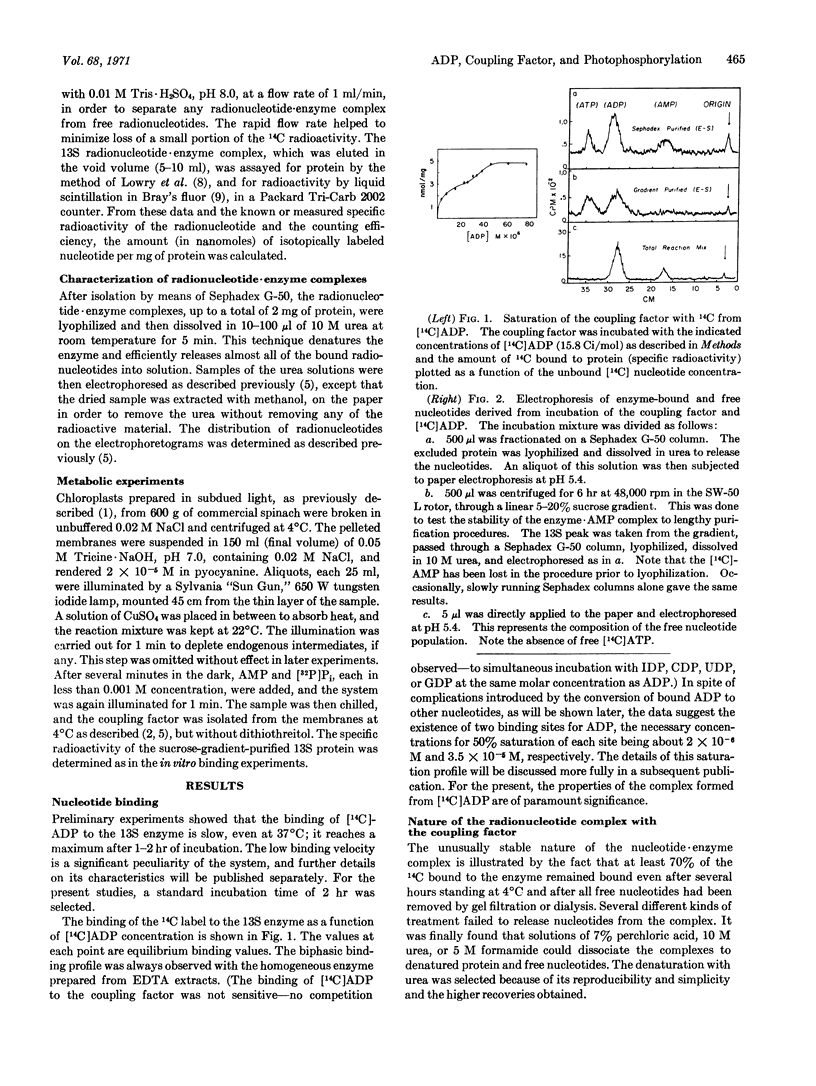
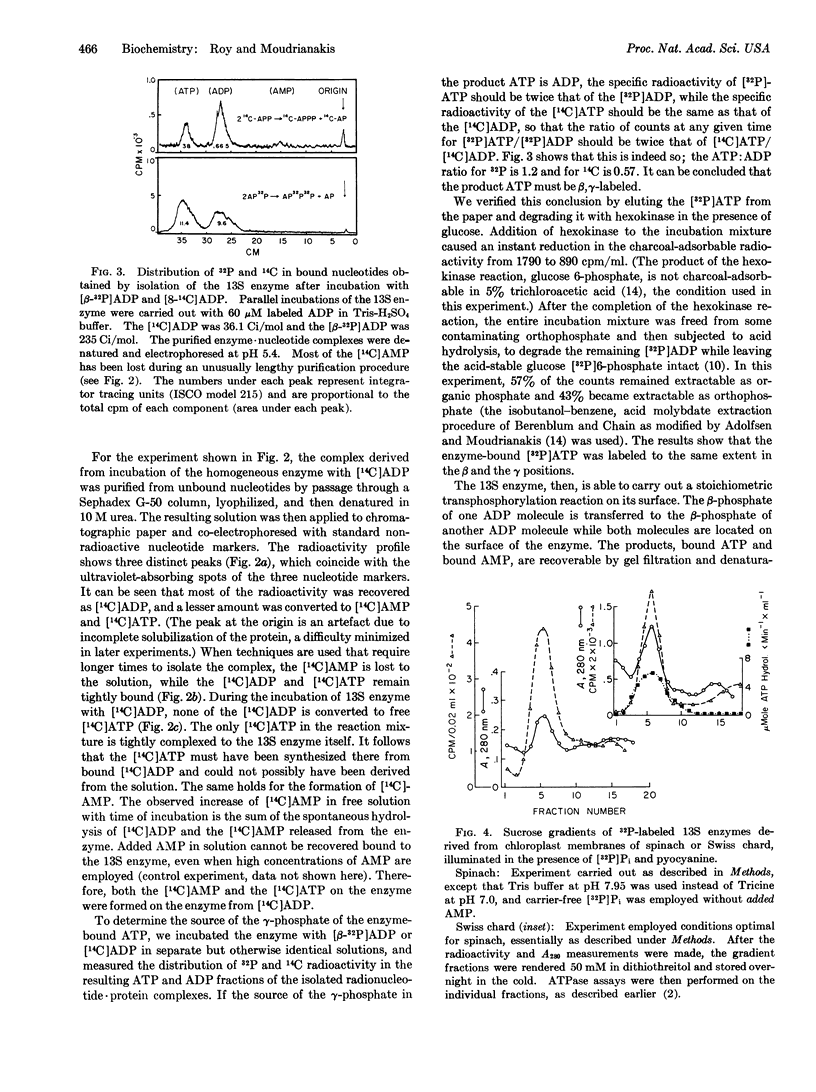
Abstract
The coupling factor of photophosphorylation, which carries out the terminal steps in the light-dependent synthesis of ATP in spinach chloroplasts, forms tight complexes with [14C]ADP in vitro. The bound [14C]ADP undergoes a transphosphorylation reaction to give [14C]AMP and [14C]ATP. The [14C]ATP remains tightly bound, and can be recovered conveniently only by denaturation of the enzyme nucleotide complex. If spinach membranes are illuminated in the presence of pyocyanine and [3H]AMP or [32P]Pi, the enzyme can be recovered as a tight complex with [3H]ADP or [32P]ADP. The evidence indicates that AMP is an earlier acceptor of phosphate than is ADP, in a light-driven phosphorylation reaction. It also suggests that AMP serves as a cofactor in photophosphorylation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Bennun A., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. IV. Interaction of coupling factor 1 from chloroplasts with components of the chloroplast membrane. J Biol Chem. 1969 Mar 10;244(5):1325–1331. [PubMed] [Google Scholar]
- Hind G., Jagendorf A. T. SEPARATION OF LIGHT AND DARK STAGES IN PHOTOPHOSPHORYLATION. Proc Natl Acad Sci U S A. 1963 May;49(5):715–722. doi: 10.1073/pnas.49.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. H., Moudrianakis E. N. Function of the "quantasome" in photosynthesis: structure and properties of membrane-bound particle active in the dark reactions of photophosphorylation. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1261–1268. doi: 10.1073/pnas.58.3.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. H., Moudrianakis E. N. Hill reaction site in chloroplast membranes: non-participation of the quantasome particle in photoreduction. J Mol Biol. 1967 Jul 28;27(2):323–333. doi: 10.1016/0022-2836(67)90023-x. [DOI] [PubMed] [Google Scholar]
- Karu A. E., Moudrianakis E. N. Fractionation and comparative studies of enzymes in aqueous extracts of spinach chloroplasts. Arch Biochem Biophys. 1969 Feb;129(2):655–671. doi: 10.1016/0003-9861(69)90226-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCarty R. E., Racker E. Effect of a coupling factor and its antiserum on photophosphorylation and hydrogen ion transport. Brookhaven Symp Biol. 1966;19:202–214. [PubMed] [Google Scholar]
- VAMBUTAS V. K., RACKER E. PARTIAL RESOLUTION OF THE ENZYMES CATALYZINE PHOTOPHOSPHORYLATION. I. STIMULATION OF PHOTOPHOSPHORYLATION BY A PREPARATION OF A LATENT, CA++- DEPENDENT ADENOSINE TRIPHOSPHATASE FROM CHLOROPLASTS. J Biol Chem. 1965 Jun;240:2660–2667. [PubMed] [Google Scholar]
- Weiss B., Live T. R., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. V. End group labeling and analysis of deoxyribonucleic acid containing single straned breaks. J Biol Chem. 1968 Sep 10;243(17):4530–4542. [PubMed] [Google Scholar]


