Abstract
Objectives
53BP1, a critical mediator of the DNA damage response, functions by regulating the balance between homologous recombination (HR) and the more error-prone non-homologous endjoining (NHEJ). Deletion of 53BP1 in brca1 (but not brca2) null cells partially restores HR and reverses sensitivity to poly-ADP-ribose polymerase inhibitors (PARPi). We characterized 53BP1 and BRCA1 expression and their association with clinical outcomes in sporadic and inherited ovarian carcinomas.
Methods
We evaluated 53BP1 and BRCA1 protein expression using immunohistochemistry in 248 ovarian carcinomas and mRNA expression in 89 cases with quantitative reverse transcriptase PCR. All subjects were comprehensively characterized for germline mutations in BRCA1 and BRCA2.
Results
BRCA1-mutated (but not BRCA2-mutated) ovarian carcinomas had significantly higher 53BP1 protein expression than wildtype carcinomas. 53BP1 message levels were significantly associated with BRCA1 message levels in wildtype and BRCA1-mutated but not BRCA2-mutated carcinomas. In wildtype carcinomas, lower 53BP1 message predicted improved survival (p=0.02, median survival 74 vs. 41 months, HR 0.49, 95% CI 0.27–0.88). Survival was not impacted by BRCA1 message level. 53BP1 expression was not associated with primary platinum resistance. In 54 paired primary and recurrent cases, 53BP1 protein expression was equally likely to decrease or increase, and there was no association between decreased 53BP1 at recurrence and the development of platinum resistance.
Conclusions
BRCA1-mutated ovarian carcinomas have higher 53BP1 protein expression than wildtype or BRCA2-mutated carcinomas, in opposition to previous findings in breast carcinomas. Higher 53BP1 protein, which promotes NHEJ, could explain the frequent chromosomal aberrations that are characteristic of BRCA1-mutated ovarian carcinomas. In wildtype ovarian carcinomas, decreased 53BP1 message predicts improved survival, but message and protein expression were not associated.
Keywords: 53BP1, BRCA1, BRCA2, ovarian carcinoma, homologous recombination, non-homologous endjoining
INTRODUCTION
In mammalian cells, homologous recombination (HR) and non-homologous end joining (NHEJ) are the two major pathways responsible for the repair of DNA double-strand breaks. BRCA1 and BRCA2 (BRCA1/2) are required for error-free repair via HR. In BRCA1/2 mutation carriers, primary carcinomas develop when the wildtype BRCA1/2 allele is lost, leading to a deficiency of functional BRCA1/2 protein. Cells deficient in BRCA1/2 have defective HR, which results in increased sensitivity to DNA crosslinking agents, such as cisplatin and carboplatin. In addition, loss of HR-mediated DNA repair is synthetically lethal with exposure to poly-ADP-ribose polymerase inhibitors (PARPi) [1–3].
Cells with defective HR preferentially undergo DNA repair through the NHEJ pathway. In BRCA1/2-deficient cells, p53-binding protein 1 (53BP1) occupies sites of damage and promotes error-prone NHEJ, which results in mutations and radial chromosome formation. While 53BP1 promotes NHEJ, loss of 53BP1 promotes HR [4–8]; thus, 53BP1 appears to be a key transducer of the cellular response to DNA damage. Investigators have shown that deletion of 53BP1 in brca1 (but not brca2) null cells rescues embryonic lethality, partially restores HR, and reverses sensitivity to PARPi [9, 10]. However, while 53BP1 knockdown or deletion rescues HR deficiency and sensitivity to PARPi, it is insufficient to reverse sensitivity to agents that cause interstrand DNA cross-links, including cisplatin [11].
BRCA1/2-mutated ovarian carcinomas have aneuploidy and frequent chromosomal aberrations, consistent with NHEJ being the predominant DNA repair pathway in these carcinomas. Most studies report improved survival in women with BRCA1/2-mutated ovarian carcinomas compared to women with sporadic ovarian carcinomas, consistent with increased sensitivity to platinum-based chemotherapy secondary to HR deficiency [12–14]. Although somatic mutations in BRCA1/2 are relatively rare in sporadic ovarian carcinomas, loss of BRCA1 protein is common [15]. BRCA1 methylation, which occurs in 15–20% of ovarian carcinomas [16–19], is associated with decreased protein expression, but explains only a fraction of sporadic carcinomas with decreased BRCA1 message [15]. Decreased BRCA1 protein expression, but not BRCA1 methylation, is associated with improved overall survival in sporadic ovarian carcinomas [15, 20, 21].
A better understanding of the role of 53BP1 in sporadic and inherited ovarian carcinoma could have important therapeutic implications. We evaluated protein and mRNA expression of 53BP1 and BRCA1 in a large number of primary and recurrent ovarian, fallopian tube, and peritoneal carcinomas to determine whether 53BP1 expression is associated with clinical outcomes in sporadic and inherited ovarian carcinoma.
METHODS
Subjects
Primary or recurrent epithelial ovarian, fallopian tube, and peritoneal carcinomas that were completely characterized for germline mutations in BRCA1 and BRCA2 were included in the study. All tissues and clinical information were obtained from the University of Washington Gynecologic Oncology Tissue Bank according to an institutional review board-approved protocol. BRCA1/2 genetic testing information was obtained from clinical records or from comprehensive genomic analysis using targeted capture and massively parallel sequencing, as previously described [22]. All cases with negative genetic testing were evaluated for BRCA1/2 gene rearrangements. 194 subjects were included in the study. 112 primary, 28 recurrent, and 54 paired primary-recurrent carcinomas were analyzed.
Only germline mutations in BRCA1 and BRCA2 were considered for the study, as it is not established that somatic and germline mutations would necessarily behave in an equivalent manner. However, the majority of subjects (129 out of 194, 66%) underwent comprehensive genomic analysis for somatic mutations, and only three subjects were identified as having somatic mutations in BRCA1 or BRCA2.
53BP1 Immunohistochemistry (IHC)
IHC analysis was performed on 4-μm-thick tissue sections prepared from formalin-fixed, paraffin-embedded tissue. Tissue sections were deparaffinized in xylene and sequentially rehydrated. Heat-mediated antigen retrieval was performed in a Tris–EDTA buffer (10 mM Tris Base, 1 mM EDTA, pH 9.0) containing 0.05% Tween 20. Slides were allowed to cool to room temperature, washed in PBS, incubated in 3% hydrogen peroxide to block endogenous peroxidase, blocked with 2.5% horse serum, and incubated with mouse primary antibody diluted 1:20 (Anti-53BP1, a kind gift from Thanos Halazonetis, University of Geneva [23]) at 4°C overnight. Secondary antibody was anti-mouse immunoglobulin (ImmPRESS Anti-Mouse IgG, Rat adsorbed peroxidase Polymer Detection Kit; Vector Laboratories, Burlingame, CA, MP-7422-15), and was applied for 30 minutes at room temperature. DAB substrate (Dako) was used to visualize antibody complexes. Sections were counterstained with hematoxylin. Negative (no primary antibody) and positive (normal fallopian tube epithelium) controls were included in each staining run. For each slide, the percentage of positive tumor cells was defined as the percent that stained moderately to strongly positive (nuclear staining) and was treated as a continuous variable for some analyses. The percent of positive neoplastic cells was dichotomized as normal (≥40% moderate or strongly positive) or decreased (<40% moderate or strongly positive) in most analyses, although other cut-offs besides 40% were also examined. The 40% cut-off has been used in other studies which used this same antibody [10, 24], and the relatively high cut-off of 40% was originally chosen because this 53BP1 antibody is very sensitive for detection of low levels of 53BP1.
BRCA1 Immunohistochemistry
BRCA1 protein was detected in formalin fixed paraffin sections using the mouse monoclonal antibody MS110 diluted 1:250 (previously called Ab-1, Oncogene Research Products), as previously described [15]. The MS110 antibody recognizes an amino terminal epitope at BRCA1 (amino acid residues 89–222) [25]. We used non-tumor inflammatory and stromal cells as internal positive control cells for BRCA1. Decreased protein was only scored if normal cells on the same section were positive. Primary and recurrent tumors were stained side by side under identical conditions.
Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction
Real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was used to determine expression of BRCA1 and 53BP1 in a subset of 89 cases for which fresh frozen neoplasm was available. RNA was isolated from frozen tissue using the RNeasy Maxi kit (Qiagen, Valencia, CA). Reverse-transcription was performed with the Superscript VILO cDNA synthesis kit (Invitrogen Life Technologies, Grand Island, NY). TaqMan Gene Expression Assays (Applied Biosystems, Carlsbad, CA) were used for BRCA1 (Hs00173233_m1), 53BP1 (Hs00996818_m1) and GAPDH (PN 4352934E). All samples were run in triplicate, and the Comparative CT (ddCT) method was used for relative quantitation using ABI Sequence Detection Software v2.4 (Applied Biosystems). Target gene CT values were normalized to GAPDH. GAPDH was chosen as our reference gene based on prior experiments evaluating the stability of various candidate reference genes among our samples: We previously tested 7 of our own ovarian cancer specimens (6 of which overlapped with our current study) along with paired normal ovarian epithelial tissue for expression of 10 housekeeping genes (ACTB, B2M, GAPDH, GUSB, HPRT, PGK, PPIA, RPLPO, TBP, and TFRC) as well as 18S rRNA. Among the 11 reference genes tested, GAPDH was the most consistently expressed across malignant and non-malignant ovarian specimens.
Statistical Analysis
Significance of contingency tables was analyzed by Chi squared or Fisher’s exact test. Comparison of 53BP1 protein expression as a continuous variable in subgroups was performed with unpaired t-test. 53BP1 and BRCA1 mRNA associations were evaluated with Pearson correlation analysis. Overall survival was evaluated according to the method of Kaplan and Meier; differences were assessed by the log-rank test. In cases with paired primary and recurrent samples, individuals were counted only once and the primary carcinoma of the pair was used for analysis. Primary platinum sensitivity was defined as a complete response to treatment maintained without progression for at least six months after platinum therapy. Primary platinum resistance was defined as progressive disease on platinum therapy, less than a complete response to platinum therapy, or progression within 6 months of completing platinum therapy. When paired primary and recurrent samples were evaluated to determine if 53BP1 status could predict the development of platinum resistance at time of recurrence, the actual response to platinum-based chemotherapy was used. A case was considered platinum sensitive at recurrence if the subject had a complete response to platinum therapy administered for that recurrence, followed by a progression free interval of six months or greater. The case was considered platinum resistant at recurrence if the subject had less than a complete response to the platinum therapy or recurrence within six months. All P values were two-tailed with alpha set at 0.05. GraphPad Prism software (La Jolla, CA) was used for all statistical analyses.
RESULTS
Case characteristics
194 subjects and 248 carcinomas were included in this study: 112 subjects with primary carcinoma, 28 with recurrent carcinoma, and 54 with a paired primary and recurrent carcinoma (thus, a total of 166 cases were primary and 82 cases were recurrent). Of the 194 subjects, 66 had a deleterious mutation in BRCA1, 23 in BRCA2, and 105 were wildtype for BRCA1/2. Supplementary Table 1 provides the specific germline BRCA1/2 mutations observed. Individuals with variants of uncertain significance were excluded from the study. For primary carcinomas, the median age at diagnosis was 57 years (range, 27–88 years), 89% were advanced stage and had serous histology, and 71% had optimal cytoreduction (<1 cm maximum residual tumor diameter) at the time of primary surgery (Table 1). Table 1 reflects characteristics of the 166 cases with primary carcinoma.
Table 1.
Clinical characteristics of primary carcinomas with normal and decreased 53BP1 expression.
| Total (N) | 53BP1 protein expression | P | ||
|---|---|---|---|---|
| Decreased (<40%) | Normal (≥40%) | |||
|
| ||||
| Median age (years) | 57 y | 60 y | 55.5 y | 0.31 |
|
| ||||
| Stage | ||||
| Early (I/II) | 18 | 1 | 17 | 0.12 |
| Advanced (III/IV) | 148 | 35 | 113 | |
|
| ||||
| Histology | ||||
| Serous | 148 | 32 | 116 | 1.0 |
| Non-serous | 18 | 4 | 14 | |
| Endometrioid | 9 | |||
| Clear cell | 3 | |||
| MMMT | 5 | |||
| Transitional cell | 1 | |||
|
| ||||
| Cytoreduction | ||||
| Optimal | 118 | 22 | 96 | 0.14 |
| Suboptimal | 46 | 14 | 32 | |
| Unknown | 2 | 0 | 2 | |
|
| ||||
| Genetic status | ||||
| Wildtype | 85 | 25 | 60 | |
| BRCA1-mutated | 59 | 7 | 52 | *0.01 |
| BRCA2-mutated | 22 | 4 | 18 | †0.42 |
|
| ||||
| Total | 166 | 36 (22%) | 130 (78%) | |
| Total (N) | 53BP1 mRNA expression | P | ||
|---|---|---|---|---|
| Low | High | |||
|
| ||||
| Median age (years) | 56 y | 57 y | 56 y | 0.73 |
|
| ||||
| Stage | ||||
| Early (I/II) | 2 | 1 | 1 | 1.0 |
| Advanced (III/IV) | 63 | 32 | 31 | |
|
| ||||
| Histology | ||||
| Serous | 59 | 30 | 29 | 1.0 |
| Non-serous | 6 | 3 | 3 | |
| Endometrioid | 2 | |||
| Clear cell | 1 | |||
| MMMT | 3 | |||
|
| ||||
| Cytoreduction | ||||
| Optimal | 40 | 18 | 22 | 0.31 |
| Suboptimal | 25 | 15 | 10 | |
|
| ||||
| Genetic status | ||||
| Wildtype | 53 | 26 | 27 | |
| BRCA1-mutated | 8 | 5 | 3 | *0.71 |
| BRCA2-mutated | 4 | 2 | 2 | †1.0 |
|
| ||||
| Total | 65 | 33 (51%) | 32 (49%) | |
Comparison between wildtype and BRCA1-mutated
Comparison between wildtype and BRCA2-mutated
85 subjects had 53BP1 and BRCA1 mRNA expression evaluated. In this subset, 61 subjects had primary carcinoma, 20 had recurrent carcinoma, and 4 subjects had paired primary-recurrent carcinomas (thus, a total of 65 primary and 24 recurrent cases). 10 subjects were BRCA1 mutation carriers, 5 were BRCA2 mutation carriers, and 70 were wildtype for BRCA1/2. Characteristics of the 65 primary carcinoma cases with mRNA expression data are displayed in Table 1.
53BP1 protein expression
Representative pictures of normal and decreased 53BP1 protein expression in carcinomas are shown in Figure 1. Decreased 53BP1 protein (<40% of cancer cells stained positive) was noted in 22% of all primary carcinomas and 29% of all recurrent carcinomas (p=0.27). When primary carcinomas were stratified by decreased or normal 53BP1 protein expression, there was no significant difference in age at diagnosis, stage, histology, or optimal cytoreduction rates (Table 1). A higher proportion of wildtype primary carcinomas (29.4%) had decreased 53BP1 protein (<40% staining) compared to BRCA1-mutated ovarian carcinomas (11.9%, p=0.01). There was no significant difference in 53BP1 protein expression between BRCA2-mutated and wildtype ovarian carcinomas (Table 1). Using protein expression as a continuous variable, BRCA1-mutated ovarian carcinomas had significantly higher 53BP1 protein expression than wildtype carcinomas in both primary (p=0.001) and recurrent (p=0.04) carcinomas (Figure 2). BRCA2-mutated ovarian carcinomas had a trend toward higher 53BP1 expression than wildtype carcinomas in primary (p=0.07) but not recurrent (p=0.9) carcinomas.
Figure 1.
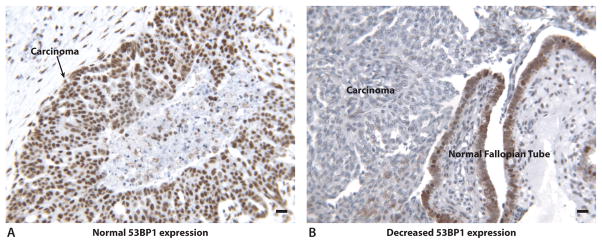
Representative protein expression of 53BP1 in ovarian carcinomas. Protein expression is represented by brown stain. Black bars in lower right corners represent 10 microns. A. Normal 53BP1 protein expression in an ovarian carcinoma. B. Decreased 53BP1 expression in an ovarian carcinoma. Adjacent normal fallopian tube epithelium has normal 53BP1 protein expression.
Figure 2.
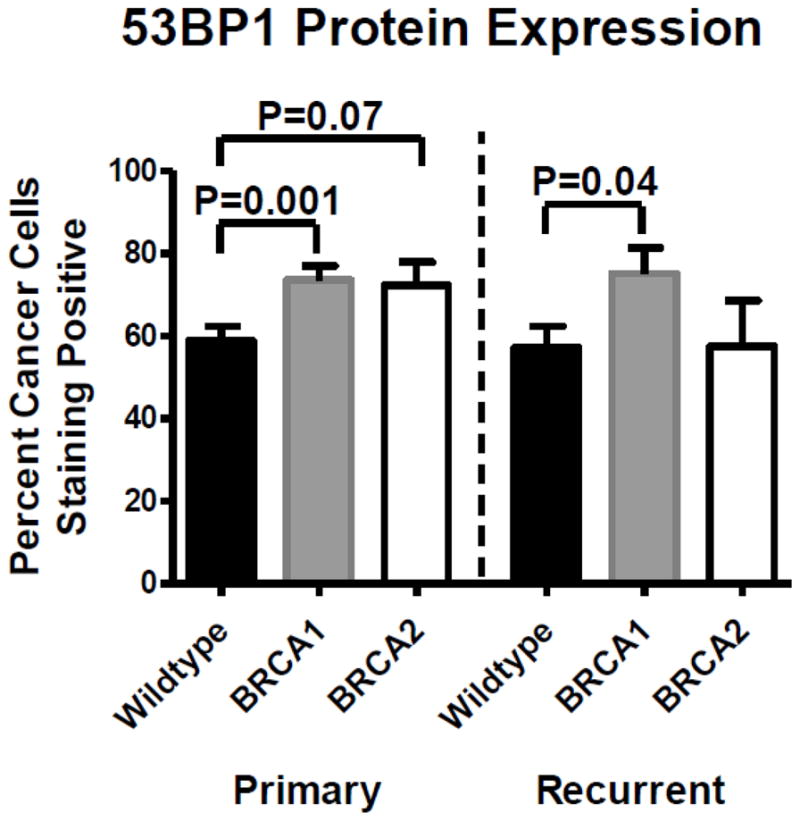
53BP1 protein expression by genetic status. Primary and recurrent BRCA1-mutated carcinomas had significantly higher 53BP1 protein expression compared to wildtype carcinomas. BRCA2-mutated carcinomas had a trend toward higher 53BP1 expression compared to wildtype carcinomas in primary but not recurrent carcinomas. There was no significant difference between primary and recurrent carcinomas for any genetic subgroup.
There was no difference in 53BP1 protein expression among primary wildtype carcinomas when samples with low BRCA1 protein expression (≤30% staining of tumor cells) versus normal BRCA1 protein expression were compared. Likewise, there was also no difference in 53BP1 protein expression when samples were stratified by BRCA1 mRNA expression (data not shown).
53BP1 mRNA expression
In the 65 primary carcinomas with 53BP1 and BRCA1 mRNA expression data, there was no difference in clinical characteristics for carcinomas with low versus high 53BP1 mRNA expression when dichotomizing samples around median 53BP1 mRNA expression (Table 1). There was also no difference in 53BP1 message levels between BRCA1, BRCA2, or wildtype carcinomas, but these analyses were limited by the small number of cases in this series with BRCA1/2 mutations. When dichotomizing samples around median 53BP1 mRNA expression, 16 out of 20 (80%) of recurrent carcinomas had low 53BP1, compared to 33 out of 65 (51%) primary carcinomas (p=0.04). When analyzing mRNA expression as a continuous variable, recurrent carcinomas had slightly lower 53BP1 message expression compared to primary carcinomas (p=0.03); recurrent carcinomas had a median expression of 5.01, compared to 7.93 in the primary carcinomas (1.6-fold lower).
53BP1 message levels were significantly associated with BRCA1 message levels in wildtype and in BRCA1-mutated, but not in BRCA2-mutated, ovarian carcinomas (Figure 3).
Figure 3.
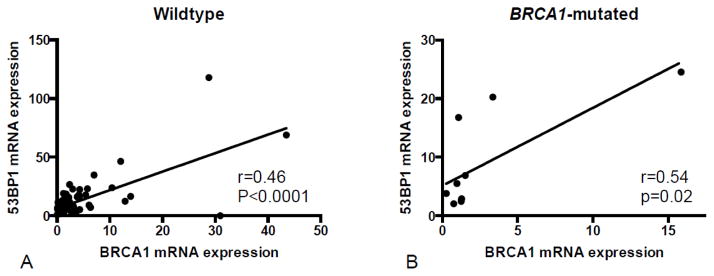
Correlation between expression of 53BP1 and BRCA1 messages. There was a significant correlation between 53BP1 and BRCA1 message expression in wildtype carcinomas (A) and in BRCA1-mutated carcinomas (B). Removing the outliers with high BRCA1 and 53BP1 mRNA expression did not change statistical significance.
Importantly, there was no significant correlation between 53BP1 mRNA and protein expression for cases for which both were evaluated, including whether mRNA and protein expression were considered as continuous variables or as dichotomous variables.
53BP1 and clinical outcomes
Lower 53BP1 message in wildtype carcinoma was associated with an improved overall survival (P=0.02), with a median survival of 74 months versus 41 months (Hazard ratio=0.49, 95% confidence interval 0.27–0.88, Figure 4). Too few carcinomas with BRCA1/2 mutations were available for 53BP1 mRNA assessment to allow survival estimates in those subsets. In contrast to message expression, 53BP1 protein expression did not impact overall survival for subjects with primary wildtype, BRCA1-mutated, or BRCA2-mutated carcinomas, whether analyzed as a dichotomous or continuous variable.
Figure 4.
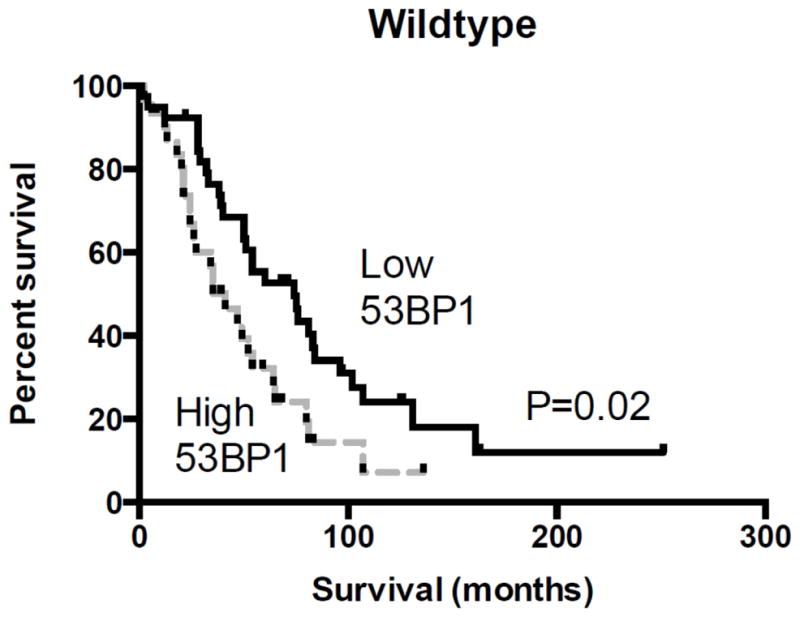
53BP1 message and overall survival. There was a significant association between low 53BP1 mRNA expression and improved survival in subjects with wildtype carcinomas, with a median survival of 74 months versus 41 months (HR=0.49, 95% CI=0.27–0.88, P=0.02).
Since mRNA levels of BRCA1 and 53BP1 were correlated, we assessed whether the improved survival observed for wildtype ovarian carcinomas with low 53BP1 message could be attributed to low BRCA1 message. However, survival was not significantly impacted by BRCA1 message level when carcinomas were dichotomized around median BRCA1 message expression.
For primary carcinomas, there was no significant association between decreased 53BP1 protein expression or low 53BP1 message and primary platinum resistance. There was also no significant association between decreased BRCA1 protein expression (≤30% staining) or low BRCA1 message and platinum resistance in wildtype carcinomas.
53BP1 protein expression in primary-recurrent pairs
Fifty-four subjects had both a primary and a recurrent carcinoma available for evaluation; of these, 18 had a germline BRCA1 mutation, 9 had a germline BRCA2 mutation, and 27 were wildtype. We classified 53BP1 protein expression as decreased or normal and examined whether 53BP1 levels varied at the time of recurrence compared to the untreated primary carcinoma (Table 2). In 43 cases in which the primary carcinoma had normal 53BP1 expression, 28 (65%) maintained normal protein expression, and 15 (35%) demonstrated decreased 53BP1 expression in the recurrence. In 11 cases in which the primary carcinomas had decreased 53BP1 expression, 6 (55%) had similar and 5 (45%) had increased (i.e. normal) 53BP1 expression in the recurrence. Therefore, it was not more likely for 53BP1 protein to increase or decrease in a paired recurrence. Only four pairs had 53BP1 mRNA levels evaluated for both the primary and recurrent sample (one BRCA1-mutated carcinoma, and three wildtype carcinomas), and all four demonstrated a relative decrease in 53BP1 mRNA levels in the recurrence.
Table 2.
53BP1 protein expression and platinum response in 54 paired primary and recurrent carcinomas, by genetic subtype.
| Wildtype pairs | ||||
|---|---|---|---|---|
| 53BP1 status | Total (N) | Number platinum resistant (%)* | ||
| Primary | Recurrent | Primary | Recurrent | |
| Normal | Normal | 11 | 3/7 (43%) | 3/7 (43%) |
| Normal | Decreased | 9 | 2/8 (25%) | 4/8 (50%) |
| Decreased | Decreased | 5 | 1/5 (20%) | 3/5 (60%) |
| Decreased | Normal | 2 | 1/2 (50%) | 2/2 (100%) |
| BRCA1-mutated pairs | ||||
|---|---|---|---|---|
| 53BP1 status | Total (N) | Number platinum resistant (%)* | ||
| Primary | Recurrent | Primary | Recurrent | |
| Normal | Normal | 14 | 1/10 (10%) | 3/10 (30%) |
| Normal | Decreased | 3 | 1/3 (33%) | 2/3 (66%) |
| Decreased | Decreased | 1 | 0/1 (0%) | 1/1 (100%) |
| Decreased | Normal | 0 | 0/0 | 0/0 |
| BRCA2-mutated pairs | ||||
|---|---|---|---|---|
| 53BP1 status | Total (N) | Number platinum resistant (%)* | ||
| Primary | Recurrent | Primary | Recurrent | |
| Normal | Normal | 3 | 0/2 (0%) | 1/2 (50%) |
| Normal | Decreased | 3 | 0/1 (0%) | 0/1 (0%) |
| Decreased | Decreased | 0 | 0/0 | 0/0 |
| Decreased | Normal | 3 | 0/2 (0%) | 0/2 (100%) |
Not all pairs with 53BP1 assessment had complete clinical information available
Although not statistically significant, there was a trend that BRCA1-mutated carcinomas were more likely to retain normal 53BP1 protein expression in recurrence compared to wildtype carcinomas, with 53BP1 decreasing in only three out of 17 (17.6%) BRCA1-mutated carcinomas, compared to nine out of 20 (45%) wildtype carcinomas (p=0.09).
22 wildtype, 14 BRCA1-mutated, and five BRCA2-mutated pairs had complete clinical information available regarding their history of recurrence and platinum sensitivity (Table 2). There was no association between decreased 53BP1 protein expression (<40%) at time of recurrence and development of platinum resistance. In wildtype carcinomas, 13 patients had decreased 53BP1 at recurrence, and three (23%) developed platinum resistance (six remained platinum-sensitive and three had primary platinum resistance); 9 patients had normal 53BP1 expression in their recurrence, and one (11%) developed platinum resistance (four remained platinum-sensitive and four had primary platinum resistance).
In the BRCA1-mutated carcinomas, four patients had decreased 53BP1 at recurrence, and two (50%) developed platinum resistance (one patient remained platinum-sensitive and one had primary platinum resistance); ten patients had normal 53BP1 expression in their recurrence, and two (20%) developed platinum resistance (seven remained platinum-sensitive and one had primary platinum resistance). 12 of 14 BRCA1-mutated carcinomas also were evaluated for secondary somatic mutations that restored BRCA1 in the recurrence, which we have previously shown to predict platinum resistance [26]. For those with decreased 53BP1 expression at recurrence, both recurrent carcinomas (100%) which had developed platinum resistance had somatic mutations that restored BRCA1. Of those which retained normal 53BP1 expression, two of three (66%) which had developed platinum resistance had secondary somatic mutations that restored BRCA1. (No secondary mutations were found in the patients who remained platinum sensitive, regardless of 53BP1 expression.) Thus, a decrease in 53BP1 protein at recurrence did not predict platinum resistance in BRCA1-mutated carcinomas, in contrast to the development of reversion mutations, which was highly correlated with platinum resistance.
Exploratory analyses using different definitions of decreased 53BP1 protein expression
53BP1 protein expression was considered decreased when less than 40% of malignant epithelial cells stained positive. We explored whether other cut-offs, including 30% and 50%, would change our results. When using a cut-off of 50%, a higher proportion of wildtype primary carcinomas (29.4%) had decreased 53BP1 protein compared to BRCA1-mutated ovarian carcinomas (13.6%, p=0.03), and there was no significant difference in 53BP1 protein expression between BRCA2-mutated and wildtype ovarian carcinomas, similar to the findings seen when using a cut-off of 40%. When using a cut-off of 30%, overall fewer primary cases had decreased 53BP1 expression (16.9% compared to 21.7% using the 40% cut-off), and there was no longer a statistically significant difference between primary wildtype and BRCA1-mutated (or BRCA2-mutated) carcinomas, although there was a trend towards significance (p=0.07).
There was no association between decreased 53BP1 protein expression and primary platinum sensitivity or survival for any cut-off evaluated. When evaluating 53BP1 protein expression in primary-recurrent pairs, it was not more likely for 53BP1 protein to increase or decrease in a paired recurrence. Of the cases in which the primary carcinoma had normal 53BP1 expression, 33%, 35%, and 32% of cases demonstrated decreased 53BP1 expression at recurrence, when using cut-offs of 30%, 40%, and 50%, respectively. Of the small number of cases in which the primary carcinomas had decreased 53BP1 expression, 36%, 45%, and 50% had increased (i.e. normal) expression in the recurrence, respectively. Thus, findings were very similar when using different cut-offs.
DISCUSSION
We evaluated 53BP1 expression in a large set of ovarian, fallopian tube, and peritoneal carcinomas, and correlated 53BP1 expression with clinical outcomes in sporadic and inherited carcinomas. Primary and recurrent BRCA1-mutated (but not BRCA2-mutated) ovarian carcinomas had higher 53BP1 protein expression compared to wildtype carcinomas. In primary carcinomas, decreased 53BP1 expression was noted in 29% of wildtype compared to only 12% of BRCA1-mutated carcinomas. However, although these results were statistically significant, the lack of an association of 53BP1 protein expression with clinical outcomes calls into question the clinical importance of this finding.
In contrast to our findings of higher 53BP1 protein expression in BRCA1-mutated compared to wildtype ovarian carcinomas, Bouwman et al reported that both BRCA1-mutated and BRCA2-mutated breast carcinomas had lower 53BP1 expression compared to non-BRCA1/2 breast carcinomas [10]. These investigators hypothesized that loss of 53BP1 promoted survival, allowing BRCA1/2-deficient neoplastic cells to proliferate. It is not immediately clear why 53BP1 protein expression would have an opposite association with BRCA1-mutation status in breast versus ovarian carcinomas. Notably, we used the same antibody, dilution, and definition of decreased expression.
We did not find as pronounced an association for BRCA2-mutated compared to BRCA1-mutated ovarian carcinomas and 53BP1 expression, which is in line with in vitro data that demonstrates a differential effect for 53BP1 in a BRCA1-mutated compared to a BRCA2-mutated background. The increased 53BP1 protein expression in BRCA1-mutated ovarian cancers could merely reflect dominance of the NHEJ DNA repair pathway, which is promoted by 53BP1, in these cancers. The frequently observed chromosomal aberrations and high aneuploidy seen in BRCA1-mutated ovarian carcinomas are consistent with NHEJ being the primary DNA repair pathway in these cells. Indeed, our finding of increased 53BP1 protein in BRCA1-mutated ovarian carcinomas is concordant with laboratory findings by Bouwman et al, who showed that brca1-null conditional mouse embryonic stem cells accumulate in G2, likely reflecting a checkpoint response induced by unrepaired DNA damage, and display increased 53BP1 protein levels in response to DNA damage [10]. The G2 arrest of brca1-null cells was abrogated by shRNA-mediated depletion of 53BP1.
Because in vitro studies have shown that loss of 53BP1 partially restores HR [9–11], we predicted that decreased 53BP1 would correlate with worsened survival. However, we did not observe any correlation of 53BP1 protein expression (whether considered as a dichotomous or continuous variable) with outcomes. Instead, we observed that low 53BP1 mRNA levels significantly correlated with improved survival for subjects with wildtype ovarian carcinoma, with a median survival of 74 months in tumors with low 53BP1 mRNA compared to 41 months in tumors with high 53BP1 mRNA (p=0.02). Notably, 53BP1 mRNA and protein levels were not associated, implying that 53BP1 protein expression is dependent on regulatory mechanisms other than mRNA level, such as microRNAs or protein stability and turnover. Since 53BP1 mRNA was significantly associated with BRCA1 mRNA expression in wildtype carcinomas, we evaluated whether the improved survival associated with low 53BP1 message could be attributed to low BRCA1 message. However, that was not the case. Given the lack of correlation between 53BP1 mRNA and protein levels, it seems doubtful that the improved survival associated with decreased message is directly related to 53BP1 activity, but instead reflects some other gene regulatory process associated with outcome, such as alterations of transcription factors or microRNAs that simultaneously impact both 53BP1 and other genes that impact survival.
In a recent analysis of expression of DNA repair genes using data from the Cancer Genome Atlas (TCGA) analysis of serous ovarian carcinomas [20], Kang et al found that higher expression of 10 of 11 HR genes was associated with improved survival, an effect in opposite direction to their predicted hypothesis [28]. The notable exception was BRCA2, in which lower expression was associated with longer survival. Kang’s findings that higher HR gene expression was associated with improved survival runs counter to the current predictive model of HR defective ovarian carcinomas and predicted platinum sensitivity. Possibly, cells that are defective in HR have higher expression of HR genes and lower 53BP1 message in an ineffective attempt to alter protein expression and increase HR function. The DNA damage response pathway and how alterations in individual components might impact therapeutic response appears more complex than our underlying assumptions; regulatory mechanism will require significant further investigation.
A hallmark of BRCA1/2-mutated carcinomas is hypersensitivity to platinum-based chemotherapy and PARP inhibitors. Investigators have shown that deletion of 53BP1 in brca1 (but not brca2) null cells partially restores HR and reverses sensitivity to PARP inhibitors [9–11], but does not reverse sensitivity to cisplatin [11]. Furthermore, data from Patel et al suggest that response to PARP inhibitors requires an intact NHEJ pathway [29]. In our study, decreased 53BP1 expression did not correlate with platinum resistance in either primary or paired primary and recurrent cases, concordant with recent in vitro data [11]. However, it will be of interest to correlate 53BP1 protein expression with in vivo response to PARPi.
In BRCA1/2 mutation carriers, the development of secondary somatic mutations that restore BRCA1/2 in recurrent ovarian carcinoma is highly predictive of developing platinum resistance [26]. We hypothesized that loss of 53BP1 could be a complementary resistance mechanism. However, in our set of paired primary and recurrent carcinomas, most of the BRCA1-mutated recurrent carcinomas that developed platinum resistance had a secondary somatic mutation that restored BRCA1, likely explaining platinum resistance. In these cases, 53BP1 protein alterations did not add further predictive information. Thus, a decrease in 53BP1 protein at recurrence compared to untreated primary carcinoma does not appear to be an important mediator of platinum resistance in BRCA1-mutated carcinomas.
In summary, BRCA1-mutated ovarian carcinomas have higher 53BP1 protein expression compared to wildtype or BRCA2-mutated ovarian carcinomas, which contrasts to previous data in breast carcinomas. Decreased 53BP1 mRNA expression predicts improved overall survival in wildtype carcinomas. Decreased 53BP1 does not appear to be a mediator of platinum resistance, but it will be important to assess whether 53BP1 expression is more predictive of clinical PARPi response or resistance.
Supplementary Material
Research Highlights.
Decreased 53BP1 mRNA expression is associated with improved survival in sporadic ovarian carcinomas
53BP1 protein expression is higher in BRCA1-mutated ovarian carcinomas
Acknowledgments
Role of the funding source
This work was supported by NIH grants R01CA131965, and P50CA083636, and the Wendy Feuer Ovarian Cancer Research Fund.
The authors would like to thank Dr. Thanos Halazonetis for the generous donation of 53BP1 antibody and for editorial suggestions, and Dr. Scott Kaufmann for a thoughtful review and editorial suggestions.
Footnotes
Conflicts of Interest Statement
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 2.McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi T, Tischkowitz M, Ameziane N, Hodgson SV, Mathew CG, Joenje H, et al. Disruption of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian tumors. Nat Med. 2003;9:568–574. doi: 10.1038/nm852. [DOI] [PubMed] [Google Scholar]
- 4.Bothmer A, Robbiani DF, Di Virgilio M, Bunting SF, Klein IA, Feldhahn N, et al. Regulation of DNA end joining, resection, and immunoglobulin class switch recombination by 53BP1. Mol Cell. 2011;42:319–329. doi: 10.1016/j.molcel.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunting SF, Callen E, Wong N, Chen HT, Polato F, Gunn A, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Difilippantonio S, Gapud E, Wong N, Huang CY, Mahowald G, Chen HT, et al. 53BP1 facilitates long-range DNA end-joining during V(D)J recombination. Nature. 2008;456:529–533. doi: 10.1038/nature07476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward IM, Reina-San-Martin B, Olaru A, Minn K, Tamada K, Lau JS, et al. 53BP1 is required for class switch recombination. J Cell Biol. 2004;165:459–464. doi: 10.1083/jcb.200403021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie A, Hartlerode A, Stucki M, Odate S, Puget N, Kwok A, et al. Distinct roles of chromatin-associated proteins MDC1 and 53BP1 in mammalian double-strand break repair. Mol Cell. 2007;28:1045–1057. doi: 10.1016/j.molcel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunting SF, Callen E, Wong N, Chen HT, Polato F, Gunn A, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouwman P, Aly A, Escandell JM, Pieterse M, Bartkova J, van der Gulden H, et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat Struct Mol Biol. 2010;17:688–695. doi: 10.1038/nsmb.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunting SF, Callen E, Kozak ML, Kim JM, Wong N, Lopez-Contreras AJ, et al. BRCA1 functions independently of homologous recombination in DNA interstrand crosslink repair. Mol Cell. 2012;46:125–135. doi: 10.1016/j.molcel.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolton KL, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382–390. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chetrit A, Hirsh-Yechezkel G, Ben-David Y, Lubin F, Friedman E, Sadetzki S. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: the national Israeli study of ovarian cancer. J Clin Oncol. 2008;26:20–25. doi: 10.1200/JCO.2007.11.6905. [DOI] [PubMed] [Google Scholar]
- 14.Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swisher EM, Gonzalez RM, Taniguchi T, Garcia RL, Walsh T, Goff BA, et al. Methylation and protein expression of DNA repair genes: association with chemotherapy exposure and survival in sporadic ovarian and peritoneal carcinomas. Mol Cancer. 2009;8:48. doi: 10.1186/1476-4598-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catteau A, Harris WH, Xu CF, Solomon E. Methylation of the BRCA1 promoter region in sporadic breast and ovarian cancer: correlation with disease characteristics. Oncogene. 1999;18:1957–1965. doi: 10.1038/sj.onc.1202509. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin RL, Nemeth E, Tran H, Shvartsman H, Cass I, Narod S, et al. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000;60:5329–5333. [PubMed] [Google Scholar]
- 18.Strathdee G, Appleton K, Illand M, Millan DW, Sargent J, Paul J, et al. Primary ovarian carcinomas display multiple methylator phenotypes involving known tumor suppressor genes. Am J Pathol. 2001;158:1121–1127. doi: 10.1016/S0002-9440(10)64059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teodoridis JM, Hall J, Marsh S, Kannall HD, Smyth C, Curto J, et al. CpG island methylation of DNA damage response genes in advance ovarian cancer. Cancer Res. 2005;65:8961–8967. doi: 10.1158/0008-5472.CAN-05-1187. [DOI] [PubMed] [Google Scholar]
- 20.The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thrall M, Gallion HH, Kryscio R, Kapali M, Armstrong DK, DeLoia JA. BRCA1 expression in a large series of sporadic ovarian carcinomas: a Gynecologic Oncology Group study. Int J Gynecol Cancer. 2006;16:166–171. doi: 10.1111/j.1525-1438.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 22.Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108:18032–18037. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schultz LB, Chehab NH, Malikzay A, Halazonetis TD. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J Cell Biol. 2000;151:1381–1390. doi: 10.1083/jcb.151.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartkova J, Horejsi Z, Sehested M, Nesland JM, Rajpert-De Meyts E, Skakkebaek NE, et al. DNA damage response mediators MDC1 and 53BP1: constitutive activation and aberrant loss in breast and lung cancer, but not in testicular germ cell tumors. Oncogene. 2007;26:7414–7422. doi: 10.1038/sj.onc.1210553. [DOI] [PubMed] [Google Scholar]
- 25.Wilson CA, Ramos L, Villasenor MR, Anders KH, Press MF, Clarke K, et al. Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet. 1999;21:236–240. doi: 10.1038/6029. [DOI] [PubMed] [Google Scholar]
- 26.Norquist B, Wurz KA, Pennil CC, Garcia R, Gross J, Sakai W, et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol. 2011;29:3008–3015. doi: 10.1200/JCO.2010.34.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang ZC, Birkbak NJ, Culhane A, Drapkin RI, Fatima A, Tian R, et al. Profiles of genomic instability in high-grade serous ovarian cancer predict treatment outcome. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang J, D’Andrea AD, Kozono D. A DNA repair pathway-focused score for prediction of outcomes in ovarian cancer treated with platinum-based chemotherapy. J Natl Cancer Inst. 2012;104:670–681. doi: 10.1093/jnci/djs177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci USA. 2011;108:3406–3411. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


