Abstract
Background
Strictures develop in >30% of Crohn’s disease. No available medication prevents stricture development in susceptible patients. In Crohn’s strictures, but not adjacent normal intestine, TGF-β1 increases in muscularis smooth muscle, increasing collagen I production and strictures. Muscle cells express αVβ3 integrin containing an RGD binding domain. The aim was to determine whether increased TGF-β1 levels in strictures was the result of latent TGF-β1, which contains an RGD sequence , binding to and activation by αVβ3; and whether cilengitide, which is an RGD-containing αVβ3 integrin inhibitor, decreases TGF-β1 activation and development of fibrosis in chronic TNBS-induced colitis
Design
Muscle cells were isolated from Crohn’s disease strictures and normal resection margin and from colon of rats after 42 d of chronic TNBS-induced colitis were used to prepare RNA, protein lysates and initiate primary cultures. The mechanisms leading to increased TGF-β1 activation, collagen I production and fibrosis were examined in human muscle and in rats. Human cultured cells in vitro and rats in vivo were treated with cilengitide to determine it efficacy to decrease TGF-β1-activation, collagen production and decrease the development of fibrosis.
Results
Latent TGF-β1 is activated by the αVβ3 RGD domain in human and rat intestinal smooth muscle. Increased activation of TGF-β1 in Crohn’s disease and in TNBS-induced colitis causes increased collagen production, and fibrosis that could be inhibited by cilengitide.
Conclusions
Cilengitide, a αVβ3 integrin RGD inhibitor, could be a novel treatment to diminish excess TGF-β1 activation, collagen I production and development of fibrosis in Crohn’s disease.
Keywords: Smad 3, RGD domain, TNBS colitis, collagen, proximity ligation assay
Introduction
Smooth muscle cells of the muscularis propria play key roles in three pathophysiologic processes occurring in the >30% of patients with Crohn’s disease who develop fibrosis and strictures. Muscle cells of strictured intestine produce excess extracellular matrix particularly collagen, undergo excess cellular hyperplasia and develop cellular hypertrophy. Expression and production of the fibrogenic cytokine, TGF-β1, is increased in smooth muscle cells of strictures compared to histologically normal adjacent resection margin.1, 2 In these patients ongoing TGF-β1-dependent extracellular matrix production of collagen IαI, the primary collagen isoform expressed in the intestine, lead to fibrosis rather than wound healing. TGF-β1, in addition, stimulates expression of other fibrogenic factors: fibronectin, CTGF and IGF-I and plays a pivotal role in Treg immune function.3, 4
All three isoforms, TGF-β1, TGF-β2, and TGF-β3, are expressed in intestinal muscle and secreted as heterotrimeric complexes derived from the same gene. The C-terminal protein sequence encodes the active 25 kDa homodimeric TGF-β protein, and the N-terminal sequence encodes the 90 kDa homodimeric latency-associated protein (LAP) and combine to form the latent form of TGF-β1, LAP-β1.5, 6 Sequestration and regulated release of active TGF-β from LAP in this complex provide a mechanism by which the biologic function of TGF-β is controlled at the cellular level.7, 8
Latent TGF-β1 can be activated by both proteolytic and non-proteolytic mechanisms. 9–11 LAP-β1 can bind to any of the αV-containing integrins: αVβ1, αVβ3, αVβ6 and αVβ8 via its Arg-Gly-Asp (RGD) binding motif. In vitro, binding of LAP-β1 to αVβ6, αVβ8 or thrombospondin has been shown to result in TGF-β1 activation.8 Although this interaction may occur, not all integrins that bind LAP-β1 activate latent TGF-β1. Activation of LAP-β1 occurs in cells expressing the appropriate integrin in a specifically relevant physiological context. Neither in vivo nor ex vivo activation of LAP-β1 by αVβ3 has been directly demonstrated, nor has its physiologic role been established.
We have already shown that αVβ3 integrin regulates IGF-I-dependent proliferation of muscle cells and contributes to excess hyperplasia in intestinal strictures in Crohn’s disease. Occupancy of αVβ3 (the cognate vitronectin receptor) by integrin ligands, e.g. vitronectin and fibronectin, stimulates smooth muscle proliferation by maximizing the intensity and duration of IGF-I-stimulated, IGF-I receptor activation and effects.2
Our current results indicate that tissue levels of active TGF-β1 and the resulting collagen production are higher in strictured intestinal muscle in Crohn’s disease than in adjacent proximal normal intestine. In a model of stricturing colitis in rats, chronic TNBS-induced colitis, cilengitide, an RGD-containing αVβ3 integrin inhibitor, by binding competitively to the same RGD-binding domain of αVβ3 integrin, decreases LAP-β1 activation, normalize levels of active TGF-β1, decreases collagen I production and inhibits the development of fibrosis over a 6-week period. This model was used because the mechanisms of chronic TNBS is similar to stricturing Crohn’s disease including increased active TGF-β1, excess collagen production and fibrosis.
In this paper we demonstrate that LAP-β1 is activated by the RGD domain of αVβ3 integrin in both human and rat intestinal smooth muscle. In strictures of patients with Crohn’s disease and in rat TNBS-induced chronic colitis, the sequence of disordered wound healing: excess LAP-β1 activation, increased levels of activated TGF-β1 in smooth muscle, excess collagen production and fibrosis are reversed by the αVβ3 RGD inhibitor, cilengitide. This model was used because the immunologic and biochemical program that develops in response to chronic TNBS is similar to that present in muscle cells of stricturing Crohn’s disease including increased active TGF-β1, excess collagen production and fibrosis. The clinical significance of these findings is the possibility that a non-toxic, RGD inhibitor can be used to diminish TGF-β1 activation, TGF-β1-dependent collagen IαI production, and the fibrosis that complicates Crohn’s disease and leads to stricture formation in these patients.
Methods
Isolation of Muscle Cells from Patients with Crohn’s disease and from Rat Colon after TNBS-induced colitis
Intestine was obtained from patients undergoing ileal/ileal-colonic resection for stricturing Crohn’s disease (Table 1). All patient specimens including in this analysis were from patients with Montreal Classification L1,B2 or L2,B2 determined by CT enterography or MR enterography. Patients undergoing resection with other phenotypes of Crohn’s disease, B1 and B3, are also reported for comparison purposes. The patients analyzed in this study expressed a stricturing phenotype without penetrating or purely inflammatory features. Fibrosis in the ileal portion of the resection specimen and normal proximal resection margin used in this study were confirmed histologically. Muscle cells were enzymatically isolated from the circular muscle layer of ileal strictures and histologically normal proximal ileal resection margin as described previously 2. Intestinal specimens were also obtained for comparison from patients undergoing surgery for reasons other than Crohn’s disease, eg normal intestine or diverticular disease.
Table 1.
Demographics of patients with Montreal B2 stricturing Crohn’s disease.
| Age (years) |
Patient No. (% of total) |
|---|---|
| under 20 | 1 (5.6) |
| 20–29 | 2 (11.1) |
| 30–39 | 4 (22.2) |
| 40–49 | 5 (27.8) |
| 50–59 | 5 (27.8) |
| over 60 | 1 (5.6) |
| Sex | |
| Male | 6 (33.3) |
| Female | 12 (67.7) |
| Race | |
| White | 11 (61.1) |
| Black or African | 6 (33.3) |
| Other/unknown | 1 (5.6) |
Chronic TNBS-induced colitis was established in Sprague-Dawley rats by weekly intra-rectal instillation of escalating doses of TNBS: 60, 60, 67.5, 67.5, 75, 75 mg/kg/week in 50% EtOH or EtOh vehicle alone.12–18 Some rats were also administered the αVβ3 integrin RGD inhibitor, cilengitide (Creative Dynamics BOC Sciences Inc, Shirley, NY) 18mg/kg/d i.p. or PBS vehicle starting with the initial TNBS treatment.19, 20 The number of rats were examined in each group was: 12-EtOH, 12-TNBS, 6-EtOH/PBS, 6-TNBS/PBS, 6-EtOH/Cilengitide, 6-TNBS/Cilengitide.
Intestinal specimens were opened longitudinally along the mesenteric border and the circular muscle layer of muscularis propria was micro-dissected from the outer longitudinal/serosal layer including myenteric plexus and the underlying submucosal tissues. Smooth muscle cells from human and rat intestine enzymatically digested from the circular muscle layer were used to prepare RNA, whole cell lysates, or placed into primary cell culture as reported and validated previously 2, 21. Epithelial cells, endothelial cells, neurons, and interstitial cells of Cajal are not detected in cells isolated in this fashion. These cells possess a smooth muscle phenotype: immunostaining for smooth muscle markers but not fibroblast markers, expression of γ-enteric actin, and the physiologic characteristics of contractile intestinal smooth muscle 2, 22. Each characteristic is retained by the muscle cells in culture 2, 22.
Ethical Considerations
Human studies were approved by the VCU Institutional Review Board; all patients provided informed consent. Animal studies were approved by the VCU Institutional Animal Care and Use Committee.
Quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was used to measure RNA transcripts of TGF-β isoforms and Collagen IαI. Primers were purchased from Applied Biosystems (Foster City, CA ) (including human TGF-β1: Hs00998133_m1, TGF-β2: Hs00234244_m1, TGF-β3: Hs01086000_m1; GAPDH: Hs03929097_g1; Rat TGF-β1: Rn00572010_m1; Collagen Iα1: Rn01462662_g1; GAPDH: Rn01462662_g1) using the 2−ΔΔCt method based on GAPDH amplification as previously reported.1, 23
In situ Hybridization Proximity Ligation Assay
Protein-protein interaction between LAP-β1 and integrin β3 was determined using in situ hybridization proximity ligation assay.24 Histologic sections or primary cultures of human smooth muscle cells were incubated with 1:100 goat anti-human LAP-β1 (R&D Systems, Minneapolis, MN) and 1:200 murine anti-human integrin β3 (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C and ligation-hybridization performed according to manufacturer’s directions (Olink Bioscience, Uppsala, Sweden). Images were obtained by indirect immunofluorescence using a Zeiss AxioImager Z1 Microscope and AxioVision 4.6.3-SP1 software (Carl Zeiss Microscopy GmBH, Jena, Germany). Images were analyzed with Duolink-II Imaging Software (Ver 1.0.1.2, Olink Bioscience, Uppsala, Sweden). Results were reported as blobs per cell minus background for 5 smooth muscle cells counted in 10 successive high power fields by a blinded observer.
Measurement of TGF-β1 and cytokines by ELISA
TGF-β1 levels were measured using isoform-specific ELISA (R&D Systems, Minneapolis, MN) in muscle cells of stricturing Crohn’s disease, normal proximal resection margin and normal intestine from patients without Crohn’s disease, and also from muscle cells of rats treated with TNBS or ethanol controls in the presence and absence of cilengitide as described previously1. Tissue active TGF-β1 (already active in the patient’s muscle cells) was measured in untreated samples. Total TGF-β1 was measured in samples treated with acid activation and represents TGF-β1 already active in the patient’s muscle cells + LAP-β1 (that was activated by the acid-treatment). Absorbance was read using a Wallac Victor2 1420 Multilabel counter (Perkin Elmer Life Sciences, Waltham, MA). Wavelength correction was calculated by subtracting readings at 570nm from the reading at 450nm to eliminate background readings in the ELISA plate. The TGF-β1 assay has no appreciable cross reactivity with TGF-β2 or TGF-β3, activins, inhibins, or BMPs. Results were calculated as ng/mg protein.
Cytokine Multi-analyte ELISArray
Colonic smooth muscle cell lysates were used for Multi-Analyte ELISArray analysis of rat cytokines (SA Biosciences, Frederick, MD) according to the manufacturer’s instructions. Absorbance was read using a Wallac Victor2 1420 Multilabel counter (Perkin Elmer Life Sciences, Waltham, MA). Wavelength correction was calculated by subtracting readings at 570nm from the reading at 450nm to eliminate background readings in the ELISA plate.
Immunoblot Analysis
Cell lysates were prepared as described previously.25–27 Collagen I, total Smad 3, phospho-Smad 3(Ser423/425) levels and β-actin were measured in lysates by immunoblot analysis using standard methods.25, 28
Masson’s Trichrome Staining and measurement of fibrosis
Fresh tissue was fixed in 4% paraformaldehyde and 10 µm cryostat sections prepared as described previously.17, 23 Collagen deposition was measured as previously described in tissue sections stained with Masson’s trichrome.1, 23, 29 Results were calculated as collagen area within the muscularis propria in three consecutive microscopic fields per section measured by a blinded reviewer and reported as percent of total muscularis propria area.
Thickness of the muscularis propria was also measured at the base of 5 villi/section using image scanning micrometry by a blinded observer.
Sircol collagen assay
Total collagen content in the muscularis propria of human intestine and rat colon was detected with Sirius red collagen detection kit (Chondrex, Inc, Redmond, WA). Muscle cells of rat colon was homogenized in T-PER buffer (Thermal Science, Amarillo, Texas), incubated on ice for 15min, and centrifuged for 5 min at 10,000 rpm at 4°C. Each protein sample was diluted in 0.5M acetic acid to a final concentration (100µg/ml). Optical density was read at 530 nm. Results were calculated based on collagen per 100µg/ml protein.
CD11b immunofluorescence
Leukocytes in the intestinal wall of human intestine and rat colon were identified as CD11b positive cells (1:1000 dilution; AbD Serotec, Raleigh, NC); smooth muscle cells were identified using an antibody against smooth muscle actin (1:100 dilution, Sigma-Aldrich, St Louis, MO) and nuclei counterstained with DAPI.
MPO assay
MPO activity was quantified in human intestine and rat colonic muscle cell homogenates using MPO assay kit (Invitrogen, Grand Island, NY). Briefly, equal protein amounts (100 µg/ml) of muscle cell lysate of rat colon were homogenized in 30 µl of T-PER buffer/mg of lysate, incubated on ice for 15min, and centrifuged for 5 min at 10,000 rpm at 4°C. Fluorescence was measured with a Wallac Victor2 1420 Multilabel counter (Perkin Elmer Life Sciences, Waltham, MA) with excitation and emission at 485 and 530 nm, respectively, for the APF assay, excitation and emission at 530 and 590 nm, respectively, for the Amplex® UltraRed assay. Results were expressed as fold change when normalized to the control sample.
Confocal microscopy
Cryosections of human intestine tissue were prepared and blocked as described elsewhere. Goat anti-human integrin ανβ3 antibody (Santa Cruz, Dallas, TX) and mouse monoclonal antibody against alpha-smooth muscle actin (Sigma-Aldrich, St. Louis, MO) were incubated at 4°C for overnight and then incubated for 2hours at room temperature in Alexa Fluor 594- and 488-conjugated secondary antibodies against the primary species antibodies (Molecular Probes). The slides were analyzed using a Leica TCS-SP2 AOBS Confocal Laser Scanning Microscope.
Statistical analysis
Values represent means ± SE of n experiments, where n represents the number of experiments on cells derived from separate subjects or animals. Statistical significance was tested by Student’s t-test for either paired or unpaired data as appropriate.
RESULTS
LAP-β1 binds to αVβ3 integrin in human intestinal smooth muscle
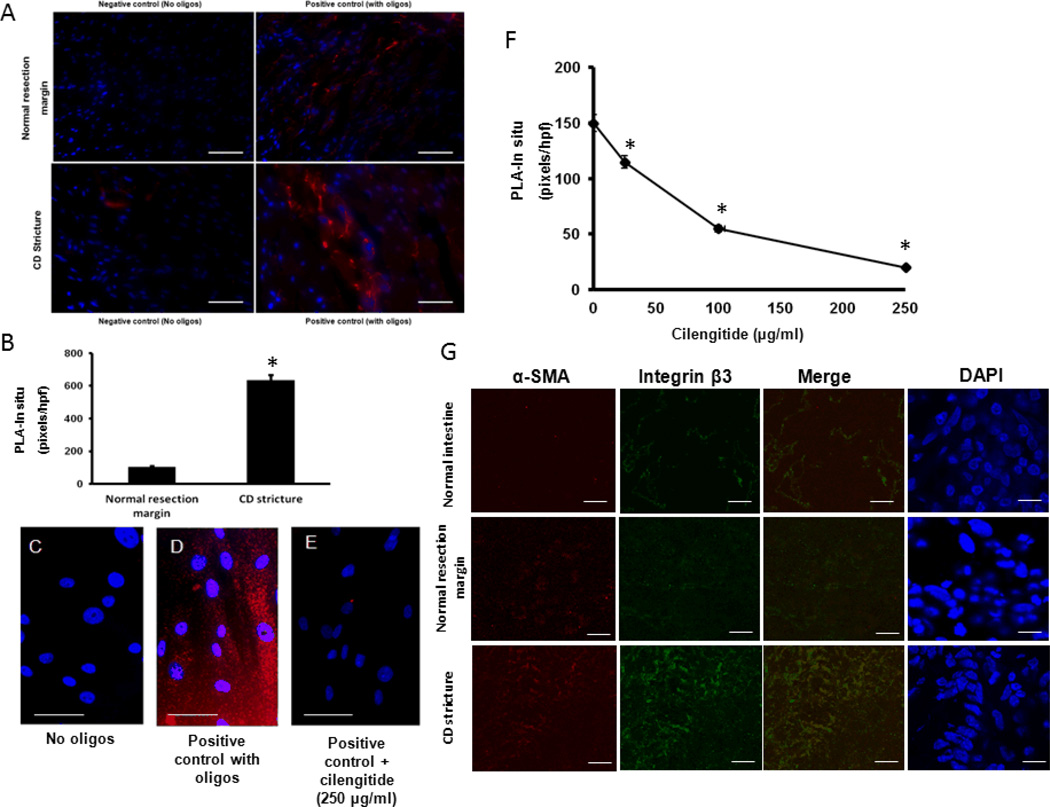
The physical association of LAP-β1 and αVβ3 integrin was demonstrated using in situ Proximity Ligation Assay (PLA). The direct interaction, within ~15 nm, between LAP-β1 and integrin β3 subunit was demonstrated in histologic sections of normal ileal resection margin and of strictured ileum in Crohn’s disease (Fig. 1A–B). In human intestine, there is 5.95±0.3 fold higher binding of LAP-β1 to αVβ3 in strictured intestine compared to normal resection margin (Fig. 1B).
Figure 1. LAP-β1 interaction with αVβ3 integrin in human intestinal muscle is inhibited by cilengitide.
A: Representative images of negative controls (without oligonucleotides) and positive controls (with oligonucleotides) from normal resection margin and strictured intestine in the same patient with Crohn’s disease, B: Quantification of fluorescence staining of LAP-β1 and integrin β3 interaction in normal resection margin and strictured intestine in patients with Crohn’s disease (CD), C-E: Representative image of LAP-β1 and integrin β3 interaction in primary cultures of human intestinal smooth muscle cells fromnegative control, positive control and cilengitide treated smooth muscle cells (250 µg/ml F: LAP-β1 and integrin β3 interaction is inhibited in a concentration-dependent fashion by cilengitide (0–250 µg/ml). ). In situ hybridization pixels in red and DAPI counterstained nuclei in blue. G: Integrin β3 and α-smooth actin co-localize in the human intestine. Data are expressed as pixels per hpf in each of 5 consecutive high power fields with similar numbers of cells. Results are mean ± SE of 6 separate patients or experiments, * denotes P < 0.05 vs untreated, scale bar = 50 µm.
The direct interaction of LAP-β1 and αVβ3 integrin in human intestinal smooth muscle was also examined in cells in primary culture (Fig. 1C–E). The interaction was inhibited in a concentration-dependent fashion by the αVβ3 inhibitor, cilengitide with EC50 66 ± 0.5 µM (Fig. 1F).
It is noteworthy that αVβ3 co-localizes with alpha smooth muscle actin on smooth muscle cells in the intestine (Fig 1G). We have previously shown that in addition to increased binding of LAP-β1 to αVβ3 integrin present on intestinal muscle cells there is a concomitant increase in the numbers of muscle cells in the muscularis propria, i.e. hyperplasia. 2
Increased TGF-β1 expression in stricturing Crohn’s disease and chronic TNBS-induced colitis
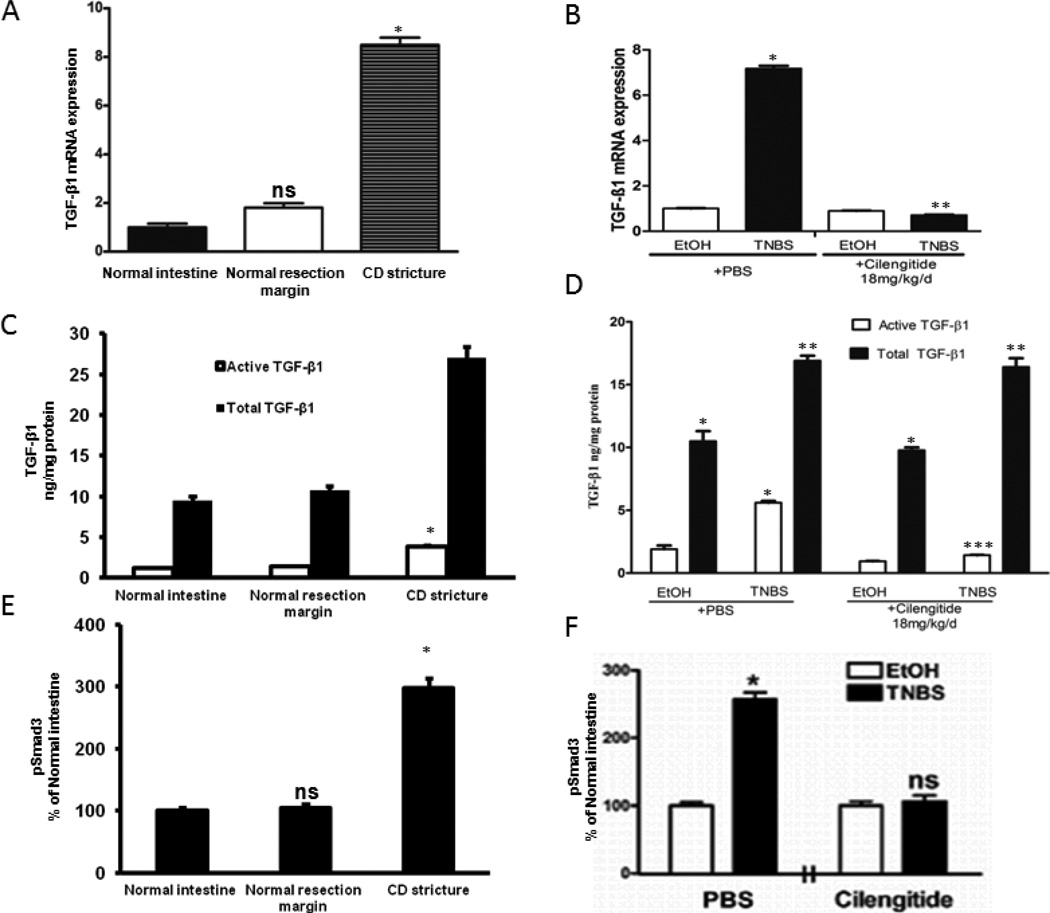
TGF-β1 expression in muscle cells of normal human intestine (patients undergoing resection for non-Crohn’s diseases) was similar to that in muscle cells of histologically normal proximal resection margin in patients undergoing ileal resection (Fig. 2A). TGF-β1 expression increased 8.2 ± 0.4 fold in muscle cells of the stricture in the same patient (Fig. 2A). By comparison, TGF-β2 transcripts decreased 0.58 ± 0.1 fold and TGF-β3 transcripts increased only 1.41 ± 0.19 fold in strictured muscle cells compared to that in normal resection margins. This study therefore focused on TGF-β1.
Figure 2. Increased TGF-β1 expression and Smad3 phosphorylation in stricturing Crohn’s disease and in chronic TNBS colitis.
A&B: Increased TGF-β1 transcripts in smooth muscle cells of human strictured intestine compared to normal margin (A) and rat colon after 42 d of chronic TNBS colitis compared to EtOH controls (B). C-F: Tissue levels of active TGF-β1 and of phosphorylated Smad3 in strictured regions of Crohn’s disease in humans (C&E) and after 42 d of chronic TNBS-induced colitis in rats (D&F). Both active TGF-β1 and pSmad3(Ser423/425) levels are αVβ3 RGD-dependent and inhibited by cilengitide. Tissue active and total TGF-β1 levels were measured by ELISA in 9 paired human samples, and in 12 EtOH and TNBS naïve rats or rats treated with PBS or Cilengitide (18 mg/kg/d i.p). * denotes P < 0.05 vs normal margin in humans or EtOH control in rats; ** denotes P < 0.05 vs total TGF-β1 in normal margin in humans, or EtOH/PBS treated rats; *** denotes P < 0.05 vs TNBS/PBS treated rats; ns denotes no significant difference vs TNBS/PBS control rats.
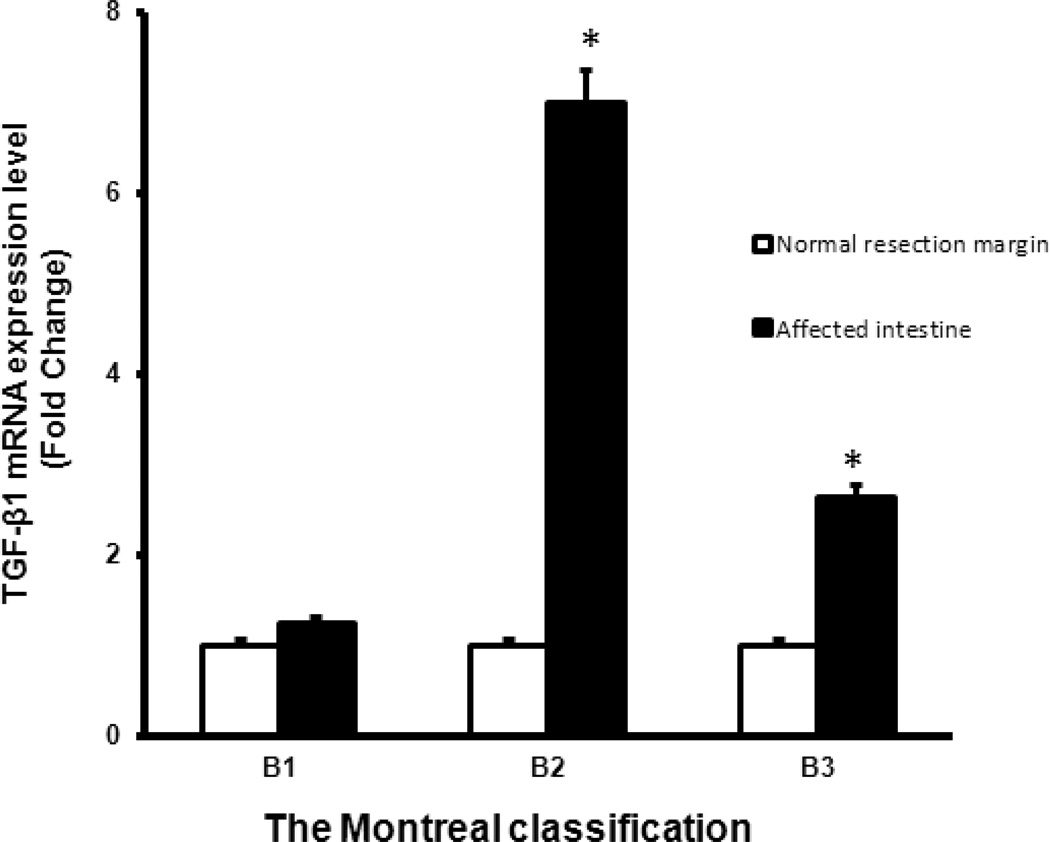
The expression of TGF-β1 in muscle cells was also measured in patients undergoing resection for Crohn’s disease with other Montreal Classification phenotypes, solely medically refractory inflammation (B1), and solely penetrating disease (B3) for comparison to the patients with stricturing disease (B2). In contrast to patients with solely stricturing Crohn’s disease, the expression of TGF-β1 was significantly increased in strictured intestine of patients with B2 phenotype Crohn’s disease but not increased in of the affected intestine in patients with a B1 phenotype Crohn’s disease compared to normal proximal resection margin in the same patient (Figure 3). A small increase in TGF-β1 transcripts was seen in the affected intestine of patients with B3 phenotype of Crohn’s disease.
Figure 3. TGF-β1 transcript levels by Montreal Classification Phenotype.
TGF-β1 transcript levels were in smooth muscle cells isolated from affected intestine were compared to the levels in the normal proximal resection margin in the same patient. TGF-β1 transcripts were increased in patients with stricturing disease (B2) and to a lesser extent penetrating disseae (B3) but not different in inflammatory disease (B1). TGF-β1 transcripts were measured by qRT-PCR. Results represent the mean ± SE. * denotes P < 0.05 vs normal resection margin in the same patient, *** denotes P < 0.001 vs normal resection margin in the same patient.
TGF-β1 expression in colonic smooth muscle cells in rats was examined after 42 d of chronic TNBS-induced colitis. TGF-β1 expression increased 7.5 ± 0.4 fold over than that in EtOH vehicle treated rats (Fig. 2B).
Active TGF-β1 levels increase in intestinal strictures of Crohn’s disease
In muscle cells isolated ex vivo from the normal resection margin active TGF-β1 was 0.25 ± 0.05 ng/mg protein, total TGF-β1 (latent + active) was 10.5 ± 0.9 ng/mg protein and the ratio of active to total was 0.024 ± 0.001. In contrast, in muscle cells from strictures active TGF-β1 increased to 2.63 ± 0.16 ng/mg protein, total TGF-β1 increased to 27.8 ± 2.6 ng/mg protein and the ratio was 0.10 ± 0.02 representing a 4.2 ± 0.2 fold increase compared to normal margin the same patient (Fig. 2C).
TGF-β1 activity was also assessed from the levels of phosphorylated Smad3(Ser432/425) which increased 175 ± 5% in ex vivo muscle cells isolated from strictured intestine compared to adjacent normal resection margin. Levels of total Smad3 were unchanged (Fig. 2E).
The levels of leukocyte invasion and inflammation were measured in the normal intestine, and in the normal proximal resection margin and strictured region in these patients from the presence of CD11b immunoreactive cells and from myeloperoxidase (MPO) activity (Fig., Supplemental Digital Content 1, http://links.lww.com/IBD/A287).
Cilengitide decreases TGF-β1 activation in chronic TNBS-induced colitis
TGF-β1 levels were measured ex vivo in isolated rat colonic smooth muscle cells after 42 days of chronic TNBS-induced colitis. In EtOH vehicle treated rats active TGF-β1 was 1.47 ± 0.46 ng/mg protein, total TGF-β1 was 8.77 ± 0.65 ng/mg protein, and the ratio was 0.14 ± 0.02. In TNBS treated rats the active TGF-β1 was 5.54 ± 0.42 ng/mg protein, total TGF-β1 was 17.3 ± 1.2 ng/mg protein, and the ratio increased to 0.32 ± 0.04 (Fig. 2D).
The ability of an αVβ3 integrin inhibitor to decrease activation of latent TGF-β1 was examined by administration of cilengitide, 18 mg/kg/d i.p or PBS vehicle, to EtOH or TNBS treated rats. In ex vivo isolated muscle cells from EtOH/PBS control animals, active TGF-β1 was 1.90 ± 0.30 ng/mg protein, total TGF-β1 was 10.5 ± 0.8 ng/mg protein, and the ratio was 0.15 ± 0.02 which was unchanged from naïve rats (Fig. 2D). In EtOH/Cilengitide treated rats, active TGF-β1 decreased to 0.94 ± 0.015 ng/mg protein, total TGF-β1 was unchanged at 9.77 ± 0.23 ng/mg protein, and the ratio decreased to 0.10 ± 0.02. In TNBS/PBS treated rats, active TGF-β1 was 5.60 ± 0.14 ng/mg protein, total TGF-β1 level was 16.9 ± 0.4 ng/mg protein, and the ratio was unchanged from TNBS treated control rats at 0.32 ± 0.05 (see above). In TNBS/cilengitide treated rats, active TGF-β1 decreased to 1.42 ± 0.03 ng/mg protein, total TGF-β1 was unaffected at 16.4 ± 0.7 ng/mg protein, and the ratio decreased to 0.09 ± 0.03 (Fig. 2D).
TGF-β1 activity was from the levels of Smad3 phosphorylation in ex vivo isolated muscle cells. In EtOH treated rats, administration of cilengitide did not affect phosphorylation of Smad3(Ser423/425) but in TNBS/PBS treated rats, Smad3(Ser423/425) phosphorylation increased 157 ± 10% over EtOH/PBS treated rats (Fig. 2F). The increase in Smad3(Ser423/425) phosphorylation was abolished in TNBS/cilengitide treated rats. Total Smad3 levels were similar in all groups.
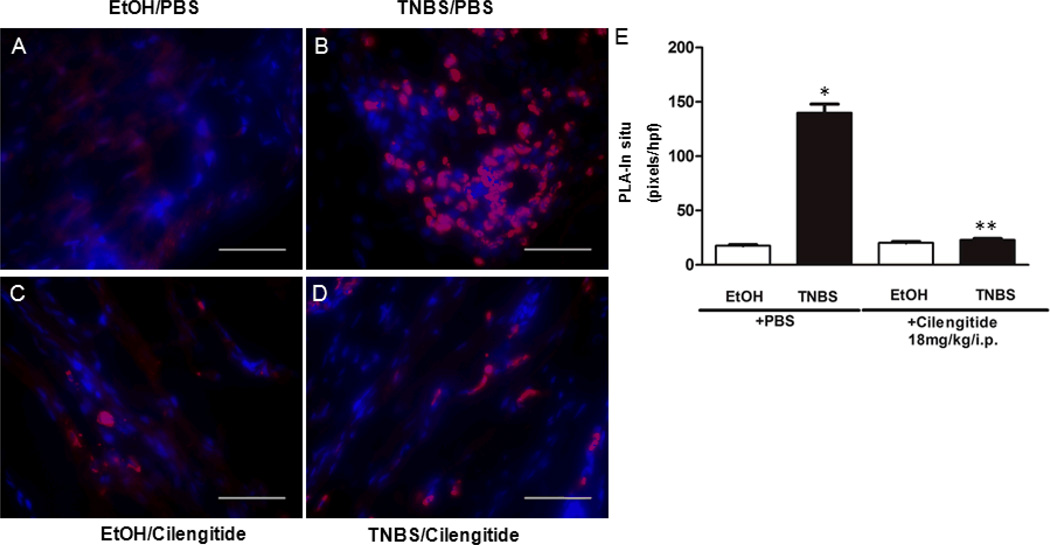
LAP-β1 binding to αVβ3 integrin increases in TNBS-induced colitis
The physical association of LAP-β1 and αVβ3 integrin in rat colon muscle cells was measured using proximity ligation assay (Fig. 3A–D). The association of LAP-β1 with integrin β3 in histologic section from EtOH/PBS treated rats was 17.5 ± 1.0 pixelss/hpf and similar to that in the EtOH/cilengitide treated rats, 20.2 ± 1.2 pixels/hpf (Fig. 3E). After 42 d of TNBS/PBS treatment the association of LAP-β1 and αVβ3 increased to 139 ± 8.0 pixels/hpf. In TNBS/cilengitide treated rats association decreased to 22.9 ± 1.3 pixels/hpf.
Cilengitide decreases excess collagen IαI production in TNBS-induced colitis
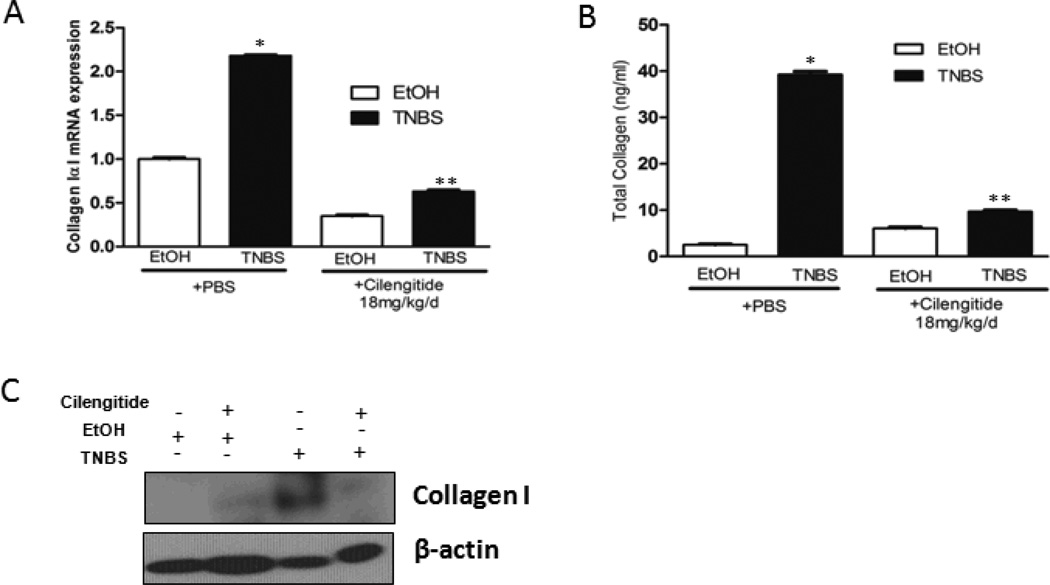
Excess collagen I production in intestinal muscle cells of Crohn’s strictures compared to normal margin is TGF-β1-dependent, and Smad3-dependent 1. Since TGF-β1 activation by αVβ3 is RGD-dependent, collagen IαI expression was measured ex vivo in muscle cells isolated from rats treated with Cilengitide (18 mg/kg/d i.p) or PBS vehicle. Collagen IαI expression increased 2.2 ± 0.1 fold in TNBS/PBS treated rats compared to EtOH/PBS treated rats (Fig. 4A). Collagen IαI expression in EtOH/Cilengitide treated rats decreased 0.65 ± 0.02 fold compared to EtOH/PBS treatment. In contrast to TNBS/PBS treatment, collagen IαI expression in TNBS/cilengitide treated rats increased only 0.35 ± 0.04 above EtOH/Cilengitide treated rats (Fig. 4A).
Figure 4. Cilengitide treatment inhibits the interaction of LAP-β1 and αVβ3 integrin in chronic TNBS-induced colitis.
Representative histologic sections showing muscularis propria from EtOH/PBS (A),TNBS/PBS (B), EtOH/cilengitide (C), and TNBS/cilengitide (D) treated rats after 42 d of colitis. The direct interaction of LAP-β1 and the integrin β3 subunit was demonstrated in histologic sections of rat colon intestine where their interaction was inhibited by the cilengitide (18mg/kg/d i.p.). E: Quantitative analysis of PLA-in situ results expressed as pixels/hpf. * denotes P < 0.05 vs EtoH control in rats; ** denotes P < 0.05 vs TNBS/PBS treated rats. Scale bar = 50 µm.
An identical pattern was measured for collagen protein in ex vivo isolated muscle cells. After 42 d of EtOH/PBS treatment collagen protein measured by Sircol assay increased from 2.5 ± 0.13 ng/ml to 38 ± 1.9 ng/ml in TNBS/PBS treated rats (Fig. 4B). In EtOH/Cilengitide treated rats collagen protein was 4.5 ± 0.23 ng/ml and increased to only 9.5 ± 0.48 ng/ml in TNBS/cilengitide treated rats (Fig. 4B). Similar results were obtained when collagen I protein was measured by immunoblot analysis (Fig. 4C).
Cilengitide diminishes development of fibrosis in TNBS-induced colitis
The ability of cilengitide to reduce the development of fibrosis in chronic TNBS-induced colitis was examined in two ways: measurement of collagen deposition as a percent of total muscularis propria volume, and measurement of muscularis propria thickness. Histologic sections from rats in all four groups after 42 d of TNBS colitis were used for analysis of fibrosis (Fig. 5A)
Figure 5. Increased collagen IαI expression and collagen I production in TNBS-induced colitis is inhibited by cilengitide.
The increase in collagen IαI after 42 days of TNBS-induced colitis in rats treated with daily PBS vehicle is significantly diminished in rats treated with daily a selective αVβ3 RGD inhibitor, cilengitide (18mg/kg/d i.p.). A: Collagen IαI transcripts were measured in isolated smooth muscle cells by qRT-PCR from EtOH or TNBS treated rats receiving daily PBS or cilengitide. Results are expressed as mean ± SE for 12 animals in each group. B: Collagen I protein levels were measured by Sircol assay and C: Collagen protein measured by immunoblot analysis. * denotes P < 0.05 vs EtOH/PBS treated rats. ** denotes P < 0.05 vs TNBS/PBS treated rats.
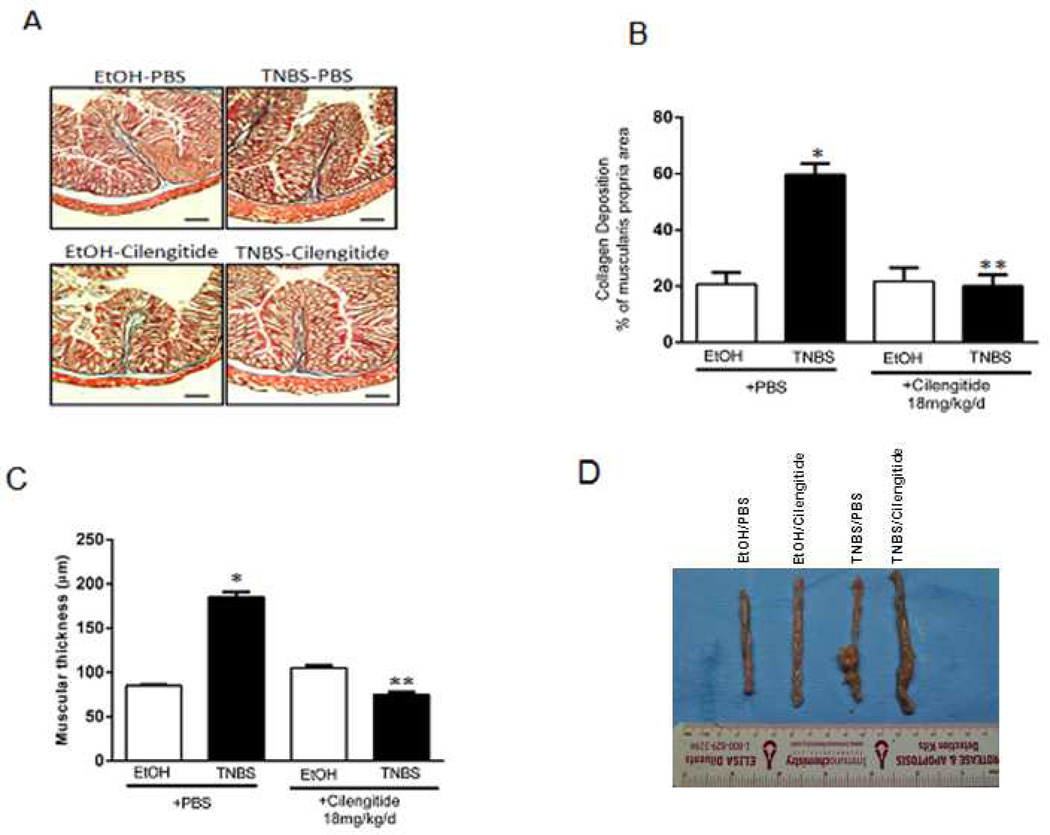
In EtOH/PBS and EtOH/Cilengtide treated rats collagen deposition was similar: 20 ± 2.5% and 21 ± 2.2%, respectively, of muscularis propria volume which increased to 60 ± 3% in TNBS/PBS treated rats. Collagen deposition after 42 d in the TNBS/cilengitide group was 18 ± 2% of muscularis propria volume and not different than control (Fig. 5B).
Similarly, muscularis propria thickness in EtOH/PBS and EtOH/cilengitide treated rats were similar, 90 ± 4 µm and 100 ± 5, respectively. In the TNBS/PBS group muscularis propria thickness increased to 185 ± 5 µm after 42 d. The increase was inhibited in TNBS/cilengitide treated rats with a muscularis propria thickness of: 80 ± 4 µm (Fig. 5C and D).
Effect of Cilengitide on leukocyte invasion, inflammation and immunologic responses
The possibility that the effects of cilengitide on development of fibrosis were due to inhibition of leukocyte attraction, inflammation or altered cytokine activation was specifically investigated.
The effect of cilengitide on leukocyte attraction were measured by immunofluorescent staining of CD11b which is expressed on monocytes, neutrophils, natural killer cells, granulocytes and macrophages, T-cells and B-cells. After 42 d of TNBS-induced colitis, levels of CD11b immunofluorescence measured in histologic section prepared from the EtOH/PBS group were 450 ± 23 pixels/hpf and 550 ± 28 pixels/cells in TNBS/PBS treated rats. Levels of CD11b immunofluorescence were similar in EtOH/cilengitide (585 ± 30 pixels/hpf) and TNBS/cilengitide (500 ± 25 pixels/hpf) treated rats (Fig. A, Supplemental Digital Content 2, http://links.lww.com/IBD/A288).
The effect of cilengitide on inflammation was measured by MPO activity in ex vivo isolated muscle cells. After 42 d of TNBS-induce colitis, levels of MPO measured in the EtOH/PBS group of rats was 3.10 ± 0.15 ng/µg protein and increased to 6.06 ± 0.30 ng/µg protein in TNBS/PBS treated rats. MPO activity did not increase in either EtOH/cilengitide (4.41 ± 0.22 ng/µg protein) or TNBS/cilengitide (4.49 ± 0.23 ng/µg protein) treated rats (Fig. B, Supplemental Digital Content 2, http://links.lww.com/IBD/A288).
The effect of cilengitide on cytokine activation was also examined in ex vivo isolated muscle cells by measurement of Th1, Th2, Th17 and IL-1 related cytokines (Fig. C–E, Supplemental Digital Content 2, http://links.lww.com/IBD/A288). After 42 d of TNBS-induced colitis levels of most Th1, Th2 and Th17 cytokines were similar between the EtOH/PBS and TNBS/PBS treated groups of rats (Fig. C–E, Supplemental Digital Content 2, http://links.lww.com/IBD/A288). The levels of Il-1α and IL-1β were increased in the TNBS/PBS treated group compared to the EtOH/PBS treated group (Fig. F, Supplemental Digital Content 2, http://links.lww.com/IBD/A288). After 42 d of treatment with cilengitide the levels of many cytokines were not altered, however, in the TNBS/cilengitide treated group levels of IFN-γ, TNF-α, IL-6 and IL-10 as well as IL-1α and IL-1β were significantly lower than controls (Fig. C–F, Supplemental Digital Content 2, http://links.lww.com/IBD/A288).
Discussion
Excess extracellular matrix production is central to the disordered wound healing process leading to fibrosis in many organs, including the intestine. In liver cirrhosis, pulmonary fibrosis, or systemic sclerosis the whole organ can be affected by fibrosis but is a segmental process as in Crohn’s disease implying that the process is fundamentally different. In addition, only ~30–50% of patients with CD develop stricturing disease. Additional factors are involved that are uniquely local or patient specific in nature including the 163 disease susceptibility and modifying gene polymorphisms which include Smad3 polymorphisms.30
Levels of TGF-β gene expression are increased in strictured intestine muscle in Crohn’s disease and in the rat model of Crohn’s disease used in this study. Similar events are also present in the submucosal myofibroblasts of Crohn’s strictures.31 This paper shows that increased levels of LAP-β1 in strictures results in increased levels of active TGF-β1 compared to normal resection margin in the same patient. Binding and activation is blocked by the RGD-domain specific inhibitor, Cilengitide. Cilengitide is selective for αVβ3 integrin with IC50 of 3 nM with lower affinity for αVβ5 with IC 50 of 37 nM. However, β5 integrin is nearly 2-fold less abundant than β5 in strictured intestine than in normal resection margin in the same patient (data not shown).
It is noteworthy that this analysis of human subjects used paired patient samples: strictured ileal intestine and normal proximal ileal resection margin. In each paired sample examined TGF-β1 expression, tissue TGF-β1 levels, collagen IαI abundance and Smad3 activation were greater in muscle cells of strictured intestine than in adjacent normal proximal margin. These changes occurred without regard to medications used, smoking status or years of disease. However, since smooth muscle cell hyperplasia and excess extracellular matrix production per mg protein occur concomitantly with increased levels of active TGF-β1 the tissue levels of active TGF-β1 would be even higher on a per cell basis and therefore could be expected to result in greater collagen production and fibrosis.2, 17, 23
This mechanism has important therapeutic implications for stricturing Crohn’s disease. 32 Intestinal smooth muscle cells express TGF-βRI/II receptors linked to canonical Smad2/3 signaling.33 Binding of active TGF-β1 to TGF-βRI/II receptors in human intestinal muscle activates canonical Smad signaling, r-Smad2/3 phosphorylation and increased collagen IαI expression which accounts for ~70% of collagen in the intestine.3, 34 With the exception of Smad3 deletion, deletion of TGF-β protein, its receptors or other Smad proteins are generally lethal mutations or a feature of neoplastic cells. In two of the three Smad3 deletion mutants, a lethal wasting syndrome develops early in association with immunodeficiency.35, 36 In the third Smad3 mutant, while the effects of TNBS-induced colitis were reduced, metastatic adenocarcinoma of the colon developed within 4–6 months of age.37 Overall these observations suggest that pharmacologic targeting of the TGF-β system in Crohn’s disease patients to decrease the development of fibrosis could have untoward effects. In the present study, we have explored an alternative approach utilizing cilengitide to decrease TGF-β1 activation and thereby decrease collagen production and fibrosis. Cilengitide also possesses anti-angiogenic effects which have also been implicated in the pathogenesis of Crohn’s disease and could have combined beneficial effects.38
The findings of low MPO levels, minimal CD11b positive leukocyte recruitment, and similar levels of Th1, Th2 and Th17 cytokines indicate that the TNBS model used in these studies is a model of chronic inflammation and fibrosis and not acute inflammation. This chronic TNBS model recapitulates the process of chronic stricture formation and progression in Crohn’s disease where it is increasingly clear that once fibrosis is initiated in the susceptible patient, it is self-perpetuating and can proceed absent active inflammation. The effects of cilengitide not only diminish fibrosis but also inflammation and alter cytokine activation (IFN-γ, TNF-α, IL-6 , IL-10, IL-1α and IL-1β, and increase the levels of GM-CSF) suggest that αVβ3 integrin plays a role in both processes.39 It is worth noting that while cilengitide suppression of TGF-β1 activation effectively diminishes fibrosis it could result in activation of pro-inflammatory cytokines due to loss of TGF-β1’s Treg function.
Integrins expressed on smooth muscle cells are key elements of mechano-transduction pathways that communicate with and are regulated by the focal adhesion complex that includes FAK, c-Src, and paxillin, and proteins mediating cytoskeletal remodeling.40 In cultured rat lung myofibroblasts, contractile agonists increased the release of active TGF-β1 from LAP-β1 via an αVβ5 integrin-dependent mechanism, whereas, treatment of these cells with an inhibitor of actin polymerization, such as cytochalasin D, reduced agonist-induced TGF-β1 release. Altered smooth muscle contractility leading to diarrhea is a common symptom in Crohn’s disease.41 When coupled with altered compliance of the fibrotic intestine these represent two key components that could increase mechanical tension and lead to increased non-proteolytic cleavage of LAP-β1, increased active TGF-β1 in muscle tissue leading to excess collagen IαI production and fibrosis. When examined in the rat liver, cilengitide was anti-fibrotic when examined in vitro with cultured hepatic stellate cells but when examined in vivo in rat bile duct ligation model of cirrhosis fibrosis worsened.42 The differing results between liver and intestine may reflect differences between non-motile liver and contractile intestine or the coupling of αVβ3 (Ser752) to c-Src and the actin cytoskeleton in muscle cells that actively contract and relax. The effects of RGD mimetic peptides, like cilengitide, can be pleiotropic with inhibition of αVβ3 integrin function at the high concentrations, as used in this study, but activation of αVβ3 integrin at lower concentrations and thereby account for different effects in other studies.43
In summary, we have shown that active TGF-β1 is generated by the binding of LAP-β1 directly to αVβ3 integrin. Binding of LAP-β1 and activation of TGF-β1 could be inhibited by the selective αVβ3 RGD inhibitor, cilengitide. Increased active TGF-β1 levels are higher in strictured intestinal muscle than in muscle from normal margin in Crohn’s disease and in a rat model of Crohn’s disease where they regulate excess collagen IαI expression and cause fibrosis. These findings have clinical relevance in patients expressing a B2 phenotype of fibrosis who characteristically have increased TGF-β1 expression at regions of stricture formation compared to patients with other Crohn’s disease phenotypes. Our results point to a novel effective therapy directed toward limiting the development of fibrosis and stricture formation in susceptible patients with Crohn’s disease.
Supplementary Material
Figure 6. Development of ibrosis in TNBS-induced colitis is inhibited by cilengitide.
A: Representative Masson’s trichrome stained colon sections from rats after 42 d of intra-rectal EtOH or TNBS in the presence of 18 mg/kg/d i.p. cilengitide or PBS. Scale bar = 200 µm. B: Collagen deposition after 42 days of TNBS-induced colitis in rats treated with daily PBS vehicle is diminished in rats treated with daily 18 mg/kg/d i.p. cilengitide. Collagen deposition was measured by Red-Green-Blue (RGB) segmentation using fixed thresholding values and calculated as collagen area within the muscularis propria in each of three consecutive microscopic fields per section in each of 12 animals per group measured by a blinded reviewer and reported as percent of the total muscularis propria area. Panel C: Increase muscularis propria thickness after 42 days of TNBS-induced colitis in rats treated with daily PBS vehicle is diminished by 18 mg/kg/d i.p. cilengitide. The thickness of the muscularis propria was measured by image scanning micrometry at the base of 5 villi per section. Results were calculated as mean ± SE of the muscularis propria thickness in each of three consecutive sections in each of 12 animals per group measured by a blinded reviewer and reported in µm. Panel D: Macroscopic presentation of colon harvested from rats in these studies after 42 d. From left to right: EtOH/PBS; EtOH/cilengitide 18 mg/kg/d i.p; TNBS/PBS; TNBS/cilengitide, 18 mg/kg/d i.p.* denotes P < 0.05 vs EtOH/PBS treated rats. ** denotes P < 0.05 vs TNBS/PBS treated rats.
ACKNOWLEDGEMENTS
Supported by Grant DK49691 from NIH: National Institutes for Diabetes, Digestive and Kidney Diseases (JFK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributionship: CL: acquisition and analysis of data
RSF: acquisition and analysis of data
JRG: experimental design
KSM: experimental design
JMK: experimental design
HA: experimental design
JFK: experimental design and manuscript writing
Competing interests: No author has any competing interest to disclose
REFERENCES
- 1.Flynn RS, Mahavadi S, Murthy KS, et al. Endogenous IGFBP-3 regulates excess collagen expression in intestinal smooth muscle cells of Crohn's disease strictures. Inflammatory Bowel Diseases. 2011;17:193–201. doi: 10.1002/ibd.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flynn RS, Murthy KS, Grider JR, et al. Endogenous IGF-I and [alpha]V[beta]3 Integrin Ligands Regulate Increased Smooth Muscle Hyperplasia in Stricturing Crohn's Disease. Gastroenterology. 2010;138:285–293. doi: 10.1053/j.gastro.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham MF, Diegelmann RF, Elson CO, et al. Collagen content and types in the intestinal strictures of Crohn's disease. Gastroenterology. 1988;94:257–265. doi: 10.1016/0016-5085(88)90411-8. [DOI] [PubMed] [Google Scholar]
- 4.John PB, Marc F, Karen D, et al. Transcriptomic analysis of intestinal fibrosis-associated gene expression in response to medical therapy in Crohn's disease. Inflammatory Bowel Diseases. 2008;14:1197–1204. doi: 10.1002/ibd.20482. [DOI] [PubMed] [Google Scholar]
- 5.Munger JS, Harpel JG, Gleizes PE, et al. Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney international. 1997;51:1376–1382. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 6.Sinha S, Nevett C, Shuttleworth CA, et al. Cellular and extracellular biology of the latent transforming growth factor-beta binding proteins. Matrix biology : journal of the International Society for Matrix Biology. 1998;17:529–545. doi: 10.1016/s0945-053x(98)90106-8. [DOI] [PubMed] [Google Scholar]
- 7.Munger JS, Harpel JG, Giancotti FG, et al. Interactions between Growth Factors and Integrins: Latent Forms of Transforming Growth Factor-beta Are Ligands for the Integrin alpha vbeta 1. Mol. Biol. Cell. 1998;9:2627–2638. doi: 10.1091/mbc.9.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wipff P-J, Hinz B. Integrins and the activation of latent transforming growth factor [beta]1 - An intimate relationship. European Journal of Cell Biology. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Mu D, Cambier S, Fjellbirkeland L, et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. The Journal of Cell Biology. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munger JS, Huang X, Kawakatsu H, et al. A Mechanism for Regulating Pulmonary Inflammation and Fibrosis: The Integrin [alpha]v[beta]6 Binds and Activates Latent TGF [beta]1. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 11.Wipff P-J, Rifkin DB, Meister J-J, et al. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. The Journal of Cell Biology. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosseini JM, Goldhill JM, Bossone C, et al. Progressive alterations in circular smooth muscle contractility in TNBS-induced colitis in rats. Neurogastroenterol Motil. 1999;11:347–356. doi: 10.1046/j.1365-2982.1999.00165.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamada Y, Marshall S, Specian RD, et al. A comparative analysis of two models of colitis in rats. Gastroenterology. 1992;102:1524–1534. doi: 10.1016/0016-5085(92)91710-l. [DOI] [PubMed] [Google Scholar]
- 14.Zeeh JM, Mohapatra N, Lund PK, et al. Differential expression and localization of IGF-I and IGF binding proteins in inflamed rat colon. J Recept Signal Transduct Res. 1998;18:265–280. doi: 10.3109/10799899809047747. [DOI] [PubMed] [Google Scholar]
- 15.Zeeh JM, Riley NE, Hoffmann P, et al. Expression of insulin-like growth factor binding proteins and collagen in experimental colitis in rats. Eur J Gastroenterol Hepatol. 2001;13:851–858. doi: 10.1097/00042737-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Qiao LY, Grider JR. Up-regulation of calcitonin gene-related peptide and receptor tyrosine kinase TrkB in rat bladder afferent neurons following TNBS colitis. Exp Neurol. 2007;204:667–679. doi: 10.1016/j.expneurol.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazelgrove KB, Flynn RS, Qiao LY, et al. Endogenous IGF-I and alpha v beta3 integrin ligands regulate increased smooth muscle growth in TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1230–G1237. doi: 10.1152/ajpgi.90508.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuemmerle JF. Synergistic regulation of NOS II expression by IL-1 beta and TNF-alpha in cultured rat colonic smooth muscle cells. Am J Physiol. 1998;274:G178–G185. doi: 10.1152/ajpgi.1998.274.1.G178. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee S, Matsumura A, Schradermeier J, et al. Human Malignant Glioma Therapy Using Anti-αVβ3 Integrin Agents. Journal of Neuro-Oncology. 2000;46:135–144. doi: 10.1023/a:1006444300504. [DOI] [PubMed] [Google Scholar]
- 20.Shimamura N, Matchett G, Yatsushige H, et al. Inhibition of Integrin {alpha}v{beta}3 Ameliorates Focal Cerebral Ischemic Damage in the Rat Middle Cerebral Artery Occlusion Model. Stroke. 2006;37:1902–1909. doi: 10.1161/01.STR.0000226991.27540.f2. [DOI] [PubMed] [Google Scholar]
- 21.Kuemmerle JF. Autocrine regulation of growth in cultured human intestinal muscle by growth factors. Gastroenterology. 1997;113:817–824. doi: 10.1016/s0016-5085(97)70176-8. [DOI] [PubMed] [Google Scholar]
- 22.Teng B, Murthy KS, Kuemmerle JF, et al. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol. 1998;275:G342–G351. doi: 10.1152/ajpgi.1998.275.2.G342. [DOI] [PubMed] [Google Scholar]
- 23.Mahavadi S, Flynn RS, Grider JR, et al. Amelioration of excess collagen IαI, fibrosis, and smooth muscle growth in TNBS-induced colitis in IGF-I(+/−) mice. Inflammatory Bowel Diseases. 2011;17:711–719. doi: 10.1002/ibd.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fredriksson S, Gullberg M, Jarvius J, et al. Protein detection using proximity-dependent DNA ligation assays. Nat Biotech. 2002;20:473–477. doi: 10.1038/nbt0502-473. [DOI] [PubMed] [Google Scholar]
- 25.Kuemmerle JF. Occupation of alphavbeta3-integrin by endogenous ligands modulates IGF-I receptor activation and proliferation of human intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1194–G1202. doi: 10.1152/ajpgi.00345.2005. [DOI] [PubMed] [Google Scholar]
- 26.Kuemmerle JF, Murthy KS. Coupling of the insulin-like growth factor-I receptor tyrosine kinase to Gi2 in human intestinal smooth muscle: Gbetagamma -dependent mitogen-activated protein kinase activation and growth. J Biol Chem. 2001;276:7187–7194. doi: 10.1074/jbc.M011145200. [DOI] [PubMed] [Google Scholar]
- 27.Kuemmerle JF, Zhou H. Insulin-like growth factor-binding protein-5 (IGFBP-5) stimulates growth and IGF-I secretion in human intestinal smooth muscle by Ras-dependent activation of p38 MAP kinase and Erk1/2 pathways. J Biol Chem. 2002;277:20563–20571. doi: 10.1074/jbc.M200885200. [DOI] [PubMed] [Google Scholar]
- 28.Kuemmerle JF. IGF-I elicits growth of human intestinal smooth muscle cells by activation of PI3K, PDK-1, and p70S6 kinase. Am J Physiol Gastrointest Liver Physiol. 2003;284:G411–G422. doi: 10.1152/ajpgi.00310.2002. [DOI] [PubMed] [Google Scholar]
- 29.Ortolan EVP, Spadella CT, Caramori C, et al. Microscopic, Morphometric and Ultrastructural Analysis of Anastomotic Healing in the Intestine of Normal and Diabetic Rats. Exp Clin Endocrinol Diabetes. 2008;116:198–202. doi: 10.1055/s-2007-993147. [DOI] [PubMed] [Google Scholar]
- 30.Lee JC, Parkes M. Genome-wide association studies and Crohn’s disease. Briefings in Functional Genomics. 2011;10:71–76. doi: 10.1093/bfgp/elr009. [DOI] [PubMed] [Google Scholar]
- 31.Di Sabatino A, Jackson CL, Pickard KM, et al. Transforming growth factor {beta} signalling and matrix metalloproteinases in the mucosa overlying Crohn's disease strictures. Gut. 2009;58:777–789. doi: 10.1136/gut.2008.149096. [DOI] [PubMed] [Google Scholar]
- 32.Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflammatory Bowel Diseases. 2000;6:8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Kuemmerle JF, Murthy KS, Bowers JG. IGFBP-3 activates TGF-beta receptors and directly inhibits growth in human intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G795–G802. doi: 10.1152/ajpgi.00009.2004. [DOI] [PubMed] [Google Scholar]
- 34.Flynn RS, Mahavadi S, Murthy KS, et al. Endogenous IGFBP-3 Regulates Excess Collagen Expression in Intestinal Smooth Muscle Cells of Crohn's Disease Strictures. Inflammatory Bowel Diseases. 2010 doi: 10.1002/ibd.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datto MB, Frederick JP, Pan L, et al. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X, Letterio JJ, Lechleider RJ, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latella G, Vetuschi A, Sferra R, et al. Smad3 loss confers resistance to the development of trinitrobenzene sulfonic acid<i>–induced</i>colorectal fibrosis. European Journal of Clinical Investigation. 2009;39:145–156. doi: 10.1111/j.1365-2362.2008.02076.x. [DOI] [PubMed] [Google Scholar]
- 38.Danese S, Sans M, de la Motte C, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein JI, Kominsky DJ, Jacobson N, et al. Defective Leukocyte GM-CSF Receptor (CD116) Expression and Function in Inflammatory Bowel Disease. Gastroenterology. 2011;141:208–216. doi: 10.1053/j.gastro.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerthoffer WT, Gunst SJ. Invited Review: Focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. Journal of Applied Physiology. 2001;91:963–972. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- 41.Akiho H, Ihara E, Motomura Y, et al. Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders. World J Gastrointest Pathophysiol. 2011;2:72–81. doi: 10.4291/wjgp.v2.i5.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patsenker E, Popov Y, Stickel F, et al. Pharmacological inhibition of integrin αvβ3 aggravates experimental liver fibrosis and suppresses hepatic angiogenesis. Hepatology. 2009;50:1501–1511. doi: 10.1002/hep.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Legler DF, Wiedle G, Ross FP, et al. Superactivation of integrin (α)v(β)3 by low antagonist concentrations. Journal of Cell Science. 2001;114:1545–1553. doi: 10.1242/jcs.114.8.1545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.