Abstract
Objective
Whether or not cisplatin and cetuximab are similarly effective in improving outcomes when added to radiation therapy(RT) in HNSCC is unknown.
Methods
Retrospective analysis of patients treated with definitive RT and cisplatin(n=18) or cetuximab(n=29).
Results
T and N classifications, stage, HPV status, and smoking history were balanced in the two groups; however, patients in the cisplatin group were younger, and had better performance status. Delivery of RT was similar between the two groups. Median follow-up was 23(4–64) months. Disease-specific(DSS) survival at 3 years was 83% in the cisplatin group and 31% in the cetuximab group. Recurrent disease was more common in the cetuximab group compared with the cisplatin group(17 versus 4 patients). Propensity score analysis to adjust for differences in patient characteristics which influenced treatment selection showed that DSS was indeed longer with cisplatin than with cetuximab(DSS Hazard Ratio[HR] 0.15{Confidence Interval[CI]0.033,0.66},p=0.012).
Conclusions
DSS was superior in the patients given cisplatin with definitive RT compared to cetuximab with definitive RT due to a lower risk of recurrent disease in the cisplatin group. These observations could not be explained by differences between the two groups in the patient and tumor characteristics or in treatment delivery.
Keywords: Radiation, Cetuximab, Cisplatin, Head and Neck, Neoplasms
Introduction
Squamous cell carcinoma of the head and neck(HNSCC) is a common malignancy, with more than 500,000 newly diagnosed cases worldwide in 2002 [1]. A meta-analysis of 93 randomized trials showed that chemotherapy given concurrently with radiation therapy(RT) improved overall survival(OS) over RT alone in patients with locally advanced HNSCC [2]. The meta-analysis demonstrated that platinum-based therapy and monotherapy were the most efficacious treatment options when combined concurrently with radiation. Cisplatin is commonly considered to be the gold standard that other regimens are compared to; however, the acute toxicity of cisplatin and RT is significant, and limits its application to younger patients with minimal co-morbidities.
More recently, cetuximab given concurrently with RT was also shown to improve OS over RT alone in patients with locally advanced HNSCC [3, 4]. Whether or not cisplatin and cetuximab are similarly effective in improving outcomes when added to RT is unknown as there are no published controlled randomized trials to guide decision-making. Herein, we report a single institution retrospective analysis of 47 patients with locally advanced HNSCC treated with definitive RT and either concurrent cisplatin or cetuximab.
Materials and Methods
Study Design and Patient Selection
This was an Institutional Review Board(IRB)–approved retrospective analysis of all patients with locally advanced HNSCC treated with definitive RT concurrently with either cisplatin or cetuximab at a single institution between 2005 and 2010. Patients were identified from the IRB-approved HNSCC registry protocol initiated in 2005. Eligibility criteria for this retrospective analysis included: Stage III, IVa or IVb HNSCC that was treated with definitive RT concurrently with either scheduled cisplatin(100 mg/m2 on days 1, 22, and 43 of RT) or cetuximab(400 mg/m2 loading dose before RT, then 250 mg/m2 per week during RT for 7 doses). HNSCC subsites included oral cavity, oropharynx, larynx, hypopharynx, and unknown primary with a level II and/or III neck mass. Intensity modulated radiation therapy(IMRT) was administered once daily, five days weekly, using either a Varian Linear Accelerator(Varian Medical Systems, Inc) or a Tomotherapy Hi-ART System®(Tomotherapy, Inc). The total dose of RT to gross disease was 6600–7000 cGy in 33–35 fractions of 200 cGy each over 7 weeks in all but 1 patient(unknown primary site received 30 fractions). Additional areas of intermediate risk received 6300 cGy and regions in the ipsilateral and contralateral neck at risk for microscopic disease received 5600 cGy.
Exclusion criteria included primary surgical resection, induction chemotherapy or alternative chemotherapy given with RT. Forty-seven patients met the entry criteria for the retrospective analysis and are the subject of this report: 18 received cisplatin and 29 received cetuximab concurrently with RT.
Standard Assessments
The institution employed a standardized initial evaluation, treatment, supportive care, and long-term surveillance approach for these patients. The initial evaluation included a multi-disciplinary team and tumor board assessment involving otolaryngology, radiation oncology, medical oncology, and pathology. The primary tumor site was identified and biopsied and the cancer was staged by experienced oncologic otolaryngologists using clinical (fiber optic endoscopy [FOE] and/or laryngoscopy) and radiographic (CT, MRI and/or FDG-PET/CT) methods.
In general, cisplatin was given concurrent with definitive RT in those patients with a favorable ECOG performance status (0–1), age <70 years, and with low co-morbidity burden and cetuximab was given with definitive RT in patients with an unfavorable ECOG performance status (2–3), age >70 years, and/or with high co-morbidity burden. Due to the presence of the latter patient characteristics in the cetuximab group, once daily fractionation of radiation was chosen because of concerns for excessive toxicity with altered fractionation of radiation.
During definitive RT with concurrent cisplatin or cetuximab, adverse events(AE’s) were monitored. Following completion of definitive therapy, patients underwent an office-based FOE and neck exam, and CT of the neck at 6–8 weeks followed by a clinical exam and FDG-PET/CT at 10–16 weeks. Subsequently, patients underwent office exams every 1–3 months for 3 years along with CT of the neck and chest every 6 months for 3 years. After 3 years, exams occurred 1–2 times per year for at least 2 additional years.
Data Captured
Baseline clinical and pathologic data collected included age, gender, race, smoking history, ECOG performance status, ACE-27 co-morbidity index [5], insurance(Medicare, Medicaid, Private, Other/none), primary site, TNM classification [6], and HPV-relationship(based on p16 by immunohistochemistry[IHC] and/or non-keratinizing squamous cell carcinoma on histology, both surrogate markers for HPV) [7]. Treatment data collected included variables related to radiation(total dose, number of fractions, dose per fraction, elapsed days), cisplatin(total dose, number of doses, mg per dose), and cetuximab(total dose, number of doses, mg per dose). Selected AE’s captured included incidence and grade of mucositis, incidence and grade of acneiform rash, weight change from start to end of RT, and requirement for and duration of PEG tube. AE’s were graded using NCI-CTC Version 3.0.
Other data collected included overall survival([OS] interval from diagnosis to either death or last follow-up alive) and disease-specific survival([DSS] time from initiation of CRT to death due to disease). Causes of death were determined including primary cancer, secondary cancer, treatment-related mortality(TRM), inter-current illness, and other/unknown.
Statistical Plan
Patients were stratified by chemotherapy regimen(cisplatin or cetuximab) given with definitive RT. Survival outcomes were estimated using the Cox proportional hazards methods, adjusted using a propensity score for sex, race, age, ECOG performance score, primary site, overall stage, HPV status, type of insurance, smoking status and comorbidity score. Baseline clinical and pathologic data were tabulated for each chemotherapy group and compared using Fisher’s Exact test, a nonparametric test for trend over an ordinal covariate(Jonckeheere-Terpstra test), nonparametric Wilcoxon rank sum and t-tests. Treatment delivery(RT; cisplatin or cetuximab) was analyzed using descriptive statistics, and a comparison of RT delivery by chemotherapy regimen was performed by Wilcoxon rank sum test. Selected AE’s were analyzed by descriptive statistics and were compared between chemotherapy groups by Wilcoxon rank sum test, Fisher’s Exact test or a test for the difference of proportions over an ordinal scale(Jonckheere-Terpstra test). Causes of death were tabulated and stratified by chemotherapy group.
Results
Patient Characteristics
Of the 47 patients identified, 18 received cisplatin and 29 received cetuximab concurrently with definitive RT(Table 1). Tumor T and N classifications, stage, gender, race, smoking history and HPV status were balanced in the two treatment groups. Patients in the cisplatin group were younger(median 55 versus 62 years, respectively; p=0.015) and had better performance status(median 0 versus 1, respectively; p=0.042) compared to those in the cetuximab group.
Table 1.
Patient and Tumor Characteristics
| Regimen | RT with Cisplatin (n=18) |
RT with Cetuximab (n=29) |
p-value | ||
|---|---|---|---|---|---|
| Characteristic | No. | % | No. | % | |
| Age (years) Median | 55 | 62 | 0.015 | ||
| Range | 35–78 | 46–86 | |||
| Sex | 0.32 | ||||
| Male | 15 | 83.3 | 19 | 65.5 | |
| Female | 3 | 16.7 | 10 | 34.5 | |
| Race | 0.99 | ||||
| Caucasian | 14 | 77.8 | 21 | 72.4 | |
| African American | 3 | 16.7 | 6 | 20.7 | |
| Native American | 1 | 5.6 | 2 | 6.9 | |
| Smoking History | 0.94 | ||||
| Yes | 14 | 77.8 | 26 | 89.7 | |
| No | 4 | 22.2 | 3 | 10.3 | |
| Insurance | 0.076 | ||||
| Private | 5 | 27.8 | 4 | 13.8 | |
| Medicare | 3 | 16.7 | 15 | 51.7 | |
| Medicaid | 9 | 50.0 | 9 | 31.0 | |
| None | 1 | 5.6 | 1 | 3.4 | |
| ECOG performance status | 0.042 | ||||
| 0 | 11 | 61.1 | 8 | 27.6 | |
| 1 | 4 | 22.2 | 13 | 44.8 | |
| 2 | 3 | 16.7 | 6 | 20.7 | |
| 3 | 0 | 0.0 | 2 | 6.9 | |
| ACE Comorbidity Index | 0.083 | ||||
| 0 (none) | 5 | 27.8 | 5 | 17.2 | |
| 1 (mild) | 7 | 38.9 | 8 | 27.6 | |
| 2 (moderate) | 4 | 22.2 | 5 | 17.2 | |
| 3 (severe) | 2 | 11.1 | 11 | 37.9 | |
| Primary Site | 0.29 | ||||
| Oropharynx | 10 | 55.6 | 13 | 44.8 | |
| Oral Cavity | 0 | 0.0 | 3 | 10.3 | |
| Larynx | 7 | 38.9 | 7 | 24.1 | |
| Level II/III Neck Mass (Unknown Primary) | 1 | 5.6 | 2 | 6.9 | |
| Hypopharynx | 0 | 0.0 | 4 | 13.8 | |
| T Classification | 0.58 | ||||
| T1 | 0 | 0.0 | 1 | 3.4 | |
| T2 | 0 | 0.0 | 3 | 10.3 | |
| T3 | 8 | 44.4 | 10 | 34.5 | |
| T4 | 9 | 50.0 | 13 | 44.8 | |
| Tx | 1 | 5.6 | 2 | 6.9 | |
| N Classification | 0.66 | ||||
| N0 & N1 | 6 | 33.3 | 12 | 41.4 | |
| N2a-c | 11 | 61.1 | 15 | 51.7 | |
| N3 | 1 | 5.6 | 2 | 6.9 | |
| Overall Stage | 0.55 | ||||
| III | 3 | 16.7 | 7 | 24.1 | |
| IVa/IVb | 15 | 83.3 | 22 | 75.9 | |
| HPV-Related | 0.99 | ||||
| Oropharynx | 7 | 7 | |||
| Level II/III neck mass (Unknown primary) | 1 | 1 | |||
| Hypopharynx | 0 | 1 | |||
Treatment Delivery
Delivery of RT as measured by median total dose, proportion of patients who received 33–35 fractions, elapsed days, and daily dose was similar between the two treatment groups(Table 2). The planned RT dose was delivered in 94.4% of the cisplatin group and 93.1% of the cetuximab group(p=0.90). Three patients(1 cisplatin group and 2 cetuximab group) received an abbreviated course of RT due to social/transportation issues, respiratory failure, and hospitalization for opiate withdrawal. The median(range) number of doses of cisplatin or cetuximab given were 2(1–3) and 8(1–15), respectively.
Table 2.
Treatment Delivery of Radiation
| Treatment Variable | Radiation with Cisplatin (n=18) |
Radiation with Cetuximab (n=29) |
p-value |
|---|---|---|---|
| Elapsed Daysa | 49 (25–62) | 49 (29–72) | 0.77 |
| # Fractions(FX)a | 35 (17–35) | 35 (17 – 35) | -- |
| Proportion of Patients who Received 33–35 FX | 16/18 (89%) | 27/29 (93%) | 0.63 |
| Daily Dosea | 2.0 (2.0–2.10) | 2.0 (2.0–2.22) | 0.86 |
| Total Dosea | 70 (34–71) | 70 (34–73) | 0.96 |
| % Completed Planned Dosea | 94.4% | 93.1% | 0.90 |
Median (Range)
Survival
At last follow-up, 20 patients were alive without primary cancer, 2 patients were alive with primary cancer, and 25 patients expired(due to primary cancer in 19, second cancer in 1, intercurrent illness in 2, and other causes in 3)(Table 3). Sites of recurrent disease are shown in Table 3. Recurrent disease was more common in the cetuximab group compared to the cisplatin group(p=0.018). The most common site of recurrent disease was local-regional only, and most(8 of 10) of the local-regional only recurrences occurred in the cetuximab group. The median(range) follow-up of all patients was 23(4–64) months: 35(7–64) months in the cisplatin group and 18(4–54) months in the cetuximab group.
Table 3.
Status at Last Follow-up and Site of Recurrent Disease
| Variable | RT with Cisplatin (n=18) |
RT with Cetuximab (n=29) |
|---|---|---|
| Status | ||
| Alive without disease | 13(72.2%) | 7 (24.1%) |
| Alive with disease | 1 (5.6%) | 1 (3.4%) |
| Deceased | 4 (22.2%) | 21 (72.4%) |
| Primary Cancer | 3 | 16 |
| Secondary Cancer | 0 | 1 |
| Intercurrent Illness | 0 | 2 |
| Other/Unknown | 1 | 2 |
| Site of Recurrence | ||
| Total(p=0.018) | 4 (22.2%) | 17 (58.6%) |
| Local-Regional Only | 2 | 8 |
| Distant Only | 0 | 2 |
| Both | 2 | 7 |
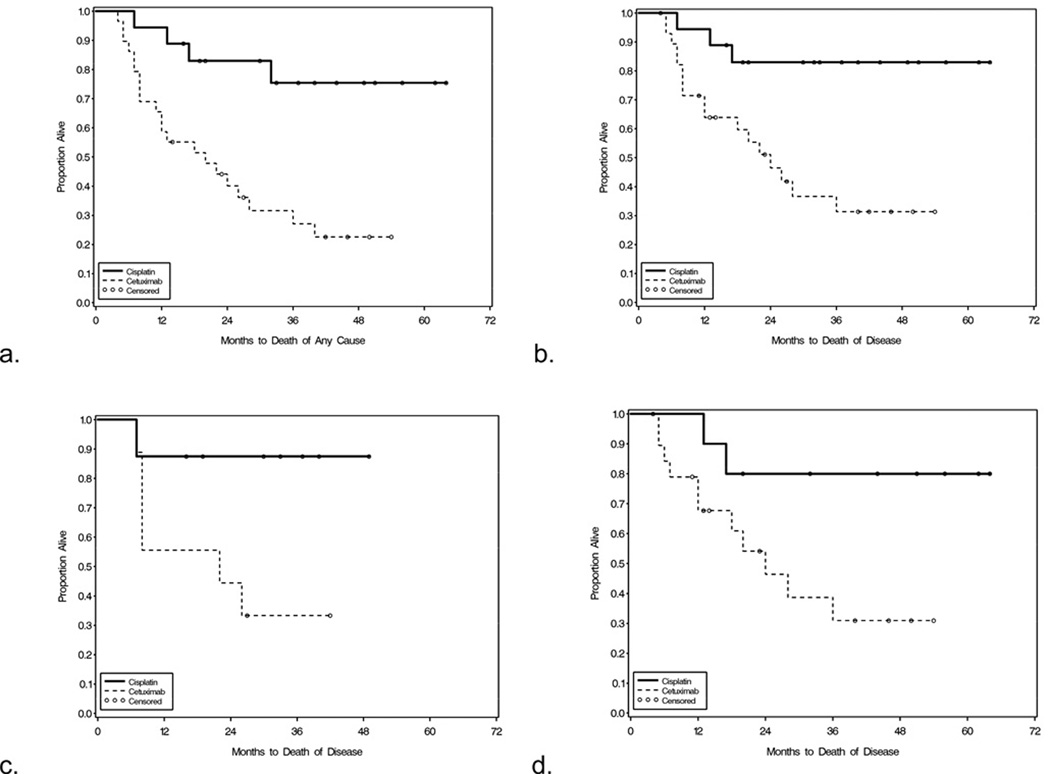
DSS at 3 years was 83% in the cisplatin group and 31% in the cetuximab group. OS at 3 years was 75% in the cisplatin group and 27% in the cetuximab group(Figure 2). Propensity score analysis to adjust for differences in patient characteristics which influenced treatment selection showed that DSS was indeed longer with cisplatin than with cetuximab(DSS Hazard Ratio[HR] 0.15{Confidence Interval[CI]0.033,0.66}, p=0.012). A similarly adjusted analysis also showed that OS was longer with cisplatin than with cetuximab(OS HR 0.24{CI0.067,0.89}, p=0.033).
DSS for HPV-related(n=17) and HPV-unrelated(n=30) HNSCC were stratified by treatment group(Figure 1). In the HPV-related and HPV-unrelated cohorts, a trend to better DSS was observed in the cisplatin group compared to the cetuximab group(p=0.058 and p=0.036, respectively).
Figure 1.
Survival curves stratified by chemotherapy regimen: a)DSS for all patients, b)OS for all patients, c)DSS for the HPV-related patients, d)DSS for the HPV-unrelated patients
Selected Adverse Events
The median grade of mucositis and the proportion of patients requiring PEG tube placement were similar between the cisplatin and the cetuximab groups(Table 4). Acneiform rash developed in 22(76%) patients in the cetuximab group(Grade 1 in 8 patients and Grade 2–3 in 14 patients).
Table 4.
Selected Adverse Events
| Toxicity/Event | Radiation with Cisplatin (n=18) |
Radiation with Cetuximab (n=29) |
p-value |
|---|---|---|---|
| Mucositis Grade-Median (Range) | 2 (0–3) | 2 (0–3) | 0.82 |
| Weight Loss(kg)-Median (Range) | 9.4 (0–21) | 6 (0–22) | 0.24 |
| # with PEG tube placement (%) | 12 (66.7%) | 15 (51.7%) | 0.37 |
| Median Duration(days) of PEG (Range) | 218.5 (49–1073) | 239 (16–1088) | 0.83 |
Discussion
In this retrospective analysis, DSS was superior in patients treated with cisplatin and definitive RT compared to cetuximab and definitive RT. This DSS difference was due to a greater proportion of patients in the cetuximab group who developed recurrence of their primary cancer and died compared to the cisplatin group. The key predictors of cancer recurrence including tumor T and N classifications, stage, gender, smoking history and HPV status were balanced between the two treatment groups. However, differences in patient characteristics between the two treatment groups were present. Older age and reduced performance status in the cetuximab group could impact primary cancer recurrence and DSS by adversely affecting delivery of RT and chemotherapy or could impact survival by affecting TRM or deaths from intercurrent illness; however, the data in this analysis do not support this conclusion. Delivery of RT was similar between the two treatment groups and the delivery of cisplatin and of cetuximab was comparable to that observed in other studies [3, 8]. Deaths from intercurrent illness and TRM were proportionately similar between the two treatment groups.
The propensity score analysis showed that DSS differed between the two groups even after adjustment for differences in patient characteristics which influenced treatment selection. The choice to treat a patient with cisplatin versus cetuximab was guided, in part, by characteristics of the patient. Some of these characteristics were related to the patient's prognosis, so a direct, unadjusted comparison of the two groups would be likely to produce biased and misleading estimates. Any differences in DSS could be due to the nature of the patients undergoing each therapy and only spuriously correlated with the therapy itself. In the present patient sample, there are measurable differences in age and performance status, and possibly in ACE-27 comorbidity index. These biases can be reduced by simultaneous adjusting for several patient characteristics using a propensity score. A propensity score is the patient's probability of receiving one drug or the other based on the patient's age, performance status, and any other characteristics thought to be a source of bias. That probability is included as a covariate in the subsequent Cox proportional hazards model of DSS, so the hazard ratio for cisplatin-cetuximab describes the difference in hazard of death (that is, the instantaneous death rate) conditional upon the characteristics included in the propensity score. In effect, it allows comparison of DSS in patients of the same age, performance status, and any other characteristic thought to be a source of bias and summarizes those comparisons in a single, adjusted estimate of hazard. The adjustment, while not resulting in a perfect match, does reduce the major biases resulting from observable characteristics related to the patient's outcome.
Two other retrospective comparisons of definitive RT and either cetuximab or chemotherapy to treat patients with locally advanced HNSCC yielded conflicting conclusions. In contrast to our study, a comparison of patients treated with definitive RT and cetuximab(n=29) or chemotherapy(n=103) showed no significant differences in locoregional control, distant metastasis-free survival, DSS, or OS [9]. However, differences in patient characteristics between the two treatment groups(higher T classification and inclusion of non-protocol patients in the chemotherapy group) and the use of heterogeneous regimens in the chemotherapy group confounds the data interpretation. A strength of our study was that all patients in the chemotherapy group were treated with high dose bolus cisplatin. A comparison of patients treated at Memorial Sloan-Kettering Cancer Center(MSKCC) with definitive RT and cetuximab(n=49) or high dose bolus cisplatin(n=125) showed significant differences in 2 year locoregional failure rate(39.9% versus 5.7%, respectively p<0.0001), 2 year failure-free survival(44.5% versus 87.4%, respectively p<0.0001), and 2 year OS(66.6% versus 92.8%, respectively p=0.0003) [10]. On multivariate analysis, treatment with definitive RT and cisplatin was associated with better locoregional control and OS. The observation of better locoregional control and OS in the group treated with definitive RT and cisplatin compared to RT and cetuximab is similar to our report.
A previous analysis of the Bonner, et al trial found that the patients who benefited most from the addition of cetuximab to RT had the phenotypic features of HPV-related HNSCC: oropharynx primary, smaller T classification, younger, male, and good performance status [4]. In our study, DSS in the HPV-related cohort may be better in the cisplatin group compared to the cetuximab group(p = 0.058). Our data should be interpreted cautiously given the small sample and the retrospective nature of the analysis. The comparative effectiveness of these two agents is being addressed by the ongoing Radiation Therapy Oncology Group(RTOG) 1016 trial which is comparing outcomes of HPV-related oropharyngeal HNSCC treated with definitive RT and either concurrent cisplatin or cetuximab.
Preliminary emerging literature suggests a potential difference in the expression of the epidermal growth factor receptor(EGFR) and in the role of EGFR inhibitors in HPV-related versus HPV-unrelated HNSCC. Several, but not all, studies documented lower EGFR expression in HPV-related HNSCC in comparison to HPV-unrelated HNSCC [11–16]. However, the level of expression of EGFR by IHC has not been demonstrated to consistently correlate with tumor response to EGFR inhibitors in HNSCC [17]. A randomized trial demonstrated that the addition of the EGFR monoclonal antibody panitumumab to chemotherapy improved OS of patients with HPV-negative recurrent or metastatic HNSCC whereas no survival benefit was found in the HPV-positive cohort [18]. Also, RTOG 0522 showed a non-significant trend toward poorer progression-free survival in HPV-related HNSCC treated with cetuximab and concurrent accelerated RT plus cisplatin in comparison with RT plus cisplatin alone [19]. More data are required to clarify the role of EGFR inhibitors in HPV-related HNSCC.
The median weight loss was lower in the cetuximab group compared to the cisplatin group even though the median grades of mucositis were similar. A prospective controlled study observed similar rates of grade 3 or greater mucositis between patients randomized to receive definitive RT alone or with cetuximab [3]. Differences in the expected acute toxicity profiles between definitive RT given with cetuximab or cisplatin must be considered in the context of the patient’s characteristics when deciding which treatment approach to recommend.
In this retrospective study, we observed that DSS was superior in the patients given cisplatin with definitive RT compared to cetuximab with definitive RT due to a lower risk of recurrent disease in the cisplatin group. These observations could not be explained by differences between the two groups in the patient and tumor characteristics or in treatment delivery. Prospective validation of the findings is indicated.
Acknowledgements
The authors acknowledge the support provided by James Lewis MD, Bruce Haughey MBChB, Jason Diaz MD, Randall Paniello MD, and support from NCI Cancer Center Support Grant P30 CA091842.
Footnotes
Presented in abstract form at the 2012 Multidisciplinary Head & Neck Cancer Symposium, Phoenix, Arizona
Conflicts of Interest
The authors declare research support from Eli Lilly (Dr. Douglas Adkins).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Pignon JP, le Maitre A, Maillard E, Bourhis J. MACH-NC Collaborative group: Metaanalysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Onco. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 4.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, zhu J, Youssoufian H, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 5.Piccirillo JF, Creech C, Zequeira R, Anderson S, Johnston AS. Inclusion of comorbidity into oncology data registries. J Registry Manag. 1999;26(2):66–70. [Google Scholar]
- 6.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th Edition. New York: Springer; 2010. [Google Scholar]
- 7.Chernock RD, El-Mofty SK, Thorstad WL, Parvin CA, Lewis JS. HPV-related nonkeratinizing Squamous cell carcinoma of the Oropharynx: Utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3(3):186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, Peters G, Lee DJ, Leaf A, Ensley J, Cooper J. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 9.Caudell JJ, Sawrie SM, Spencer SA, Desmond RA, Carroll WR, Peters GE, Nabell LM, Meredith RF, Bonner JA. Locoregionally advanced head and neck cancer treated with primiary radiotherapy: A comparison of the addition of cetuximab or chemotherapy and the impact of protocol treatment. Int J Rad Oncol Biol Phys. 2008;71(3):676–681. doi: 10.1016/j.ijrobp.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 10.Koutcher L, Sherman E, Fury M, Wolden S, Zhang Z, Mo Q, Stewart L, Schupak K, Gelblum D, Wong R, Kraus D, Shah J, Zelefsky M, Pfister D, Lee N. Concurrent cisplatin and radiation versus cetuximab and radiation for locally advanced head-and-neck cancer. Int J Rad Oncol Biol Phys. 2011;81(4):915–922. doi: 10.1016/j.ijrobp.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, Wolf GT, Urba SG, Chepeha DB, Teknos TN, Eisbruch A, Tsien CI, Taylor JM, D’Silva NJ, Yang K, Kurnit DM, Bauer JA, Bradford CR, Carey TE. EGFR, p16, HPV titer, Bcl-xL and p53, sex, smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26(19):3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong CS, Narasimhan B, Cao H, Kwok S, Erickson JP, Koong A, Pourmand N, Le QT. The relationship between human papillomavirus status and other molecular prognostic markers in head & neck squamous cell carcinomas. Int J Rad Oncol Biol Phys. 2009;74(2):553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Swiahb JN, Huang CC, Fang FM, Chuang HC, Huang HY, Luo SD, Chen CH, Chen CM, Chien CY. Prognostic impact of p16, p53, epidermal growth factor receptor, and human papillomavirus in oropharyngeal cancer in a betel nut-chewing area. Arch Otolaryngol Head Neck Surg. 2010;136(5):502–508. doi: 10.1001/archoto.2010.47. [DOI] [PubMed] [Google Scholar]
- 14.Hong A, Dobbins T, Lee CS, Jones D, Jackson E, Clark J, Armstrong B, Harnett G, Milross C, O’Brien C, Rose B. Relationships between epidermal growth factor receptor expression and human papillomavirus status as markers of prognosis in oropharyngeal cancer. Eur J Cancer. 2010;46(11):2088–2096. doi: 10.1016/j.ejca.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Perrone F, Suardi S, Pastore E, Casieri P, Orsenigo M, Caramuta S, Dagrada G, Losa M, Licitra L, Bossi P, Staurengo S, Oggionni M, Locati L, Cantu G, Squadrelli M, Carbone A, Pierotti MA, Pilotti S. Molecular and cytogenetic subgroups of oropharyngeal squamous cell carcinoma. Clin Cancer Research. 2006;12(22):6643–6651. doi: 10.1158/1078-0432.CCR-06-1759. [DOI] [PubMed] [Google Scholar]
- 16.Reimers N, Kasper HU, Weissenborn SJ, Stutzer H, Preuss SF, Hoffmann TK, Speel EJ, Dienes HP, Pfister HJ, Guntinas-Lichius O, Klussmann JP. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 17.Cohen EEW. Role of epidermal growth factor receptor pathway-targeted therapy in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24(17):2659–2665. doi: 10.1200/JCO.2005.05.4577. [DOI] [PubMed] [Google Scholar]
- 18.Vermorken J, Stohlmacher J, Oliner K, et al. Safety and efficacy of panitumumab in HPV positive and HPV negative recurrent/metastatic squamous cell carcinoma of the head and neck: Analysis of the phase 3 SPECTRUM trial [abstract] 2011 European Multidisciplinary Cancer Congress. Abstract 25LBA. [Google Scholar]
- 19.Ang KK, Zhang QE, Rosenthal DI, Nguyen-Tan P, Sherman EJ, Weber RS, Galvin JM, Schwartz DL, El-Naggar AK, Gillison ML, Jordan R, List MA, Konski AA, Thorstad WL, Trotti A, Beitler JJ, Garden AS, Spanos WJ, Yom SS, Axelrod RS. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas (HNC)[abstract] J Clin Oncol. 2011;29 doi: 10.1200/JCO.2013.53.5633. (suppl;abstr 5500). [DOI] [PMC free article] [PubMed] [Google Scholar]