-bound Zn-dependent metallopeptidase YvjB, targeted by LsbB.
The identification of the membrane-bound Zn-dependent metallopeptidase YvjB as a receptor for LsbB is based on several lines of evidence: (i) by cosmid construction and gene subcloning, we were able to find the minimum genetic unit capable of recovering sensitivity to LsbB in LsbB-resistant mutants, and this minimum genetic unit contains only the gene yvjB; (ii) all 12 independently isolated LsbB-resistant mutants contain mutations within yvjB; (iii) inactivation of yvjB by directed gene disruption in LsbB-sensitive L. lactis strains BGMN1-596 and IL1403 rendered them resistant to the bacteriocin; and (iv) perhaps most convincingly, yvjB also conferred sensitivity to LsbB when expressed in heterologous hosts outside the Lactococcus genus, such as Lactobacillus paracasei and Enterococcus faecalis, which are normally resistant to LsbB. The last observation also suggests that the protein YvjB most likely serves as the sole receptor unit (i.e., without the requirement of additional factors) and acts in a species-independent manner.
Comparative DNA sequence analysis of yvjB genes from all three groups of mutants (BGMN1-596R2, BGMN1-596R3, and BGMN1-596SR) revealed two types of mutations within yvjB that gave rise to the LsbB resistance phenotype. The resistance arose either when Gly-188 was replaced with Ser, as shown for the BGMN1-596R2 group of mutants, or when truncated forms of YvjB lacking the C-terminal part were produced, as observed in the BGMN1-596R3 and BGMN1-596SR groups of mutants. Different levels of resistance to LsbB were observed for the different groups of mutants, with BGMN1-596R3 and BGMN1-596SR mutants (encoding truncated versions of YvjB) more resistant than the BGMN1-596R2 mutants (YvjB containing the Gly-to-Ser substitution at position 188). It is reasonable to believe that the truncated forms of YvjB are structurally deficient and incapable of serving as receptors. The molecular basis of the resistance resulting from the replacement of Gly-188 with Ser is less obvious, as there are several possibilities for how such a substitution might cause a resistance phenotype. The substitution might cause a structural change, somehow becoming a nonfunctional or less functional receptor for LsbB. (ii) The amino acid Gly-188 might play an important role in receptor binding; the nonconserved substitution to Ser which is both larger and more hydrophilic than Gly-188, could interfere with the interaction with LsbB. (iii) Gly-188 might directly be involved in pore formation; a nonconserved substitution at this residue might somehow affect the properties of the pore (i.e., blocking pore formation). Arguably, other possibilities might likely exist. However, shedding light on the role of this interesting residue in terms of bacteriocin sensitivity and mode of resistance development requires further and more detailed investigation. It is worth noting that Gly-188 is almost completely conserved (present in 7,947 out of 8,451 sequences) in the corresponding position in the Pfam family PF02163 (peptidase_M50), pointing to an essential role for Gly in this position.
Interestingly, the strain L. lactis MG1363 also contains a yvjB gene in its genome; the encoded YvjB protein has the same length (428 aa) and shows a relatively high sequence identity (93%; 397 of 428 aa), to the YvjB ortholog in the LsbB-sensitive strains BGMN1-596 and IL1403. Nevertheless, MG1363 is resistant to LsbB. The sequence around Gly-188 is identical in all three strains (KMLTNFGGPLNNFILG; Gly-188 is underlined), suggesting that some of the other substitutions (31 in total) in MG1363 YvjB may be responsible for the observed resistance in this particular strain.
Class II bacteriocins are numerous and very diverse in terms of sequence, structure, and physicochemical properties and are thus believed to recognize different receptors on target cells. Similarly, bacterial cells contain a plethora of membrane proteins that are involved in diverse activities across the membrane, including transport of diverse salts and sugars, energy harvesting, cell-to-cell communication, structural functions, etc. It is reasonable to believe that any of these proteins have the potential to serve as receptors for bacteriocins. However, so far only a few membrane-located proteins are known as receptors for class II bacteriocins; these comprise a man-PTS, a maltose-ABC transporter, and, from this work, a Zn-dependent metallopeptidase. The limited progress in receptor identification is probably due to a lack of efficient strategies for this purpose. In the present work, we demonstrate an efficient and novel strategy involving generation of resistant mutants, complementation by cosmid cloning, genome sequencing, and metabolic analysis to identity novel bacteriocin receptors. Indeed, we are currently exploiting this strategy to identify receptors also for other bacteriocins, and some promising preliminary results have been obtained. Importantly, the combination of the generation of resistant mutants and genome sequencing proves to be a very powerful approach to identify potential receptor loci, which can be further assessed by other molecular tools (e.g., gene cloning). As genome sequencing has become a very cost-effective and hence more available genetic tool, more receptors are likely to be discovered in the near future.
ACKNOWLEDGMENTS
The Ministry of Education and Science of the Republic of Serbia, Republic of Serbia (grant number 173019), supported this work. D.B.D. is partly funded by NOCC.
Footnotes
Published ahead of print 11 October 2013
REFERENCES
- 1.Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777–788 [DOI] [PubMed] [Google Scholar]
- 2.Breukink E, Wiedemann I, van Kraaij C, Kuipers OP, Sahl H, de Kruijff B. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361–2364 [DOI] [PubMed] [Google Scholar]
- 3.Wiedemann I, Breukink E, van Kraaij C, Kuipers OP, Bierbaum G, de Kruijff B, Sahl HG. 2001. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 276:1772–1779 [DOI] [PubMed] [Google Scholar]
- 4.Martinez B, Bottiger T, Schneider T, Rodriguez A, Sahl HG, Wiedemann I. 2008. Specific interaction of the unmodified bacteriocin lactococcin 972 with the cell wall precursor lipid II. Appl. Environ. Microbiol. 74:4666–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diep DB, Skaugen M, Salehian Z, Holo H, Nes IF. 2007. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc. Natl. Acad. Sci. U. S. A. 104:2384–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drider D, Fimland G, Héchard Y, McMullen LM, Prévost H. 2006. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 70:564–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kjos M, Salehian Z, Nes IF, Diep DB. 2011. Mechanisms of resistance to bacteriocins targeting the mannose phosphotransferase system. Appl. Environ. Microbiol. 77:3335–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrielsen C, Brede DA, Hernández PE, Nes IF, Diep DB. 2012. The maltose ABC transporter in Lactococcus lactis facilitates high-level sensitivity to the circular bacteriocin Garvicin ML. Antimicrob. Agents Chemother. 56:2908–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gajic O, Buist G, Kojic M, Topisirovic L, Kuipers OP, Kok J. 2003. Novel mechanism of bacteriocin secretion and immunity carried out by lactococcal multidrug resistance proteins. J. Biol. Chem. 278:34291–34298 [DOI] [PubMed] [Google Scholar]
- 10.Kojic M, Strahinic I, Fira D, Jovcic B, Topisirovic L. 2006. Plasmid content and bacteriocin production by five strains of Lactococcus lactis isolated from semi-hard homemade cheese. Can. J. Microbiol. 52:1110–1120 [DOI] [PubMed] [Google Scholar]
- 11.Lozo J, Vukasinovic M, Strahinic I, Topisirovic L. 2004. Characterization and antimicrobial activity of bacteriocin 217 produced by natural isolate Lactobacillus paracasei subsp. paracasei BGBUK2-16. J. Food Prot. 67:2727–2734 [DOI] [PubMed] [Google Scholar]
- 12.Holo H, Nilssen O, Nes IF. 1991. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J. Bacteriol. 173:3879–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopwood DA, Bib JM, Chater JF, Kieser T, Bruton CJ, Kieser HM, Lydiate JD, Smith CP, Ward JM, Schemph H. 1985. Genetic manipulation of Streptomyces: a laboratory manual. The John Innes Foundation, Norwich, United Kingdom [Google Scholar]
- 14.O'Sullivan DJ, Klaenhammer TR. 1993. Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl. Environ. Microbiol. 59:2730–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holo H, Nes IF. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557–580 [DOI] [PubMed] [Google Scholar]
- 17.Adelberg E, Mandel M, Chen GCC. 1965. Optimal conditions for mutagenesis by N-methyl-N′-nitro-N-nitrosoguanidine in Escherichia coli K12. Biochem. Biophys. Res. Commun. 18:788–795 [Google Scholar]
- 18.Kojic M, Jovcic B, Strahinic I, Begovic J, Lozo J, Veljovic K, Topisirovic L. 2011. Cloning and expression of a novel lactococcal aggregation factor from Lactococcus lactis subsp. lactis BGKP1. BMC Microbiol. 11:265. 10.1186/1471-2180-11-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maguin E, Prevost H, Ehrlich SD, Gruss A. 1996. Efficient insertional mutagenesis in lactococci and other Gram-positive bacteria. J. Bacteriol. 178:931–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson JT, Wong K, Jackman SD, Schein JE, Jones SJM, Birol I. 2009. ABySS: a parallel assembler for short read sequence data. Genome Res. 19:1117–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nusbaum C, Ohsumi TK, Gomez J, Aquadro J, Victor TC, Warren RM, Hung DT, Birren BW, Lander ES. 2009. Sensitive, specific polymorphism discovery in bacteria using massively parallel sequencing. Nat. Methods 6:67–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robichon D, Gouin E, Debarbouille M, Cossart P, Cenatiempo Y, Hechard Y. 1997. The rpoN (σ54) gene from Listeria monocytogenes is involved in resistance to mesentericin Y105, an antibacterial peptide from Leuconostoc mesenteroides. J. Bacteriol. 179:7591–7594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasper HE, Kramer NE, Smith JL, Hillman JD, Zachariah C, Kuipers OP, de Kruijff B, Breukink E. 2006. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313:1636–1637 [DOI] [PubMed] [Google Scholar]
- 24.Kjos M, Salehian Z, Nes IF, Diep DB. 2010. An extracellular loop of the mannose phosphotransferase system component IIC is responsible for specific targeting by class IIa bacteriocins. J. Bacteriol. 192:5906–5913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chopin A, Chopin MC, Moillo-Batt A, Langella P. 1984. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid 11:260–263 [DOI] [PubMed] [Google Scholar]
- 26.Gajic O, Kojic M, Banina A, Topisirovic L. 1999. Characterization of natural isolate Lactococcus lactis subsp. lactis BGMN1-5, a strain producing two bacteriocins, cell wall-associated proteinase and showing clumping phenotype. Arch. Biol. Sci. 51:69–78 [Google Scholar]
- 27.Kojic M, Fira D, Banina A, Topisirovic L. 1991. Characterization of the cell wall-bound proteinase of Lactobacillus casei HN14. Appl. Environ. Microbiol. 57:1753–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyer HW, Roulland-Dussoix D. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459–472 [DOI] [PubMed] [Google Scholar]
- 29.Law J, Buist G, Haandrikman A, Kok J, Venema G, Leenhouts K. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177:7011–7018 [DOI] [PMC free article] [PubMed] [Google Scholar]