Abstract
OBJECTIVES
Primary cusp repair + aortic root reimplantation in bicuspid aortic valve (BAV) disease presenting with root aneurysm with aortic insufficiency (AI) is an effective surgical treatment. We assessed whether the geometric orientation of the repaired BAV into its reimplanted neoroot affects outcomes—180°/180° orientation was compared with the 150°/210° orientation.
METHODS
From 2005 to 2012, 66 BAV repairs were performed. This is a retrospective review of all types of Ib/II BAV AI patients undergoing root reimplantation (n = 26) at two different geometric orientations: 180°/180° (n = 11) vs 150°/210° (n = 15). In the 180°/180° group, reimplantation into the neoroot was such that both conjoint and non-conjoint cusps occupied 180° of the annular circumference. In the 150°/210° group, the repaired valve was configured to the more typical native orientation of a type I BAV: the non-conjoint cusp occupied 150°, and the conjoint cusp occupied 210° of the annular circumference.
RESULTS
Preoperative characteristics were similar in both groups. In-hospital mortality, stroke, reoperation, renal failure and pacemaker rates were zero in both groups. No patient left the operating room with >1+ AI and one had a peak gradient >20 mmHg. Transvalvular gradients were higher in the 180°/180° group, but not significant (P > 0.05). M.ean follow-ups for the 180°/180° and 150°/210° group were 48 and 33 months, respectively. Actuarial freedom from AI >2+ at 5 years was 100% in both groups. Freedom from AI >1+ at 5 years was 90 ± 10% in the 150°/210° group and 86 ± 13% in the 180°/180° group (P = 0.71). Freedom from peak gradient >20 mmHg was 80% (n = 8) in the 180°/180° group and 100% in the 150°/210° group at 1-year follow-up. Transvalvular gradients were higher in the 180°/180° group (16 ± 8 vs 10 ± 4 mmHg, P = 0.02; 9 ± 3 vs 5 ± 3 mmHg, P = 0.01). Five-year actuarial survival and freedom from aortic reoperation have remained at 100% in the entire cohort.
CONCLUSION
Cusp repair + root reimplantation for BAV type Ib/II AI can be safely performed at either geometric orientation. Conceptually, 150°/210° orientation respects the natural type I BAV anatomy with regard to cusp surface area and leaflet insertion perimeter. The 180°/180° group may have higher transvalvular gradients and smaller coaptation zones than the 150°/210° group. Further follow-up may reveal the superiority of one geometric orientation over the other.
Keywords: Bicuspid aortic valve, Root reimplantation, Aortic valve repair
INTRODUCTION
Bicuspid aortic valve (BAV) disease affects 1–2% of the population [1–3]. These patients typically present in their third to fifth decades of life with a spectrum of aortopathy and/or aortic valvulopathy. In those with aortic stenosis and root aneurysm, a Bentall aortic root replacement is performed. Valve replacement in BAV patients has several disadvantages, whether they are mechanical or bioprosthetic valves [4–6]. Therefore, valve sparing root reimplantation or valve repair, if possible, is a very attractive option in BAV patients [4–12]. Although valve sparing or repair procedures are not feasible in BAV presenting with moderate to severe aortic stenosis, repair techniques for regurgitant BAVs are well described by several select centres [13–16]. In addition, recent work has shed light on the outcomes in BAV patients undergoing valve repair + annular stabilization (sub-commissural annuloplasty) vs primary valve repair + root reimplantation [17–22]. The data suggest that root stabilization is critical in the context of a repaired BAV for long-term durability (freedom from aortic insufficiency, AI).
At our institution, BAV patients presenting with AI are primarily selected for BAV repair, unless intraoperative findings contradict a successful repair technique. Primary valvulopathy is treated with primary cusp repair + sub-commissural annuloplasty (at least historically). In addition, patients with ascending aortic aneurysm (>4.5 cm) undergo a concomitant proximal aorta replacement. BAV patients with AI + aortic root aneurysm, meeting the criteria for root replacement, undergo primary cusp repair + root reimplantation (El Khoury repair technique + David V reimplantation technique).
Unlike with tricuspid aortic valve, BAV root (sinus segment) reimplantation has several differences. Although BAV is divided into type 0 or type 1 valves, patients may present with either one of the two types, or in a spectrum between type 0 and type 1 [23, 24]. Classic type 0 BAV patients have each leaflet occupying 180° of the circumferential annulus (half the surface area). Root reimplantation in these patients is performed with the repaired valve reimplanted into the neoroot at 180°/180° circumferential orientation. Initially, when we embarked on this procedure, BAV root reimplantation in both type 0 and type I BAV patients had the aortic neoroot oriented at the 180°/180° geometric configuration. Several select centres that perform this procedure also reimplant the neoroot at this orientation.
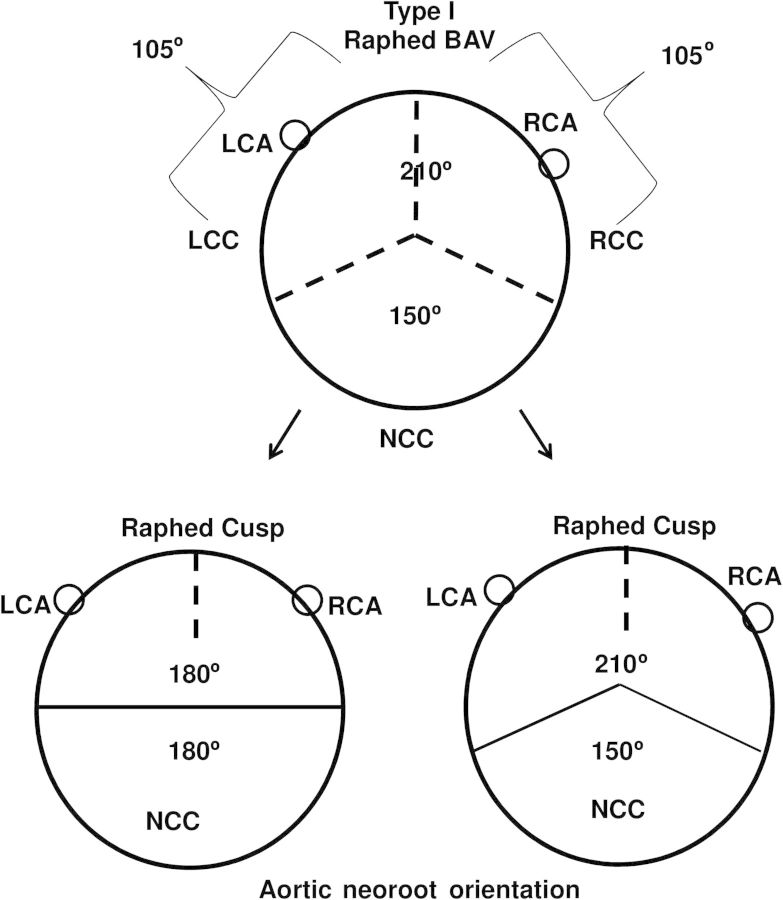
Type I BAV typically has conjoint and non-conjoint cusps, with the conjoint cusp possessing a prominent median raphe that attaches to the annular base as a pseudo-commissure [23]. Although its native circumferential orientation can be variable, classically, the conjoint cusp occupies 7/12th of the valve surface area (210° circumferential orientation) [25], and the non-conjoint cusp occupies 5/12th of the surface area (150° circumferential orientation) (Fig. 1). This 150°/210° orientation of the native type I BAV is the more common presentation than the 180°/180° configuration. Since patients presenting with valvulopathy represent a failure of the type I BAV at this native orientation, over the past 6 years we have adopted a ‘respect native orientation’ as our preferred geometric orientation of the repaired BAV reimplanted into its neoroot, rather than a 180°/180° geometric orientation (Fig. 1). We report our institutional outcomes comparing type I BAV patients (all with raphed right/left conjoint cusp) undergoing primary cusp repair + root reimplantation with the aortic neoroot reoriented at 180°/180° vs 150°/210° geometric configuration.
Figure 1:
Orientation of the aortic neoroot during type I BAV root reimplantation at the two geometric configurations: 180°/180° or 150°/210°. LCA: left coronary artery RCA: right coronary artery LCC: left coronary cusp RCC: right coronary cusp; NCC: non-coronary cusp.
MATERIALS AND METHODS
This study was approved by the institutional review board of the Hospital of the University of Pennsylvania.
Patient population
From 2005 to 2012, 162 patients were treated for BAV disease presenting with pure AI only (no mixed AI + AS). Forty underwent single cusp repair with sub-commissural annuloplasty. One hundred and twenty-two patients had concomitant aortic dilatation meeting the criteria for root replacement/root reimplantation. Twenty-six patients in this group underwent primary cusp repair + root reimplantation. The remainder had Bentall-type procedures. Data were analysed from a prospectively maintained database on all BAV patients.
Anatomical features of the BAV
Only raphed BAV patients with type Ib/II AI with root aneurysm were included in the analysis [25]. All patients in this group had a conjoint cusp with a median raphe and a pseudo-commissure. In all cases, the conjoint cusp occurred between the right and left coronary leaflets. Initially, we strictly performed reimplantation at 180°/180°. Upon re-evaluation of the BAV root geometry, we adopted an intraoperative algorithm to decide on the neoroot orientation [25]. Upon completion of the BAV repair in its native root, if the relation between the conjoint and non-conjoint cusps was closer to a 180°/180° orientation, then the reimplantation was performed at this configuration [25]. If the ‘typical’ raphe BAV relation existed between the two cusps, then the aortic neoroot was reimplanted at the 150°/210° orientation. For this reason, the total follow-up in the 180°/180° group is longer than in the 150°/210° group.
Surgical technique
Valve evaluation and cusp repair
The pathological BAV was evaluated in its native state by performing a high transverse aortotomy above the sinotubular junction. The repair was completed first with aortic root intact. The valve was then reassessed after completion of the root reimplantation. In a few cases, further minor repair of the BAV was required in the context of its neoroot. Techniques of primary cusp repair in BAV disease have been well described [7, 11, 13, 14]. In all patients, raphe release was performed to improve conjoint cusp mobility by carefully releasing the connection between the raphe and the pseudo-commissure. The majority of patients required some form of leaflet work, including leaflet plication, leaflet decalcification and fenestration closure. Patch repair of cusp free margins was not performed in any of the cases. In 3 patients, fenestrations near the middle of the leaflets were primarily repaired with Gore-Tex stitch shortening of the entire free margin, without the use of patch material (2 in 150°/210° group, 1 in 180°/180° group). Outside of leaflet decalcification to improve leaflet mobility or correct leaflet tethering, leaflets were never resected.
Subannular stabilization and root reimplantation
The repaired BAV was evaluated intraoperatively for the relationship between the conjoint and non-conjoint cusps. If the non-conjoint cusp occupied >170° of the annulus perimeter at the leaflet insertion site, and this corresponded to a relatively similar ratio of cusp surface area, then root reimplantation was performed at 180°/180° orientation, with each leaflet occupying relatively equal surface area at the annular plane. If the repaired BAV in its native root was closer to a typical raphed BAV, then the reimplantation was not surgically forced into the 180°/180° orientation, and repair was performed at a more natural 150°/210° orientation. The underlying principle was to respect the configuration of the leaflets in their native geometric orientation relative to leaflet insertion site perimeter and cusp surface area.
Subannular stabilization sutures were placed in accordance to the desired orientation of the aortic neoroot. For the 180°/180° orientation, typically four U-shaped pledget sutures were passed under the non-conjoint cusp and four were passed under the conjoint cusp (two on either side of the raphe). For the 150°/210° orientation, typically three subannular sutures were placed under the non-conjoint cusp, and four under the conjoint cusp. In all cases, the Gelweave Valsalva graft (Vascutek Ltd, Renfrewshire, Scotland) was used for reimplantation. Graft size was determined by adding 5 mm to the desired annulus diameter post reimplantation. In 4 initial cases, the root was ‘forced’ into a 180°/180° orientation from its native 150°/210° orientation.
Echocardiography
Although not initially performed in this series, intraoperative 3D echocardiography was performed in most cases. This allowed us to understand the perimeter and surface area relationships between the two cusps in its native state, and characterize the nature of AI. Postoperative echocardiography was performed in all cases to evaluate for residual AI, its orientation and degree. Coaptation height was assessed, with a goal of at least a 5-mm coaptation zone post-repair. Any residual AI >1+ mandated re-exploration of the aortic valve.
Patient follow-up
All patients underwent a transthoracic echocardiography at discharge. Patients were followed up in clinic at 1 month postoperatively and at least every 6 months thereafter. Information on survival, valve-related complications and overall clinical status was obtained. If patients did not have new onset of symptoms or changes on examination, echo was performed on a yearly basis. Clinical and echocardiographic follow-up has remained at 100% compliance.
Statistical analysis
All continuous variables are expressed as mean ± standard deviation. Univariate analysis was performed with Fisher's exact test to compare categorical variables, and Student's t-test to compare continuous variables. Statistical methods were applied to compare freedom from reintervention and late survival using Kaplan–Meier curves. SPSS software version 19.0 (SPSS, Inc., Chicago, IL, USA) was used for all calculations. The institutional review board at the University of Pennsylvania approved the study and waived the need for patient consent.
RESULTS
Demographics and preoperative characteristics
Patients were aged 45 ± 10 years with a low comorbid burden (Table 1). The majority of patients presented with AI ≥2+ (n = 25, 96%).
Table 1:
Demographics, preoperative characteristics and intraoperative outcomes
| Demographics | 150°/210° Orientation (n = 15) | 180°/180° Orientation (n = 11) | P-value |
|---|---|---|---|
| Age (years, mean ± SD) | 43 ± 11 | 48 ± 10 | 0.2 |
| Male (no, %) | 13 (87%) | 8 (72%) | 0.62 |
| Aortic insufficiency grade | |||
| ≤1+ | 0 | 1 | 0.42 |
| 2+ | 7 | 5 | 1.00 |
| 3+ | 4 | 1 | 0.36 |
| 4+ | 4 | 4 | 0.68 |
| Mean LV end-systolic diameter (mm, mean ± SD) | 34 ± 12 | 36 ± 8 | 0.37 |
| Mean LV end-diastolic diameter (mm, mean ± SD) | 67 ± 5 | 61 ± 11 | 0.14 |
| Annulus (mm, mean ± SD) | 30 ± 5 | 29 ± 4 | 0.48 |
| Sinotubular junction (mm, mean ± SD) | 42 ± 7 | 40 ± 5 | 0.26 |
| Sinus of Valsalva (mm, mean ± SD) | 48 ± 6 | 47 ± 7 | 0.41 |
| Ascending aorta (mm, mean ± SD) | 54 ± 7 | 54 ± 7 | 0.43 |
| LV ejection fraction % (mean ±SD) | 61 ± 11 | 56 ± 9 | 0.13 |
| Intraoperative outcomes | |||
| Cardiopulmonary bypass time (min, mean ± SD) | 291 ± 41 | 298 ± 70 | 0.71 |
| Aortic cross-clamp time (min, mean ± SD) | 233 ± 39 | 240 ± 52 | 0.67 |
| Circulatory arrest time (min, mean ± SD) | 19 ± 4 | 20 ± 6 | 0.63 |
| Concomitant operations | |||
| Hemiarch replacement (no, %) | 12 (80%) | 8 (73%) | 1.0 |
| Intraoperative aortic valve re-exploration (no, %) | 1 (6%) | 0 | 1.0 |
| CABG (no, %) | 1 (6%) | 0 | 1.0 |
| ASD/VSD closures (no, %) | 2 (13%) | 2 (18%) | 1.0 |
| Leaflet repair techniques | |||
| Coaptation zone (mm, mean) | 11 ± 3 | 8 ± 2 | 0.04 |
| All (no, %) | 15 (100%) | 11 (100%) | |
| Raphe release (no, %) | 13 (87%) | 11 (100%) | 0.49 |
| Raphe release with primary closure (no, %) | 2 (13%) | 0 (0%) | 0.49 |
| Gore-Tex free margin shortening for cusp perforation (no, %) | 2 (13%) | 1 (9%) | 1.00 |
| Leaflet plication (no, %) | 14 (93%) | 8 (73%) | 0.27 |
| Leaflet decalcification (no, %) | 1 (6%) | 2 (18%) | 0.56 |
| Patch repair (no, %) | 0 (0%) | 0 (0%) | 1.00 |
| Fenestration repair (no, %) | 2 (13%) | 2 (18%) | 1.00 |
All patients had BAV type Ib/II AI pathology, with 1 having leaflet tethering. In all cases, the conjoint cusp was the right–left cusp. Concomitant cardiac surgical indications included ascending aortic aneurysm requiring transverse hemiarch replacement, coronary artery disease and mitral valve disease. Average preoperative aortic annulus, sinus of Valsalva and sinotubular junction diameters were similar in both groups, and significantly larger than baseline normal parameters (Table 1). Average LV diastolic dimensions were 7 and 6 cm, and similarly enlarged in both groups. LV ejection fraction was similar in both groups.
Intraoperative and postoperative outcomes
Average cardiopulmonary bypass and aortic cross-clamp times were similar in both groups (Table 1). Of note, 76% of the cases required a brief period of circulatory arrest for transverse hemiarch distal aortic reconstruction. Average circulatory arrest period was 19 min, and was similar in both groups. One patient in the 150°/210° group required re-cross-clamping of the aorta to repair 2 + AI when weaned off bypass. Evaluation showed that the cusp repair was intact, but that one of the graft-to-graft anastomosis sutures was through the right non-coronary commissure, causing a central leak. Leaflet repair techniques were similar (Table 1).
Postoperative outcomes were satisfactory in both groups. At discharge echocardiography, no patients had AI >1+ (Table 2). Average peak gradients in the 180°/180° and 150°/210° groups were 13 ± 7.0 and 9 ± 5 mmHg, respectively (P = 0.16). Average mean gradient was 7 ± 4 and 4 ± 3 mmHg, respectively (P = 0.06). Freedoms from peak gradient >20 mmHg and mean gradient >12 mmHg were 100% (150°/210°) and 91%, respectively. Freedom from peak gradient >30 mmHg was 100% in both groups. The postoperative average coaptation zone was higher in the 150°/210° group (11 ± 3 vs 8 ± 2 mm, P = 0.04). In-hospital and 30-day mortality was zero in both groups. Stroke, renal failure, myocardial infarction, aortic valve reoperation, reoperation for bleeding and permanent pacemaker requirement rates were zero in the entire cohort.
Table 2:
Postoperative outcomes
| 150°/210° Orientation (n = 15) | 180°/180° Orientation (n = 11) | P-value | |
|---|---|---|---|
| Reoperation for bleeding | 0 | 0 | – |
| Aortic valve reoperation | 0 | 0 | – |
| Permanent pacemaker insertion | 0 | 0 | – |
| Stroke | 0 | 0 | – |
| Aortic insufficiency grade | |||
| ≤1+ | 15 (100%) | 11 (100%) | 1.0 |
| ≥2+ | 0 | 0 | – |
| Peak gradient (mmHg, mean) | 9 ± 5 | 13 ± 7 | 0.16 |
| <20 mmHg (no, %) | 15 (100%) | 10 (91%) | 0.42 |
| 20–30 mmHg (no, %) | 0 | 1 (9%) | 0.42 |
| >30 mmHg (no, %) | 0 | 0 | – |
| Mean gradient (mmHg, mean) | 4 ± 3 | 7 ± 4 | 0.06 |
| <12 mmHg (no, %) | 15 (100%) | 10 (91%) | 0.42 |
| >12 mmHg (no, %) | 0 | 1 (9%) | 0.42 |
Mid-term follow-up
Follow-up was 100% in both groups (Table 3). Patients in the 180°/180° group had longer follow-up than the 150°/210° group (mean 48 vs 33 months). Mortality has remained at zero in both groups at follow-up. All patients have remained in NYHA class I or better. Given the difference in mean follow-up between the two groups, we assessed transvalvular gradients at 1-year echo follow-up. Freedom from peak gradient >20 mmHg was 80% (n = 8) in the 180°/180° group, and 100% in the 150°/210° group (n = 14). Average peak gradient values in the 180°/180° and 150°/210° groups were 16 ± 8 and 10 ± 4 mmHg, respectively (P = 0.02). Average mean gradient values were also significantly elevated in the 180°/180° group (P = 0.01). Freedom from late aortic reoperation has remained at 100% in both groups.
Table 3:
Mid-term outcomes
| 150°/210° Orientation (n = 15) | 180°/180° Orientation (n = 11) | P-value | |
|---|---|---|---|
| Mean follow-up (months) | 33 | 48 | |
| Mortality | 0 | 0 | – |
| Aortic valve reoperation | 0 | 0 | – |
| Stroke | 0 | 0 | – |
| One-year echocardiography data | |||
| Peak gradient (mmHg, mean) | 10 ± 4 | 16 ± 8 | 0.02 |
| <20 mmHg | 14 (100%) | 8 (80%) | 0.56 |
| 20–30 mmHg | 0 | 2 (20%) | 1.0 |
| >30 mmHg | 0 | 0 | – |
| Mean gradient (mmHg) | 5 ± 3 | 9 ± 3 | 0.01 |
| <12 mmHg | 14 (100%) | 8 (80%) | 0.17 |
| >12 mmHg | 0 | 2 (20%) | 0.17 |
| LV end-systolic diameter (cm, mean ± SD) | 3 ± 1 | 4 ± 1 | 0.02 |
| LV end-diastolic diameter (cm, mean ± SD) | 5 ± 1 | 5 ± 1 | 0.21 |
Actuarial freedom from AI >2+ was 100% at 5 years in both groups (Fig. 2A). One patient presented with 3+ AI on his most recent visit, 7.5 years after his index operation. He was in NYHA class I, with LV diastolic dimension that is now enlarging (5.8 cm). Actuarial freedom from AI >1 was 90 ± 10% in the 150°/210° group, and 86 ± 13% in the 180°/180° group at 5-year follow-up (Fig. 2B). The 1 patient with 2+ AI on follow-up in the 150°/210° group had an occurrence at 32-month post-discharge and has remained stable. The LV dimensions and ejection fraction have remained normal.
Figure 2:
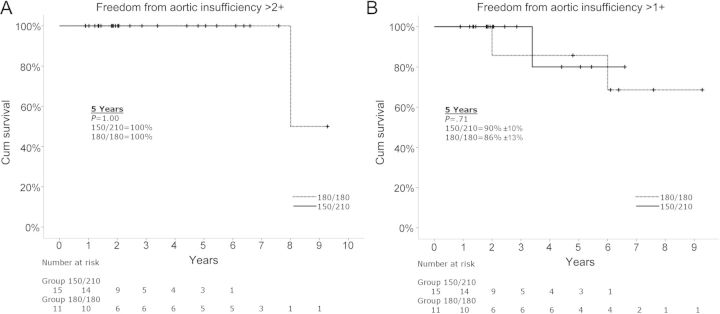
Kaplan–Meier actuarial survival curves comparing the 150°/210° orientation group to the 180°/180° orientation group. (A) Freedom from aortic insufficiency >2+. (B) Freedom from aortic insufficiency >1+.
DISCUSSION
Pioneering work by a few centres have shown that reimplantation of a repaired BAV can be safely performed with good mid-term outcomes [5, 6, 7, 17, 19, 22]. In addition, recent work by El Khoury's group has shown that the durability of a repaired BAV is more significantly improved in the context of a reimplanted aortic neoroot than with subannular stabilization techniques alone [22]. Although the evidence for root reimplantation over other annular stabilization techniques in the context of a repaired BAV is stronger, there has not been much consensus or discussion regarding the technical nuances of root reimplantation in type I BAV disease. Unlike the typical tricuspid aortic valve, where the reorientation into the neoroot is performed at a 120°/cusp orientation, a sizing and orientation algorithm does not exist in the situation of a raphed BAV. Reasons for this include (i) root reimplantation in BAV disease is a more recent undertaking compared with the tricuspid aortic valve. (ii) Fewer centres perform this procedure in smaller numbers in BAV patients; therefore, a large sample size to perform randomized studies is not possible. (iii) Unlike the tricuspid aortic valve, BAV presentation can occur in a spectrum of type 0 and I, with AI pathology presenting as a combination of type I and II lesions. Therefore, the circumferential orientation of the native BAV with regard to the conjoint and non-conjoint cusps can be variable. (iv) Reimplantation of a raphed BAV is more complex than a tricuspid aortic valve. The pseudo-commissure of the conjoint cusp is not present at the same height as the other commissures. The reimplantation technique, therefore, can be more complex in BAV. (v) Most BAV undergoing root reimplantation require some form of primary cusp repair, which can be complex and variable unlike the tricuspid aortic valve situation, where primary leaflet work is typically not required.
At our institution, we have evolved to perform root reimplantation for raphed BAV type Ib/II pathology based on a theoretical foundation to preserve the native geometric orientation of the BAV—i.e. maintain the geometric configuration between the conjoint and the non-conjoint cusps. In a type I BAV, the typical native orientation exists at 150°/210°—the non-conjoint cusp occupies 150° of the annular plane, and the conjoint cusp occupies 210° of the annular plane circumferentially, with either side of the raphe occupying 105° [25]. It is important to note that this orientation for type I BAV can vary from the 150°/210° average. In addition, aneurysmal root pathology in a type I BAV, creating the combined type/II BAV AI pathology, can further complicate the geometric orientation of the two leaflets. Root dilatation is not a symmetric process, as typically there is greater dilatation along the aortomitral continuity, and in addition, the annulus along this aspect also tends to sink posteriorly with increasing root dilatation. Finally, due to intrinsic leaflet pathology requiring cusp repair, the presentation of the raphed BAV lesion can influence the orientation of the reimplanted neoroot.
With this framework in mind, evaluation of the relation between the conjoint and non-conjoint cusps in raphed BAV correlated better with a 150°/210° orientation. This analysis was performed using mathematical models designed on 3D echocardiography-based reconstructed imaging datasets of native raphed BAV patients [25]. The ratio of the surface area of the conjoint to non-conjoint cusp typically varied from 1.3 to 1.6, and this ratio also describes the leaflet insertion perimeter at the annulus. Adopting a lesson from the mitral valve repair paradigm, where the underlying principle is the preservation of the native mitral valve orientation and structure, ‘respect’ rather than resect, and robust annular stabilization of the repaired valve along with annular stabilization, we started performing root reimplantation in raphed BAV along this same principle. Therefore, raphed BAV lesions presenting closer to a 150°/210° orientation were reimplanted at the same geometric configuration. Those presenting near a 180°/180° orientation (a minority) were reimplanted at that configuration.
Overall, our study shows that primary cusp repair and root reimplantation can be safely performed with zero mortality and without the morbidity of stroke, renal failure or pacemaker requirement. Mid-term follow-up in these patients has shown good durability of the repaired valve. We note a trend toward greater peak and mean gradients in the 180°/180° group than the 150°/210° group, along with improved leaflet coaptation zone in the latter. There was no difference in the preoperative aortic root diameters or the percentage of the annular reduction between the two groups. Although the sample number is too small, we do consider the possibility that the reorientation of the native raphed BAV into a 180°/180° orientation may affect the transvalvular gradient difference noted between the two groups. When the conjoint to non-conjoint surface area ratio is close to 1.4:1, rather than 1:1, root reimplantation at the 180°/180° orientation, without any leaflet resection, changes the ratio of the areas occupied by each leaflet at the annular plane. This change may affect the transvalvular gradient and also influence leaflet stress. Given that the primary mode of failure of the repaired BAV is from recurrent AI, the long-term significance of the difference in transvalvular gradients may not be clinically relevant. Further follow-up and larger sample size will be important in understanding the impact of a particular orientation on a reimplanted raphed BAV. Our group is currently pursuing 3D echocardiographic-guided mathematical modelling of the native, repaired and repaired + reimplanted raphed BAV to test this idea. To this effect, early results of models generated by our group suggest that leaflet tenting in the reimplanted root, which is a reflection of the leaflet stress, is better optimized at the 150°/210° orientation [25]. Correlation of the findings of the mathematical models with the clinical findings will be critical in understanding the importance of leaflet orientation in performing primary cusp repair + root reimplantation in raphed BAV with aneurysmal aortic root disease.
We believe that reimplantation of a raphed BAV into a 150°/210° or 180°/180° orientation based on the native leaflet and annulus anatomy simplifies the primary cusp repair + reimplantation procedure.
Annular reduction and stabilization suture placement are performed in a manner that preserves the native leaflet orientation and does not require any major suture manipulation of the annulus to ‘force’ a 180°/180° repair. Although our study is small, we believe that further exploration of the concept of the preservation of native BAV geometric orientation when performing primary cusp repair + root reimplantation is warranted.
Study limitations
The small sample size in each group and mid-term follow-up results are not sufficient to establish the superiority of one reimplantation method over the other. Further follow-up is required to understand whether our adopted paradigm translates into longer valve durability and improved transvalvular gradients. Lastly, rigorous mathematical modelling with follow-up assessing the physiology of the reimplanted raphed BAV would help optimize the root reimplantation technique in raphed BAV disease. Such models are currently being pursued by our group.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr G. El Khoury (Brussels, Belgium): Aortic valve repair, or any valve repair, means that we really should restore the leaflet coaptation and motion. And the leaflet motion, particularly in bicuspid aortic valve, or even in tricuspid, is really very important. The motion of this leaflet depends mainly on the free margin length, base of implantation, the STJ, and the AVG. The whole unit may influence the motion of the leaflet.
So when we are reconstructing the valve unit, we should really pay attention to this motion of the leaflet. So I agree that when you are happy with your reconstruction of the valve, when you are happy with the special configuration of the leaflet, you can respect the asymmetry of the valve and obtain good results. The problem is that if you are not happy with the special configuration of the leaflet after the repair, the debate is should we make it symmetrical or not symmetrical? But whatever we do, we should pay attention, as you will see later, to really respect or restore ideal configuration of the leaflet into the neoaortic root.
Now, back to your bicuspid aortic valve type I, I mean there is maybe some confusion. Type I is not only one type, it is a spectrum of several types. And the key issue is the size of the leaflet, the quantity of tissue we have. You have type I with a lot of tissue and the mechanism of regurgitation is really the prolapse. So in those patients we can repair the prolapse and preserve quite a good motion of the leaflet and respect the 210 and 150.
But we have other patients when the degree of fusion is very low and the leaflets are under-developed, we don't have a lot of tissue, and we feel that maybe we should add some tissue or restrict the base of implantation of the leaflets. So in those patients I think preserving the 210-150 can maybe pose a problem for the motion of the leaflet and we should do something on the base. But I agree completely that if you have enough tissue, the respected geometry is the way you have to do it.
I have two questions for you. Do all the patients you describe here have excess of tissue to respect this asymmetry?
Dr Vallabhajosyula: We have only had one patient where we found leaflet tethering. That is why I wanted to emphasize, to put it in context, that all of the other 25 patients we have had so far have presented with a combination of AI types IB and 2. These 25 out of 26 patients have excess leaflet tissue. Excess leaflet tissue gives us that freedom, as Dr. El Khoury has alluded to, to orient the valve in its natural position and respect the presenting geometry. Dilatation is usually at the annular and subannular level. Our data will determine whether or not this strategy is ultimately correct. So far from our data here we know that it is at least non-inferior.
However, in the one patient where we did have tethering, it didn't give us much room at all. We repaired the valve in the 180-180 orientation because the two leaflet insertion perimeters were approximately equal. Our approach, as Dr El Khoury is alluding to, is to first assess the valve, optimize the repair and then make a decision regarding reimplantation. We have made our decisions in that manner.
Dr El Khoury: The second question is how often you had to add some stitches after your sparing surgery or your reimplantation technique. Because we can, let's say, influence the level of coaptation or the motion of the leaflet.
Dr Vallabhajosyula: We have been fortunate in that we've only had one intraoperative re-repair, and that was in a 150-210 orientation. That was a mistake, actually made by me. When I completed the graft-to-graft anastomosis in the mid-ascending aorta, one of the bites went through the pledget at the top of the commissural suture line, so it pulled the valve distally and caused central AI.
Otherwise we have not had leaflet tethering issues and have not added any pericardial patches to the free margin so far. We either do leaflet plication or triangular resection with subsequent linear approximation. But I think it would be important once we come to that situation to see what orientation would fit better.
Dr El Khoury: One short question. For the patient with 180-180, how do you explain the presence of gradient, the stretching of the normal leaflet, or what?
Dr Vallabhajosyula: I think it probably happens at the posterior aspect of the annulus where in the 180-180 orientation you are stretching the non-conjoint cusp more, for lack of a better word, to kind of force it into that orientation. And I think the transvalvular gradient possibly comes from that.
In addition, we just started analysing some 3-D data sets comparing the two orientations. And when we do that, we're finding that the annular plane is not as orthogonal to the LV outflow tract in the 180-180 orientation compared to the 150-210 orientation. And we wonder if that also contributes to increase transvalvular gradients.
Dr H. Schaefers (Homburg/Saar, Germany): I have maybe a brief comment and a question for you.
The background is that we have over the last years switched, if at all possible, to a 180 degree orientation of the commissures. We have found that it actually leads to a very nice result because the conjoint cusp is improved in its mobility. Our gradients with the 180 degree orientation are lower compared to the 150 degree orientation.
Now, you come up with findings that are clear, but still lead to a different conclusion. At the same time you repaired only a very limited percentage of all patients with bicuspid valve and aortic regurgitation, which raises the question: Are there specific criteria that you used in order to select patients for repair or replacement, and what are the criteria? I think this needs to be taken into consideration because we are talking probably about a very selected cohort of patients in your presentation. And what do you think is the mechanism of the higher coaptation zone in the 150 degree orientation? Did you do something different regarding valve repair?
Dr Vallabhajosyula: We have repaired almost all of our aortic valves. We did have one patient in the 180-180 orientation who had no AI to begin with, so we did not perform a cusp repair in that patient. But I think it is important, and what Dr Schaefers is perhaps alluding to here, that we do not aggressively repair leaflets in terms of adding free-margin pericardial patches. We have avoided these, and generally perform an aortic root procedure in that scenario. Therefore most likely there is a difference in our patient selection in that sense, correct. If we feel a pericardial patch is required, we have opted to replace the valve.
Dr Schaefers: Any difference in repair and what is the explanation of the improved coaptation zone in the 150 degree orientation?
Dr Vallabhajosyula: There is no difference in the repair techniques for both sides. The coaptation zone, I'll be honest, we were actually quite surprised to find the difference. We are analysing our 3-D echo data sets now to try to understand why this is happening. We think that maybe not forcing that allows the non-conjoint cusp not to prolapse a little bit more than in the 180-180 orientation. But I think that we're going to need more numbers and more 3-D modelling of this to really understand why there is that difference.
REFERENCES
- 1.Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, et al. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300:1317–25. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- 2.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1–142. doi: 10.1016/j.jacc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Davies RR, Kaple RK, Mandapati D, Gallo A, Botta DM, Jr, Elefteriades JA, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg. 2007;83:1338–44. doi: 10.1016/j.athoracsur.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 4.Boodhwani M, de Kerchove L, Glineur D, Rubay J, Vanoverschelde JL, Noirhomme P, et al. Repair of regurgitant bicuspid aortic valves: a systematic approach. J Thorac Cardiovasc Surg. 2010;140:276–84.e1. doi: 10.1016/j.jtcvs.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 5.Badiu CC, Bleiziffer S, Eichinger WB, Zaimova I, Hutter A, Mazzitelli D, et al. Are bicuspid aortic valves a limitation for aortic valve repair? Eur J Cardiothorac Surg. 2011;40:1097–104. doi: 10.1016/j.ejcts.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Alsoufi B, Borger MA, Armstrong S, Maganti M, David TE. Results of valve preservation and repair for bicuspid aortic valve insufficiency. J Heart Valve Dis. 2005;14:752–9. [PubMed] [Google Scholar]
- 7.David TE, Maganti M, Armstrong S. Aortic root aneurysm: principles of repair and long-term follow-up. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S14–9. doi: 10.1016/j.jtcvs.2010.07.041. discussion S45–51. [DOI] [PubMed] [Google Scholar]
- 8.Ashikhmina E, Sundt TM, 3rd, Dearani JA, Connolly HM, Li Z, Schaff HV. Repair of the bicuspid aortic valve: a viable alternative to replacement with a bioprosthesis. J Thorac Cardiovasc Surg. 2010;139:1395–401. doi: 10.1016/j.jtcvs.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Veldtman GR, Connolly HM, Orszulak TA, Dearani JA, Schaff HV. Fate of bicuspid aortic valves in patients undergoing aortic root repair or replacement for aortic root enlargement. Mayo Clin Proc. 2006;81:322–6. doi: 10.4065/81.3.322. [DOI] [PubMed] [Google Scholar]
- 10.El Khoury G, Vanoverschelde JL, Glineur D, Pierard F, Verhelst RR, Rubay J, et al. Repair of bicuspid aortic valves in patients with aortic regurgitation. Circulation. 2006;114(1 Suppl):I610–6. doi: 10.1161/CIRCULATIONAHA.105.001594. [DOI] [PubMed] [Google Scholar]
- 11.Nash PJ, Vitvitsky E, Li J, Cosgrove DM, 3rd, Pettersson G, Grimm RA. Feasibility of valve repair for regurgitant bicuspid aortic valves–an echocardiographic study. Ann Thorac Surg. 2005;79:1473–9. doi: 10.1016/j.athoracsur.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 12.Fraser CD, Jr, Wang N, Mee RB, Lytle BW, McCarthy PM, Sapp SK, et al. Repair of insufficient bicuspid aortic valves. Ann Thorac Surg. 1994;58:386–90. doi: 10.1016/0003-4975(94)92212-8. [DOI] [PubMed] [Google Scholar]
- 13.Boodhwani M, de Kerchove L, El Khoury G. Aortic root replacement using the reimplantation technique: tips and tricks. Interact CardioVasc Thorac Surg. 2009;8:584–6. doi: 10.1510/icvts.2008.197574. [DOI] [PubMed] [Google Scholar]
- 14.de Kerchove L, Boodhwani M, Glineur D, Noirhomme P, El Khoury G. A new simple and objective method for graft sizing in valve-sparing root replacement using the reimplantation technique. Ann Thorac Surg. 2011;92:749–51. doi: 10.1016/j.athoracsur.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Price J, De Kerchove L, Glineur D, Vanoverschelde JL, Noirhomme P, El Khoury G. Risk of valve-related events after aortic valve repair. Ann Thorac Surg. 2013;95:606–12. doi: 10.1016/j.athoracsur.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Nazer RI, Elhenawy AM, Fazel SS, Garrido-Olivares LE, Armstrong S, David TE. The influence of operative techniques on the outcomes of bicuspid aortic valve disease and aortic dilatation. Ann Thorac Surg. 2010;89:1918–24. doi: 10.1016/j.athoracsur.2010.02.070. [DOI] [PubMed] [Google Scholar]
- 17.Aicher D, Kunihara T, Abou Issa O, Brittner B, Gr€aber S, Schafers HJ. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation. 2011;123:178–85. doi: 10.1161/CIRCULATIONAHA.109.934679. [DOI] [PubMed] [Google Scholar]
- 18.Kunihara T, Aicher D, Rodionycheva S, Groesdonk HV, Langer F, Sata F, et al. Preoperative aortic root geometry and postoperative cusp configuration primarily determine long-term outcome after valve-preserving aortic root repair. J Thorac Cardiovasc Surg. 2012;143:1389–95. doi: 10.1016/j.jtcvs.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 19.Sareyyupoglu B, Suri RM, Schaff HV, Dearani JA, Daly RC, Orszulak TA, et al. Survival and reoperation risk following bicuspid aortic valve-sparing root replacement. J Heart Valve Dis. 2009;18:1–8. [PubMed] [Google Scholar]
- 20.Malvindi PG, Raffa GM, Basciu A, Citterio E, Cappai A, Ornaghi D, et al. Bicuspidy does not affect reoperation risk following aortic valve reimplantation. Interact CardioVasc Thorac Surg. 2012;14:717–20. doi: 10.1093/icvts/ivs059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.le Polain de Waroux JB, Pouleur AC, Robert A, Pasquet A, Gerber BL, Noirhomme P, et al. Mechanisms of recurrent aortic regurgitation after aortic valve repair: predictive value of intraoperative transesophageal echocardiography. JACC Cardiovasc Imaging. 2009;2:931–9. doi: 10.1016/j.jcmg.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 22.De Kerchove L, Boodhwani M, Glineur D, Vandyck M, Vanoverschelde J, Noirhomme P, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg. 2011;142:1430–8. doi: 10.1016/j.jtcvs.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Sievers H, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133:1226–33. doi: 10.1016/j.jtcvs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 24.Boodhwani M, De Kerchove L, Glineur D, Poncelet A, Rubay J, Astarci P, et al. Repair-oriented classification of aortic insufficiency: impact on surgical techniques and clinical outcomes. J Thorac Cardiovasc Surg. 2009;137:286–94. doi: 10.1016/j.jtcvs.2008.08.054. [DOI] [PubMed] [Google Scholar]
- 25.Levack M, Jassar AS, Ryan L, Jackson BM, Keane NG, St John Sutton MG, et al. (2012) Quantitative three-dimensional analysis of bicuspid aortic valves: a single modality approach for characterization of valvular and proximal aortic pathology. J Am Coll Surg. 2012;215:S44. [Google Scholar]