Abstract
Urea cycle disorders (UCDs) are inherited disorders of ammonia detoxification often regarded as mainly of relevance to pediatricians. Based on an increasing number of case studies it has become obvious that a significant number of UCD patients are affected by their disease in a non-classical way: presenting outside the newborn period, following a mild course, presenting with unusual clinical features, or asymptomatic patients with only biochemical signs of a UCD. These patients are surviving into adolescence and adulthood, rendering this group of diseases clinically relevant to adult physicians as well as pediatricians. In preparation for an international workshop we collected data on all patients with non-classical UCDs treated by the participants in 20 European metabolic centres. Information was collected on a cohort of 208 patients 50% of which were ≥ 16 years old. The largest subgroup (121 patients) had X-linked ornithine transcarbamylase deficiency (OTCD) of whom 83 were female and 29% of these were asymptomatic. In index patients, there was a mean delay from first symptoms to diagnosis of 1.6 years. Cognitive impairment was present in 36% of all patients including female OTCD patients (in 31%) and those 41 patients identified presymptomatically following positive newborn screening (in 12%). In conclusion, UCD patients with non-classical clinical presentations require the interest and care of adult physicians and have a high risk of neurological complications. To improve the outcome of UCDs, a greater awareness by health professionals of the importance of hyperammonemia and UCDs, and ultimately avoidance of the still long delay to correctly diagnose the patients, is crucial.
Introduction
Urea cycle disorders (UCDs) comprise a group of inherited defects of amino acid metabolism with an estimated cumulative incidence of 1:8000 to 1:44,000 (Brusilow and Horwich 2001; Brusilow and Maestri 1996; Dionisi-Vici et al 2002; Wilcken 2004). Normal function of the urea cycle requires six enzymes: N-acetylglutamate synthase (NAGS), carbamoyl phosphate synthetase I (CPS1), ornithine transcarbamylase (OTC), argininosuccinate synthetase (ASS), argininosuccinate lyase (ASL) and arginase (ARG1). UCDs have been described resulting from the deficiency of each of these enzymes. In addition, two transporters are also necessary, because the metabolic steps are in two cellular compartments: the ornithine/citrulline antiporter ORNT1, deficiency of which causes hyperornithinemia-hyperammonemia-homocitrullinuria syndrome and the glutamate/aspartate antiporter citrin, deficiency of which gives rise to citrullinemia type 2.
Although partially expressed in many tissues, the entire urea cycle pathway is only active in periportal hepatocytes (Brusilow 1984; Brusilow and Maestri 1996; Davis and Wu 1998; Häussinger 1990). The main function of the urea cycle is the detoxification of ammonia but it is also the only pathway for endogenous synthesis of arginine (Brusilow 1984). Defects of the urea cycle result in hyperammonemia. Hyperammonemia causes encephalopathy which can progress rapidly from minor, non-specific symptoms through delirium to deep coma and death due to cerebral oedema (Bachmann 2002; Enns 2008; Msall et al 1984). Chronic encephalopathy is also seen (Häberle et al 2012; Leonard and Morris 2002; Nassogne et al 2005; Summar et al 2008). Clinical symptoms can vary and include recurrent vomiting, lethargy, personality change, agitation, protein avoidance, seizures and coma. In some patients the initial presentation is psychiatric. Prognosis mainly depends on the severity, duration and frequency of hyperammonemic decompensations, although significant cognitive impairment can occur even after a single episode of metabolic decompensation. In some patients the likely outcome can at least be predicted from the underlying molecular defect (Berning et al 2008; Ficicioglu et al 2009; Häberle et al 2003; Häberle et al 2011; Mercimek-Mahmutoglu et al 2010; Msall et al 1984; Picca et al 2001). Inheritance of all UCDs is autosomal recessive, except for OTC deficiency (OTCD), the most frequent UCD, which is X-linked (Tuchman 1992).
UCDs classically manifest themselves in the neonatal period but in fact can present at any age (Lee et al 2005; Lee 2006; Legras et al 2002; Leonard and Morris 2002; Nassogne et al 2005; Summar et al 2008). Non-classical UCDs with atypical clinical courses extend the spectrum of UCDs. This has been documented in several case reports and small case series but not yet in a cohort or larger observational study.
In 2011, in an International workshop “Non-classical Urea Cycle Disorders” held in Zurich focussing on non-classical forms of UCDs, we tried to fill this gap by collecting data from UCD patients with atypical features: onset after the newborn period; a mild disease course; uncommon presentations (at any age); asymptomatic individuals (screened because of affected relatives or identified through newborn screening, NBS) with biochemical features of a UCD. Participants from 20 metabolic centres in five European countries shared their experience on non-classical UCD patients and critically discussed how they should be managed. The primary aim of the workshop, and of this publication, was to collect and to present data on the number of patients with and the clinical course of non-classical UCDs, to improve awareness of this group of patients and to discuss suggestions for their management. Focus of the presented data is on the most important aspects of diagnosis, clinical course, and therapy.
Methods
Study population
Contributing centres cared for a significant number of patients ensuring a high level of experience and standard of care. Participants came from four centres in Austria (Bregenz, Graz, Innsbruck and Vienna), nine in Germany (Berlin, Düsseldorf, Freiburg, Hamburg, Hannover, Heidelberg, Leipzig, München, Münster), two in The Netherlands (Amsterdam/Utrecht and Rotterdam), four in Switzerland (Basel, Bern, Lausanne and Zurich) and one in the United Kingdom (London). All patients included in this study met at least one of the following criteria:
presentation after the newborn period
an unusually mild course with absence of metabolic decompensations
an uncommon presentation with signs and symptoms not frequently reported in UCDs (either in the newborn period or beyond)
having biochemical features of a UCD but no clinical symptoms.
Data were obtained from each centre on number of patients, type of UCD, mode of diagnosis, clinical course, treatment and outcome. Additional diagnoses were collected as free text. Prior to data collection, an electronic data sheet was pilot-tested in one centre (Zurich) for ease of use, followed by some adjustments. In most centres, data were collected by a trained medical student together with the local metabolic specialist using patients’ records as the source of information. Data were anonymised prior to evaluation and statistical analysis.
Statistics
Data were transferred to IBM SPSS statistics version 20 (IBM, New York) and analysed with the applications “crosstabulation”, “frequency” and “mean”. Chi-square and t-tests were used for significance testing. The present investigation was designed as an explorative study. Significance level was set at 0.05 and not adjusted for multiple testing. For diagrams, we used Microsoft Office Excel 2007 and 2010 (Microsoft Corporation, Redmond).
Results
Patients and diagnoses
Data from 208 non-classical UCD patients with deficiencies of OTC (121; 58%), ASS (ASSD; 43; 20%), ASL (ASLD; 31; 15%), ARG1 (ARG1D; 8; 4%), CPS1 (CPS1D; 2; 1%), NAGS (NAGSD; 1; 1%), and the HHH syndrome (2; 1%) were collected (Table 1).
Table 1.
Basic description of the cohort
| Disease | Number (% of total) | Gender | |
|---|---|---|---|
| Female | Male | ||
| OTCD | 121 (58%) | 83 (69%) | 38 (31%) |
| ASSD | 43 (20%) | 22 (51%) | 21 (49%) |
| ASLD | 31 (15%) | 11 (36%) | 20 (64%) |
| ARG1D | 8 (4%) | 3 (38%) | 5 (62%) |
| HHH syndrome | 2 (1%) | 0 | 2 (100%) |
| CPS1D | 2 (1%) | 1 (50%) | 1 (50%) |
| NAGSD | 1 (1%) | 1 (100%) | 0 |
| Total | 208 (100%) | 121 (58%) | 87 (42%) |
OTCD ornithine transcarbamylase deficiency; ASSD argininosuccinate synthetase deficiency; ASLD argininosuccinate lyase deficiency; ARG1D arginase deficiency; HHH syndrome hyperornithinemia-hyperammonemia-homocitrullinuria syndrome; CPS1D carbamoyl phosphate synthetase I deficiency; NAGSD N-acetylglutamate synthase deficiency
Of the 121 OTCD patients, 83 (69%) were female and 38 (31%) were male. In the 87 patients not affected by OTCD the sex distribution was more even (38 (44%) female and 49 (56%) male patients) (Table 1).
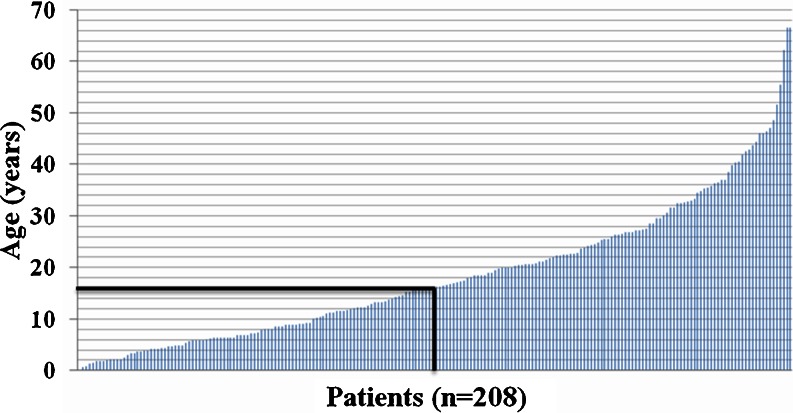
The patients’ age at the time of data collection ranged from 1 month to 66.5 years (the oldest two being female patients with OTCD) with a mean of 18.5 years (sd = 13.5) for the entire cohort. Of them, 103 (50%) were < 16 years of age (Fig. 1 and Table 2).
Fig. 1.
Age distribution of all patients (n = 208). Each column in this graph represents one patient. The line at age 16 is separating pediatric from adult patients (patient 104 being the first adult)
Table 2.
Age distribution of the cohort, abbreviations as in Table 1
| Disease | Mean age in years (range) | Pediatric patients (< 16 years) | Adult patients (≥ 16 years) |
|---|---|---|---|
| OTCD (all) | 22.2 (0.1–66.5) | 47 (39%) | 74 (61%) |
| OTCD (females) | 24.5 (2.2–66.5) | 28 (34%) | 55 (66%) |
| Cohort except OTCD | 13.4 (0.7–47.0) | 56 (64%) | 31 (36%) |
| Cohort except female OTCD | 14.5 (0.1–47.0) | 75 (60%) | 50 (40%) |
| ASLD | 16.8 (1.5–35.8) | 14 (45%) | 17 (55%) |
| ASSD | 9.0 (0.7–26.8) | 35 (81%) | 8 (19%) |
| ARG1D | 23.5 (4.2–47.0) | 4 (50%) | 4 (50%) |
| HHH syndrome | 4.7 (3.0–6.3) | 2 (100%) | 0 |
| CPS1D | 15.5 (8.1–22.9) | 1 (50%) | 1 (50%) |
| NAGSD | 27.2 | 0 | 1 (100%) |
| Total | 18.5 (0.1–66.5) | 103 (50%) | 105 (50%) |
Mode of diagnosis
Mode of diagnosis was available for 202 patients. Just over half (102; 51%) were index-patients diagnosed due to symptoms. Others were diagnosed because of a positive family history (52; 26%), as part of NBS programmes (41; 20%) (including only ASSD and ASLD patients, since neonatal screening was performed for these two diseases only) and by antenatal diagnosis in affected families (7; 3%) (Table 3).
Table 3.
Clinical data of the cohort
| Disease | Clinical course | Mode of diagnosis | Mean age at diagnosis (years)a | Mean delay (years)b | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic | Symptomatic | Mean age at first symptoms (years) | Index patients | PD | FH | NBS | Survived | Died | |||
| OTCD | 33 (28%) | 85 (72%) | 4.8 | 73 (61%) | 6 (5%) | 40 (34%) | ND | 9.1 | 1.2 | 115 (95%) | 6 (5%) |
| ASSD | 28 (65%) | 15 (35%) | 1.6 | 7 (17%) | 1 (2%) | 6 (14%) | 28 (67%) | 0.9 | 0.2 | 43 (100%) | 0 |
| ASLD | 11 (36%) | 20 (64%) | 2.1 | 14 (47%) | 0 | 3 (10%) | 13 (43%) | 2.6 | 3 | 31 (100%) | 0 |
| ARG1D | 0 | 8 (100%) | 1 | 4 (57%) | 0 | 3 (43%) | ND | 2.6 | 2.5 | 8 (100%) | 0 |
| HHH | 0 | 2 (100%) | 0.6 | 2 (100%) | 0 | 0 | ND | 1.5 | 0.8 | 2 (100%) | 0 |
| CPS1D | 0 | 2 (100%) | 0.9 | 1 (100%) | 0 | 0 | ND | 2.9 | 2 | 2 (100%) | 0 |
| NAGSD | 0 | 1 (100%) | 1 | 1 (100%) | 0 | 0 | ND | 13 | 12 | 1 (100%) | 0 |
| Total | 72 (35%) | 133 (65%) | 3.6 | 102 (51%) | 7 (3%) | 52 (26%) | 41 (20%) | 5.9 | 1.6 | 202 (97%) | 6 (3%) |
Clinical data of patients
ND not done
PD prenatal diagnosis; FH family history; NBS newborn screening
aAll types of diagnosis
bPatients with initial symptoms leading to diagnosis
Genetic analysis
From all 208 patients, 141 (68%) had genetic testing performed. This led to a confirmation of the diagnosis in 126 (89%). In 15 (11%) patients no mutations were found (13 OTCD patients and two ASLD patients).
Information on the maternal carrier status was available for 76 (63%) patients with OTCD (57 female and 19 male, after exclusion of three siblings of index patients). In 33% (19) of the female and 74% (14) of the male patients the mother was carrier for OTCD (χ2(df = 1) = 9.74; p = 0.002).
In the group of 43 ASSD patients, genetic testing was performed and fully informative in 30 (70%). Of these, 15 (50%) patients were shown to be homozygous for one of three missense mutations known to be associated with mild citrullinemia type 1 (p.Trp179Arg, p.Val263Met, or p.Gly362Val) (Häberle et al 2003). Four of these patients (of which three were identified by NBS) went on to experience symptomatic hyperammonemia (ammonia levels 146–190 μmol/L) indicating the need for medical follow-up of this group of patients.
A similar picture can be drawn for ASLD patients of whom 18 (58%; n = 31) had molecular genetic analysis, yielding the full genotype in all but two patients. In six patients, a homozygous missense mutation (p.Val178Met, p.Glu189Gly, or p.Arg379Cys) previously associated with a mild phenotype could be identified (Kleijer et al 2002; Mercimek-Mahmutoglu et al 2010). As in the ASSD patients, two of these patients with a supposedly mild genotype presented with clinical hyperammonemia (ammonia 86 and 175 μmol/L).
Clinical course
Clinical data were available for 205 patients. Asymptomatic until the age of data collection were 72 (35%) patients. Of these, 34 (47%) were diagnosed because of a positive family history, 33 (46%) by NBS programmes (10 ASLD patients and 23 ASSD patients), and five (7%) by prenatal screening.
The most frequent diagnosis, in 121 patients, was OTCD with clinical information available in 118 patients. Of these, 72% (85) had clinical symptoms and 28% (33) were asymptomatic. The proportion of clinically affected patients was independent of sex with 57 (71%) of female and 28 (74%) of male OTCD patients having symptoms (χ2(df = 1) = 0.076; p = 0.78).
For ASSD, 65% (n = 43) of patients were asymptomatic and for ASLD, 36% (n = 31) (χ2(df = 1) = 6.35; p = 0.012) (Table 3). All patients affected by ARG1D, HHH syndrome, CPS1D and NAGSD had symptoms at some stage of their clinical course that were attributed to the underlying biochemical defect (Table 3).
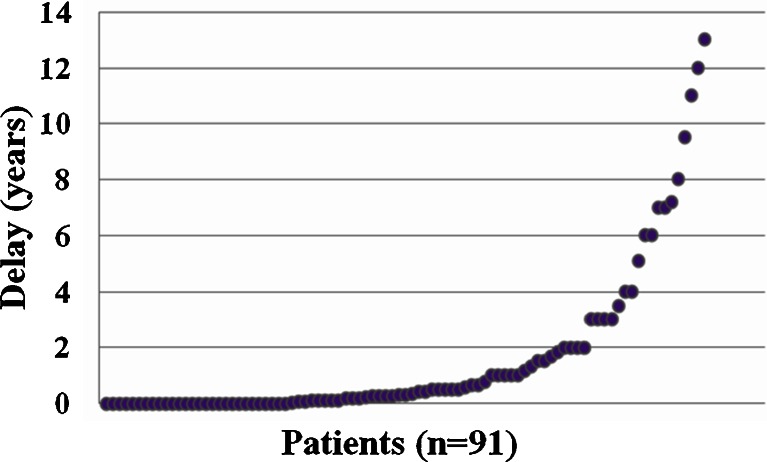
Mean age at first symptoms (not always a decompensation but any symptom attributed to a UCD) for all symptomatic patients was 3.6 years (n = 115; sd = 6.3, range 0.1-35) and mean age at diagnosis for the entire cohort was 5.9 years (n = 186; sd = 10, range 0–55) (Table 3). For patients diagnosed because of clinical symptoms, the mean delay from first symptoms to final diagnosis was 1.6 years (n = 91; sd = 2.8, range 0–13) (Table 3 and Fig. 2). A prompt diagnosis, within one month after onset of symptoms, was only made in 36 patients (40%; n = 91). A delay of > 1 month to 1 year was observed in 27 patients. In 28 patients, the delay was > 1 year.
Fig. 2.
Diagnostic delay between onset of first symptoms and diagnosis of a UCD in years for the 91 patients diagnosed because of symptoms. Each dot represents one patient
Symptoms and complications
We systematically collected data on presence of episodes of symptomatic hyperammonemia, number of hospitalisations, cognitive impairment (mental retardation (MR) of any severity (including learning difficulties) and/or developmental delay (DD)), and presence of seizures.
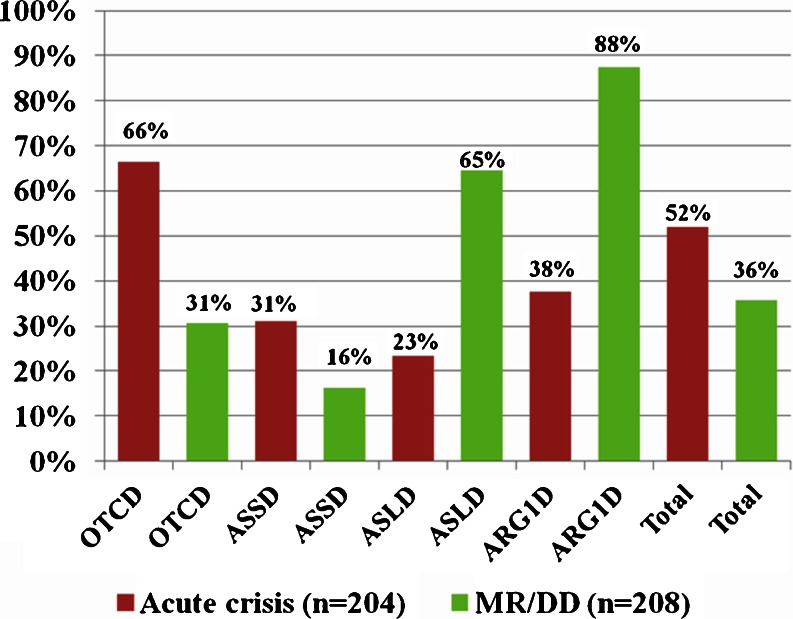
Data on symptomatic hyperammonemia were available for 204 (98%; n = 208) patients (Fig. 3). At least 106 (52%; n = 204) had one documented episode of symptomatic hyperammonemia. In female OTCD patients 64% (52; n = 81) had at least one crisis, and in male OTCD patients 71% (27; n = 38) (χ2(df = 1) = 0.81; p = 0.37). Median peak ammonia for all patients with at least one acute crisis (n=84) was 305 μmol/L (range 60-1300). The highest peak ammonia was measured in a female OTCD patient. The median peak ammonia in OTCD females with an acute crisis (n = 36; 381 μmol/L, range 121–1300) was not significantly different from the median peak ammonia in male OTCD patients with acute crisis (n = 22; 294 μmol/L, range 129–1077) (Mann–Whitney-U = 304; z = −1.474; p = 0.14).
Fig. 3.
Frequency of acute crisis in each disease (OTCD, ASSD, ASLD and ARG1D, as well as total including HHH syndrome, NAGSD, CPS1D) (data from 204 patients) and the proportions of MR/DD (data from 208 patients)
Information on the frequency of hospitalisations (“never”, “once”, “twice or more”) for acute hyperammonemic crises or beginning/imminent hyperammonemia was available for 202 (97%) patients: 93 (46%) were never hospitalised, 30 (15%) once, and 79 (39%) twice or more often. In OTCD (n = 121) 23 (20%) had one and 56 (48%) had two or more hospitalisations. In ASLD and ASSD, 23 (74%; n = 31) and 31 (74%; n = 43), respectively, never required hospitalisation.
In the entire cohort (n = 208 patients), 74 (36%) had a diagnosis of MR/DD (Fig. 3). In the subgroup of OTCD patients (n = 121), 37 were affected by MR/DD (corresponding to 31% of all and 44% of the symptomatic patients). In ASLD, all of the symptomatic patients (n = 20) were indeed affected by MR/DD; three of the ASLD patients with MR/DD were found by NBS. In ASSD, seven (16%) suffered from MR/DD including two of the patients found by NBS (carrying the homozygous mutation p.Trp179Arg and the heterozygous mutations p.Arg272Leu and Ivs13 + 5G > A, respectively). MR/DD was present in the HHH syndrome in one (50%), in NAGSD in none (0%) and in CPS1D in two (100%) patients, respectively.
If there was a diagnostic delay of > 1 year (arbitrarily set cut-off), as occurred in 28 patients, 75% (21) of patients were affected by MR/DD while only 46% (29) of 63 patients were affected by MD/DD if the delay was ≤ 1 year (χ2(df = 1) = 6.92; p < 0.01).
Seizures were present in 37 (18%) patients at some point. Patients affected by ARG1D had the highest incidence of seizures (7 patients; 88%), followed by ASLD (7; 23%), OTCD (18; 15%) and ASSD (4; 9%). Seizures have not been reported for the few patients with HHH syndrome and NAGSD in this study.
Additional symptoms collected as free text included hepatopathy/liver failure (total, n = 20; OTCD, n = 11; ASLD, n = 6; ASSD, n = 2; HHH syndrome, n = 1), hepatomegaly (total, n = 4; OTCD, n = 2; ASLD, n = 2), elevated transaminases (total, n = 6; OTCD, n = 5; ASSD, n = 1), behavioural problems (n = 16), obesity (n = 8), psychiatric symptoms (n = 6), spasticity (n = 5, four affected by ARG1D, one by OTCD), headache (n = 5), feeding problems (n = 5), trichorrhexis nodosa (n = 5, all ASLD), hyperlipidemia (hypertriglyceridemia and/or hypercholesterolemia) (n = 4), ataxia (n = 4), muscular hypotonia (n = 4), and diabetes mellitus type 1 (n = 3).
Symptoms and complications in patients diagnosed by newborn screening
Diagnosed in NBS programmes were 67% of ASSD patients (28; n = 43) and 43% of ASLD (13; n = 31) (Table 3).
In ASSD, 18% (5; n = 28) of patients were symptomatic if detected by NBS (called thereafter “NBS-patients”), versus 64% (9; n = 15) of patients found by selective screening (i.e. index patients diagnosed because of clinical symptoms, due to positive family history or prenatal screening, called thereafter “non-NBS-patients”) (χ2(df = 1) = 9.05; p = 0.003). An episode of symptomatic hyperammonemia was observed in 18% (5; n = 28) of NBS- patients and 57% (8) of non-NBS-patients (χ2(df = 1) = 6.74; p = 0.009). MR/DD was seen in 7% (2) of NBS-patients and 29% (4) of non-NBS-patients (χ2(df = 1) = 3.50; p = 0.061) and seizures were reported in only one NBS patient (4%) but 21% (3) of non-NBS-patients (χ2(df = 1) = 3.45; p = 0.063).
In ASLD, 23% (3; n = 13) of NBS-patients were symptomatic, versus 94% (16; n = 18) of non-NBS patients (χ2(df = 1) = 16.01; p = 0.000). An episode of symptomatic hyperammonemia was reported in one of the NBS-patients (8%) but in five of the non-NBS-patients (31%) (χ2(df = 1) = 2.43; p = 0.119). MR/DD was seen in 23% (3) of NBS-patients and 94% (16) of non-NBS-patients (χ2(df = 1) = 16.01; p = 0.000) and seizures were reported in 41% (7) of non-NBS-patients but not in NBS-patients (χ2(df = 1) = 4.97; p = 0.026).
Dietary treatment
For all but one patient, nutritional data were available. A protein restricted diet was prescribed for 120 (58%; n = 207) of the patients and a self chosen vegetarian diet and/or protein avoidance were reported as “additional information” in 16 patients. Of the 133 symptomatic patients, 109 (82%) were protein restricted versus 10 (14%) of the 71 asymptomatic patients. Of those treated with protein restriction, only 87 (73%) received supplements of vitamins and/or essential amino acids.
We did not systematically collect information on the compliance of the patients.
Nitrogen scavenger drugs and L-citrulline and L-arginine supplementation
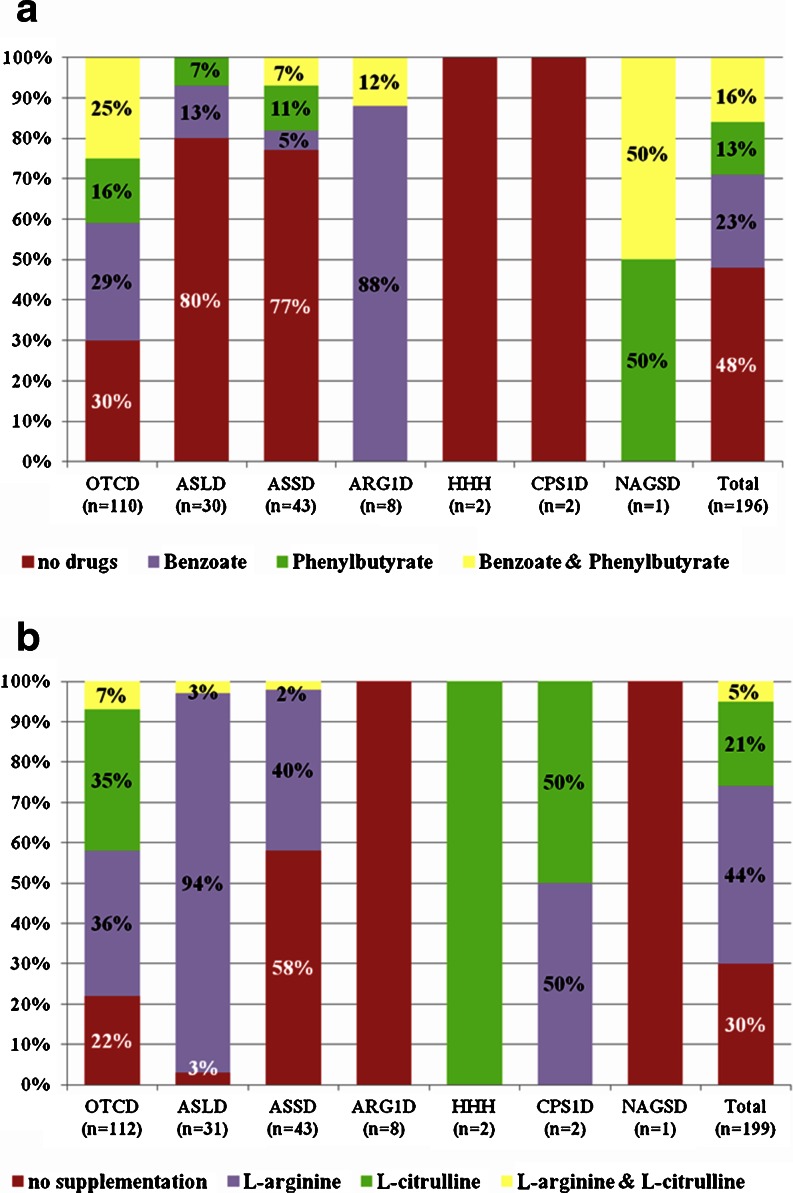
Data on current pharmacological treatment were available in most patients (concerning use of nitrogen scavenger drugs and L-citrulline and/or L-arginine supplementation in 196 and 199 patients, respectively (Table 4 and Fig. 4a, b)).
Table 4.
Nitrogen scavenger drugs and L-citrulline and L-arginine
| Patients (clinical course) | Nitrogen scavenger drugs (n = 196) | L-citrulline and L-arginine supplementation (n = 199) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SB | SP | SD & SP | None | Total | Arg | Cit | Cit & Arg | None | Total | |
| Asymptomatic | 3 (5%) | 1 (1%) | 3 (5%) | 57 (89%) | 64 (100%) | 18 (27%) | 5 (8%) | 3 (4%) | 41 (61%) | 67 (100%) |
| Symptomatic | 41 (31%) | 25 (19%) | 29 (22%) | 36 (28%) | 131 (100%) | 68 (52%) | 37 (28%) | 7 (5%) | 19 (15%) | 131 (100%) |
| Unknown | 1 | – | – | – | 1 | 1 | – | – | – | 1 |
| Total | 45 (23%) | 26 (13%) | 32 (16%) | 93 (48%) | 196 (100%) | 87 (44%) | 42 (21%) | 10 (5%) | 60 (30%) | 199 (100%) |
Data on treatment with nitrogen scavenger drugs and L-citrulline and L-arginine supplementation for patients with asymptomatic, symptomatic or unknown clinical courses
SB sodium benzoate; SP sodium phenylbutyrate; Cit L-citrulline; Arg L-arginine
Fig. 4.
Pharmacological treatment with Na-Benzoate and Na-Phenylbutyrate (4a) (data from 196 patients) as well as supplements of L-arginine and L-citrulline (4b) (data from 199 patients)
We did not systematically collect information on the compliance of the patients.
Liver transplantation
Liver transplantation, currently the only ‘cure’ for UCDs, was performed in only six (3%; n = 208) patients including four females with OTCD and two with ASSD (1F, 1M).
Mortality
Of all 208 patients, six (3%) had already died (all affected by OTCD) (Table 3). One patient died at 8.9 years during an acute crisis, one at the age of 5.3 years from brain edema, one at 12 years from abdominal bleeding and post transplantation liver failure, one at 10 years due to brain edema during an infection, one committed suicide at 33 years, and one patient died at 22 years without documentation of the cause.
Discussion
Most metabolic centres care for only small groups of UCD patients and large registries have only recently been established (Kölker et al 2011; Tuchman et al 2008). Therefore, information on the natural course of these diseases has mainly relied on case reports and case series. Traditionally, UCDs are considered as pediatric diseases and only few publications focus on adult presentations (Bogdanovic et al 2000; Felig et al 1995; Honeycutt et al 1992; Maestri et al 1998; Salek et al 2010; Saudubray et al 2006; Schimanski et al 1996; Smith et al 2005; Snebold et al 1987; Thurlow et al 2010).
However with improvements in management, more UCD patients are reaching adulthood. In recent years it has become obvious that non-classical UCD patients with later onset and/or an attenuated clinical course may be as frequent as those with classical presentations in the first weeks of life.
Data on patients with an atypical presentation, as defined above, were therefore collected from metabolic centres from five European countries. The resulting cohort of 208 patients was discussed in a workshop on non-classical UCDs held in 2011. It is important to notice that even with strict adherence to the given criteria, this study is descriptive and contains some ascertainment bias. Moreover, the definition of “non-classical UCDs” is not listed in accepted classifications such as ICD10 and this may be another confounding factor.
Similar numbers of pediatric and adult patients were present in this cohort (103 and 105, respectively). When OTCD patients were excluded from the analysis, the age distribution was slightly in favour of pediatric patients (64%). However in the largest subgroup, OTCD patients, the majority were adult (61%) with a mean age of 24.5 years in female and 17.1 years in male OTCD patients, underlining the need for physicians experienced in treating adult patients with UCDs.
Based on the high proportions of symptomatic cases (71%), female OTCD should not be regarded a benign disorder, although there may be a bias in this cohort towards symptomatic females because asymptomatic female carriers may not have come to clinical attention.
In our sample, equal proportions of female and male OTCD patients were symptomatic and also the proportion of male and female patients with at least one documented episode of symptomatic hyperammonemia was not statistically different. Likewise, the median peak ammonia for OTCD females with acute crisis (381 μmol/L) was not significantly different from that in male patients (294 μmol/L). Overall, the number of symptomatic patients was highest in OTCD (72% of all patients) compared to 65% in ASLD and 35% in ASSD. However this difference is probably explained by asymptomatic ASLD and ASSD patients identified by NBS.
Confirming data from other reports (Genet et al 2000; Yamaguchi et al 2006), the mutation detection rate in this study for OTCD was 84%, whereas it reached 97% in other UCDs. Also in line with published data (Tuchman et al 1995), maternal carrier status for OTCD was more common in male OTCD (74%) than in female OTCD (33%). This higher risk for mothers of boys to have another affected child is important for genetic counselling.
The diagnostic delay observed in this cohort is worrying albeit similar to other inborn errors of metabolism and confirming previous reports (Rowe et al 1986). In the group of patients with a delay of > 1 year, 75% were affected by cognitive impairment compared to 46% if the delay was ≤ 1 year. Assuming that severe cases reach their confirmed diagnosis earlier, this higher proportion of cognitive impairment in the presumably less severely affected patients suggests a causative relationship of time to diagnosis and likelihood of cognitive impairment. Although this finding would ideally be confirmed in larger studies, this is in favour of a need for prompt diagnosis and initiation of treatment to avoid a poor neurological outcome. If this is to be achieved, there needs to be an increased awareness of the possibility of a UCD and ammonia should be measured early in every patient with unexplained clinical features compatible with a UCD such as impaired consciousness, liver failure, or psychiatric symptoms.
A larger proportion of ASLD and ASSD patients detected by NBS as compared to those without NBS remained asymptomatic, in line with previous reports on the benign course in some patients (Ficicioglu et al 2009; Häberle et al 2003; Mercimek-Mahmutoglu et al 2010). However, some patients identified through NBS later developed symptomatic hyperammonemia. Of those, some carried a genotype predicting a “mild course” indicating that the clinical management can not entirely rely on genotypes only. This underlines the need to follow these patients regularly in a metabolic centre and to provide a sick-day protocol and emergency regimen to cover potentially catabolic episodes.
A high number of patients were affected by MR/DD in the entire cohort but especially in ASLD and ARG1D, with all ASLD patients being symptomatic and 88% of ARG1D patients. This poor outcome might in part be caused by the longer mean diagnostic delay in ASLD (3 years, sd = 4, range 0–13) and ARG1D (2.5 years, sd = 1.7, range 0–4) patients compared to OTCD (1.2 years, sd = 2.4, range 0–9.5) although symptoms in ARG1D are in addition caused by its specific pathogenesis. However, more detailed information about the neurological situation of UCD patients would be required before final conclusions can be drawn. The same refers to the relatively small number of patients with seizures (except in the few ARG1D patients of which 88% were affected). The reason for this might be the inclusion of asymptomatic patients or those with mild phenotypes.
Regarding treatment, it is interesting to see that the same number of OTCD patients was treated with either L-citrulline 39 (35%) or with L-arginine 40 (36%) and only few patients received both (Fig. 4b). This reflects the unsolved issue of using L-citrulline or L-arginine alone or a combination of both (Häberle et al 2012; Wilcken 2004). The choice of L-arginine might be influenced by the higher price and the lower availability of L-citrulline but further studies are required to determine whether one amino acid is superior to the other in treating OTCD.
In summary, this cross-sectional observational report on a large cohort of patients with non-classical UCDs highlights the high proportion of adult patients, of clinically affected female OTCD patients and of cognitive impairment as well as the often long delay in reaching the correct diagnosis that may add to a relevant extent to the morbidity of the cohort. There is a need for greater awareness that these disorders can present in a non-classical way and outside the neonatal period. Longitudinal studies are required to determine the long-term outcome for this growing group of patients.
| Clues for clinical practice |
| • Urea cycle disorders (UCDs) may be important also for adult physicians since in this study 50% of the patients were ≥ 16 years old |
| • The diagnosis should be made as early as possible to avoid neurological complications; in this study 75% were affected by cognitive impairment if the delay was >1 year, compared to 46% if the delay was ≤ 1 year |
| • In all patients with unexplained acute encephalopathy, plasma ammonia should be measured |
| • If neurological symptoms are episodic and variable, consider an underlying UCD |
| • Include UCDs to the differential diagnosis in patients with a suspicion of an intoxication |
Acknowledgment
The data presented in this paper have been collected for and were discussed at a workshop generously sponsored by Milupa Metabolics GmbH, Germany. The authors are grateful to Dr. M. Ott and Mrs. S. Vaupel-Stümke for their excellent support in organising the workshop. The workshop sponsor had however no influence on the planning and procedure of the workshop or on the content of this paper. The authors further acknowledge the technical assistance from Mrs. U. Spörri (University Children’s Hospital Zurich) in organising and conducting the data collection. Many of the authors are part of the project European registry and network for Intoxication type Metabolic diseases (E-IMD) funded by the European Union, in the framework of the Health Programme 2008–2013 (http://www.e-imd.org/en/index.phtml) which was instrumental for the success of the workshop. The work on the phenotypic variability of urea cycle disorders is supported by the Swiss National Science Foundation (grant 310030_127184/1 to JH).
Competing interest
The data presented in this study have been collected for and were discussed at a workshop held at the University Children's Hospital Zurich generously sponsored by Milupa Metabolics GmbH, Germany. In detail, the sponsor provided support for the medical student travelling to participating study centres allowing her to collect all data. Support included economy train fares, budget accommodation and meals. Moreover, the workshop sponsor provided support for the participants of the workshop by covering costs for non-luxury accommodation and standard hospital meals during the workshop. There was however no honorarium or other consulting fee included for any of the workshop participants. Likewise, the workshop sponsor had no influence and was not included other than for organisation on the planning and procedure of the workshop or on writing and revising of this paper.
Other than that, all authors declare that they have no conflict of interest.
References
- Bachmann C. Mechanisms of hyperammonemia. Clin Chem Lab Med. 2002;40:653–662. doi: 10.1515/CCLM.2002.112. [DOI] [PubMed] [Google Scholar]
- Berning C, Bieger I, Pauli S, et al. Investigation of citrullinemia type I variants by in vitro expression studies. Hum Mutat. 2008;29:1222–1227. doi: 10.1002/humu.20784. [DOI] [PubMed] [Google Scholar]
- Bogdanovic MD, Kidd D, Briddon A, Duncan JS, Land JM. Late onset heterozygous ornithine transcarbamylase deficiency mimicking complex partial status epilepticus. J Neurol Neurosurg Psychiatry. 2000;69:813–815. doi: 10.1136/jnnp.69.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusilow SW. Arginine, an indispensable amino acid for patients with inborn errors of urea synthesis. J Clin Invest. 1984;74:2144–2148. doi: 10.1172/JCI111640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusilow S, Horwich A. Urea cycle enzymes. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic & molecular bases of inherited disease. 8. New York: McGraw-Hill; 2001. pp. 1909–1963. [Google Scholar]
- Brusilow SW, Maestri NE. Urea cycle disorders: diagnosis, pathophysiology, and therapy. Adv Pediatr. 1996;43:127–170. [PubMed] [Google Scholar]
- Davis PK, Wu G. Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:527–537. doi: 10.1016/S0305-0491(98)00014-5. [DOI] [PubMed] [Google Scholar]
- Dionisi-Vici C, Rizzo C, Burlina AB, Caruso U, Sabetta G, Uziel G, Abeni D. Inborn errors of metabolism in the Italian pediatric population: a national retrospective survey. J Pediatr. 2002;140:321–327. doi: 10.1067/mpd.2002.122394. [DOI] [PubMed] [Google Scholar]
- Enns GM. Neurologic damage and neurocognitive dysfunction in urea cycle disorders. Semin Pediatr Neurol. 2008;15:132–139. doi: 10.1016/j.spen.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Felig DM, Brusilow SW, Boyer JL. Hyperammonemic coma due to parenteral nutrition in a woman with heterozygous ornithine transcarbamylase deficiency. Gastroenterology. 1995;109:282–284. doi: 10.1016/0016-5085(95)90295-3. [DOI] [PubMed] [Google Scholar]
- Ficicioglu C, Mandell R, Shih VE. Argininosuccinate lyase deficiency: longterm outcome of 13 patients detected by newborn screening. Mol Genet Metab. 2009;98:273–277. doi: 10.1016/j.ymgme.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genet S, Cranston T, Middleton-Price HR. Mutation detection in 65 families with a possible diagnosis of ornithine carbamoyltransferase deficiency including 14 novel mutations. J Inherit Metab Dis. 2000;23:669–676. doi: 10.1023/A:1005614409241. [DOI] [PubMed] [Google Scholar]
- Häberle J, Pauli S, Schmidt E, Schulze-Eilfing B, Berning C, Koch HG. Mild citrullinemia in Caucasians is an allelic variant of argininosuccinate synthetase deficiency (citrullinemia type 1) Mol Genet Metab. 2003;80:302–306. doi: 10.1016/j.ymgme.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Häberle J, Shchelochkov OA, Wang J, et al. Molecular defects in human carbamoyl phosphate synthetase I: mutational spectrum, diagnostic and protein structure considerations. Hum Mutat. 2011;32:579–589. doi: 10.1002/humu.21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häberle J, Boddaert N, Burlina A, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2012;7:32. doi: 10.1186/1750-1172-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D. Nitrogen metabolism in liver: structural and functional organization and physiological relevance. Biochem J. 1990;267:281–290. doi: 10.1042/bj2670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt D, Callahan K, Rutledge L, Evans B. Heterozygote ornithine transcarbamylase deficiency presenting as symptomatic hyperammonemia during initiation of valproate therapy. Neurology. 1992;42:666–668. doi: 10.1212/WNL.42.3.666. [DOI] [PubMed] [Google Scholar]
- Kleijer WJ, Garritsen VH, Linnebank M, et al. Clinical, enzymatic, and molecular genetic characterization of a biochemical variant type of argininosuccinic aciduria: prenatal and postnatal diagnosis in five unrelated families. J Inherit Metab Dis. 2002;25:399–410. doi: 10.1023/A:1020108002877. [DOI] [PubMed] [Google Scholar]
- Kölker S, Dobbelaere D, Chakrapani A, et al. European registry and network for intoxication type metabolic diseases (E-IMD) (Abstract) J Inher Metab Dis. 2011;34:S93. doi: 10.1007/s10545-011-9289-5. [DOI] [Google Scholar]
- Lee PJ. Hyperammonaemia in adolescence and adulthood. In: Bachmann C, Häberle J, Leonard J, editors. Pathophysiology and management of hyperammonaemia. Heilbronn: SPS Verlagsgesellschaft; 2006. pp. 93–99. [Google Scholar]
- Lee B, Singh RH, Rhead WJ, Sniderman King L, Smith W, Summar ML. Considerations in the difficult-to-manage urea cycle disorder patient. Crit Care Clin. 2005;21:S19–S25. doi: 10.1016/j.ccc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Legras A, Labarthe F, Maillot F, Garrigue MA, Kouatchet A, Ogier de Baulny H. Late diagnosis of ornithine transcarbamylase defect in three related female patients: polymorphic presentations. Crit Care Med. 2002;30:241–244. doi: 10.1097/00003246-200201000-00035. [DOI] [PubMed] [Google Scholar]
- Leonard JV, Morris AA. Urea cycle disorders. Semin Neonatol. 2002;7:27–35. doi: 10.1053/siny.2001.0085. [DOI] [PubMed] [Google Scholar]
- Maestri NE, Lord C, Glynn M, Bale A, Brusilow SW. The phenotype of ostensibly healthy women who are carriers for ornithine transcarbamylase deficiency. Medicine (Baltimore) 1998;77:389–397. doi: 10.1097/00005792-199811000-00005. [DOI] [PubMed] [Google Scholar]
- Mercimek-Mahmutoglu S, Moeslinger D, Häberle J, et al. Long-term outcome of patients with argininosuccinate lyase deficiency diagnosed by newborn screening in Austria. Mol Genet Metab. 2010;100:24–28. doi: 10.1016/j.ymgme.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED. Neurologic outcome in children with inborn errors of urea synthesis. Outcome of urea-cycle enzymopathies. N Engl J Med. 1984;310:1500–1505. doi: 10.1056/NEJM198406073102304. [DOI] [PubMed] [Google Scholar]
- Nassogne MC, Heron B, Touati G, Rabier D, Saudubray JM. Urea cycle defects: management and outcome. J Inherit Metab Dis. 2005;28:407–414. doi: 10.1007/s10545-005-0303-7. [DOI] [PubMed] [Google Scholar]
- Picca S, Dionisi-Vici C, Abeni D, et al. Extracorporeal dialysis in neonatal hyperammonemia: modalities and prognostic indicators. Pediatr Nephrol. 2001;16:862–867. doi: 10.1007/s004670100702. [DOI] [PubMed] [Google Scholar]
- Rowe PC, Newman SL, Brusilow SW. Natural history of symptomatic partial ornithine transcarbamylase deficiency. N Engl J Med. 1986;314:541–547. doi: 10.1056/NEJM198602273140903. [DOI] [PubMed] [Google Scholar]
- Salek J, Byrne J, Box T, Longo N, Sussman N. Recurrent liver failure in a 25-year-old female. Liver Transpl. 2010;16:1049–1053. doi: 10.1002/lt.22118. [DOI] [PubMed] [Google Scholar]
- Saudubray JM, Sedel F, Walter JH. Clinical approach to treatable inborn metabolic diseases: an introduction. J Inherit Metab Dis. 2006;29:261–274. doi: 10.1007/s10545-006-0358-0. [DOI] [PubMed] [Google Scholar]
- Schimanski U, Krieger D, Horn M, Stremmel W, Wermuth B, Theilmann L. A novel two-nucleotide deletion in the ornithine transcarbamylase gene causing fatal hyperammonia in early pregnancy. Hepatology. 1996;24:1413–1415. doi: 10.1002/hep.510240618. [DOI] [PubMed] [Google Scholar]
- Smith W, Kishnani PS, Lee B, et al. Urea cycle disorders: clinical presentation outside the newborn period. Crit Care Clin. 2005;21:S9–S17. doi: 10.1016/j.ccc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Snebold NG, Rizzo JF, Lessell S, Pruett RC. Transient visual loss in ornithine transcarbamoylase deficiency. Am J Ophthalmol. 1987;104:407–412. doi: 10.1016/0002-9394(87)90232-7. [DOI] [PubMed] [Google Scholar]
- Summar ML, Dobbelaere D, Brusilow S, Lee B. Diagnosis, symptoms, frequency and mortality of 260 patients with urea cycle disorders from a 21-year, multicentre study of acute hyperammonaemic episodes. Acta Paediatr. 2008;97:1420–1425. doi: 10.1111/j.1651-2227.2008.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlow VR, Asafu-Adjaye M, Agalou S, Rahman Y. Fatal ammonia toxicity in an adult due to an undiagnosed urea cycle defect: under-recognition of ornithine transcarbamylase deficiency. Ann Clin Biochem. 2010;47:279–281. doi: 10.1258/acb.2010.009250. [DOI] [PubMed] [Google Scholar]
- Tuchman M. The clinical, biochemical, and molecular spectrum of ornithine transcarbamylase deficiency. J Lab Clin Med. 1992;120:836–850. [PubMed] [Google Scholar]
- Tuchman M, Matsuda I, Munnich A, Malcolm S, Strautnieks S, Briede T. Proportions of spontaneous mutations in males and females with ornithine transcarbamylase deficiency. Am J Med Genet. 1995;55:67–70. doi: 10.1002/ajmg.1320550118. [DOI] [PubMed] [Google Scholar]
- Tuchman M, Lee B, Lichter-Konecki U, et al. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol Genet Metab. 2008;94:397–402. doi: 10.1016/j.ymgme.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcken B. Problems in the management of urea cycle disorders. Mol Genet Metab. 2004;81(Suppl 1):S86–S91. doi: 10.1016/j.ymgme.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Brailey LL, Morizono H, Bale AE, Tuchman M. Mutations and polymorphisms in the human ornithine transcarbamylase (OTC) gene. Hum Mutat. 2006;27:626–632. doi: 10.1002/humu.20339. [DOI] [PubMed] [Google Scholar]