Abstract
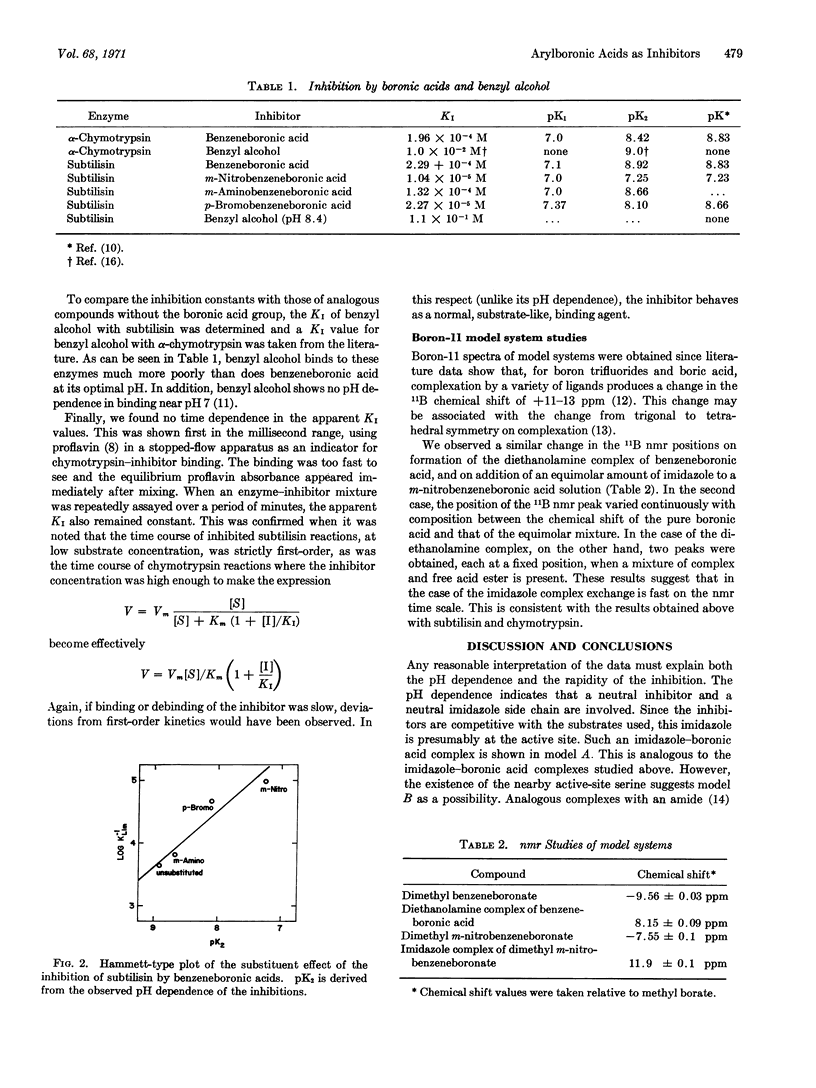
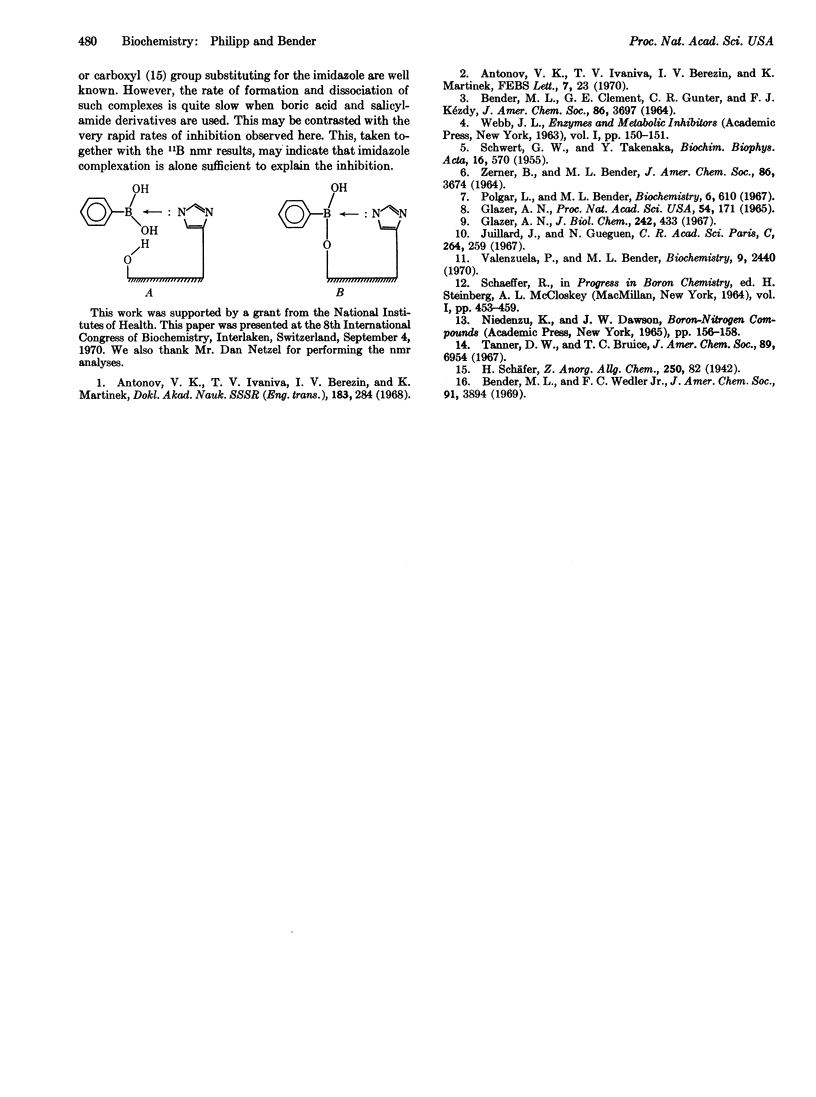
Arylboronic acids were found to be strong competitive inhibitors of subtilisin and chymotrypsin. The binding constants are strongly pH dependent and give a Hammett-type plot with a slope of -0.885. The pH dependence, the Hammett plot, and nmr model-system studies indicate that inhibition is due to electron-pair donation by the active site histidine to the bound inhibitor.
Full text
PDF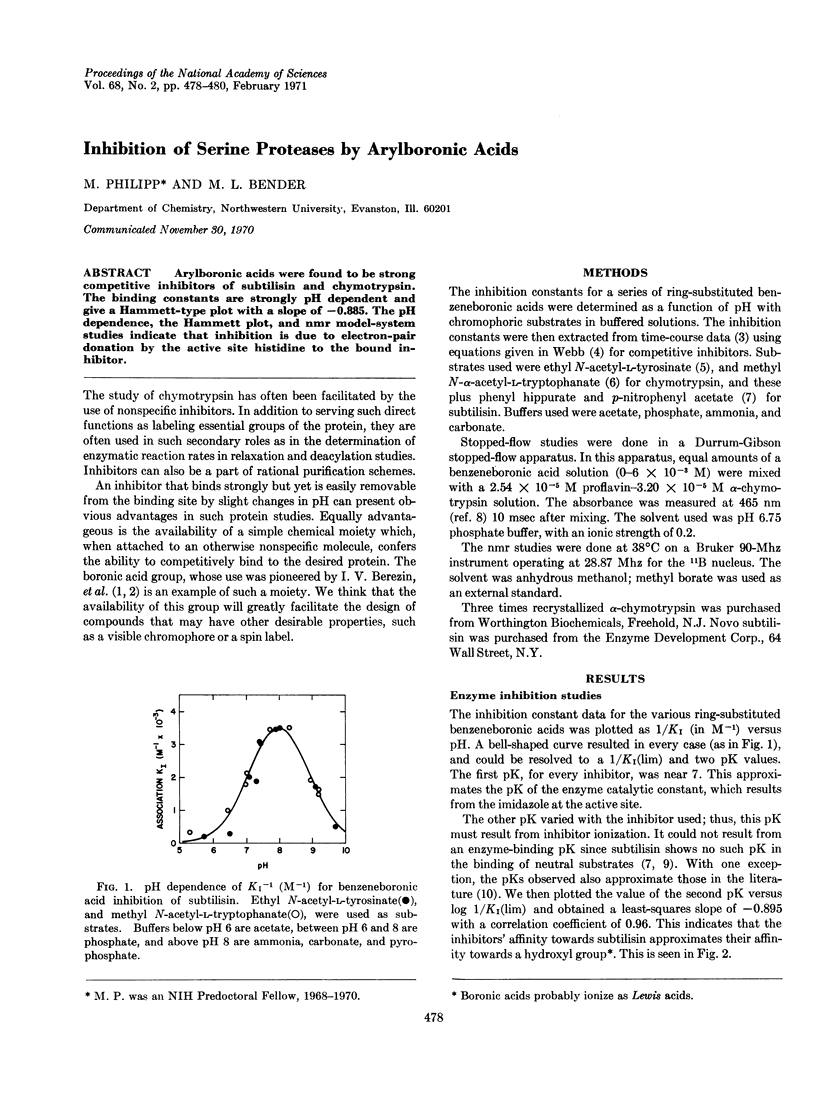
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonov V. K., Ivanina T. V., Berezin I. V., Martinek K. n-Alkylboronic acids as bifunctional reversible inhibitors of alpha-chymotrypsin. FEBS Lett. 1970 Mar 16;7(1):23–25. doi: 10.1016/0014-5793(70)80607-x. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Esteratic reactions catalyzed by subtilisins. J Biol Chem. 1967 Feb 10;242(3):433–436. [PubMed] [Google Scholar]
- Glazer A. N. Spectral studies of the interaction of alpha-chymotrypsin and trypsin with proflavine. Proc Natl Acad Sci U S A. 1965 Jul;54(1):171–176. doi: 10.1073/pnas.54.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar L., Bender M. L. The reactivity of thiol-subtilisin, an enzyme containing a synthetic functional group. Biochemistry. 1967 Feb;6(2):610–620. doi: 10.1021/bi00854a032. [DOI] [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim Biophys Acta. 1955 Apr;16(4):570–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Bender M. L. Binding of competitive inhibitors to delta-chymotrypsin in the alkaline pH region. Competitive inhibition kinetics and proton-uptake measurements. Biochemistry. 1970 Jun 9;9(12):2440–2446. doi: 10.1021/bi00814a008. [DOI] [PubMed] [Google Scholar]


