Abstract
Many applications required protein biotinylation. We routinely use biotinylated proteins to select single chain antibodies from phage and/or yeast display libraries. During phage selection the biotinylated antigens are bound to streptavidin coupled magnetic beads, while during yeast display, the biotinylated antigens are used during flow cytometry for both analysis and sorting. The Lightning-Link® Biotin kit, a rapid straightforward biotinylation kit that avoids the need for dialysis, is particularly useful when the amount of available protein is limiting. During routine screening of antibody libraries we identified a specific clone that bound a universal neo-epitope generated only when antigens are biotinylated with the commercial Lightning-Link®kit, with an affinity of ~10nM. Non-biotinylated proteins, and those biotinylated using alternative methods – the Thermo Fisher commercial kit or in vivo biotinylation using the Avitag (1)–, were not recognized by this antibody. Using deep sequence analysis, the specific antibody was identified as being the most abundant in a number of different selections. This indicates the need for caution when using such modifying reagents, because of the possibility of selecting antibodies against the modification, rather than the target protein, and also highlights the value of deep sequencing analysis during display based selections. Furthermore, this antibody may have great utility in the analysis of proteins biotinylated using this method.
Keywords: Phage display, yeast display, protein conjugation, biotinylation
1 Introduction
Phage antibody library selections have been largely used as an efficient way of discovery for high-quality reagents with wide use in biological, diagnostic and therapeutic applications. The success of the entire process is largely based on the quality of the antigens used as selection targets and the way in which they interact with the phage library during selection. A rapid successful method has consisted in the direct coating of target protein on a plastic surface (immunotubes, microtiter plate well etc.)(2). However, this can be a problem for particular proteins or peptides that are affected by immobilization-associated issues (3, 4). Peptides can be poorly coated on the plastic, making access to their epitopes difficult while proteins coated on plastic can assume an unfolded (denaturated) conformation that can prevent the selection for conformational antibodies (3, 4). Biotinylating target antigens is probably the most successful way to overcome this problem, allowing biotinylated proteins to be either indirectly coated on plastic previously saturated with either streptavidin or neutravidin, or bound to paramagnetic streptavidin-coated microbeads. The latter approach is particular popular, allowing: i) the phage-antigen interaction to occur in solution, ii) a short incubation time with the beads, iii) precise antigen quantification during selection, iv) preservation of the target structure and v) optimal epitope exposure (5).
The biotinylation process is straightforward: several commercial kits are available. At the end of the reaction, a dialysis step is usually required to remove excess biotin and carry out buffer exchange. However, this can be problematic when the quantity of available protein is limiting, and there is the danger of protein loss during the dialysis procedure. A recent commercial product - Lightning-Link® Biotin (Innova Bioscience) – overcomes this problem by allowing direct biotinylation of biomolecules in an easy-to-use, one-step procedure. The conjugated protein is obtained by simply pipetting it into a vial of lyophilized mixture containing biotin. The protocol does not require purification/desalting steps, a major advantage over traditional labeling methods. The reaction is highly efficient, and optimized to have only a very low amount of free label left at the end of the conjugation. Any potential remaining free label is blocked by the quencher provided in the kit, and is washed away during the relevant wash step of the application where the protein will be used. By avoiding the need for dialysis or desalting, it is possible to work with very low amounts of protein with full recovery of all the starting material.
We used antigens conjugated using Lightning-Link® Biotin in an antibody selection strategy we recently developed which combines phage and yeast display (6). This procedure has been successful in the screening and identification of hundreds of different antibodies. The biotinylated antigen is necessary in both the phage selection, performed using streptavidin-magnetic-beads, and in the yeast display where the interaction between antibody and biotinylated antigen is detected by fluorescently conjugated streptavidin and the use of fluorescent activated cell sorting (FACS). During the course of the analysis of the enriched population of potential binders for several proteins we identified a binder specific for any molecule biotinylated with this specific kit and not with standard biotinylation procedures. Here we report the sequence analysis and characterization of the binding activity of this clone.
2 Results and Discussion
2.1 The problem of cross-reaction
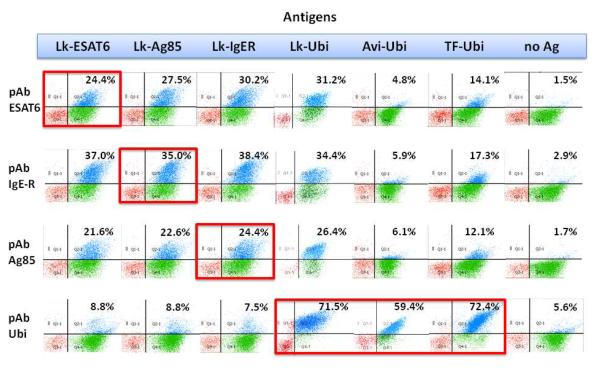
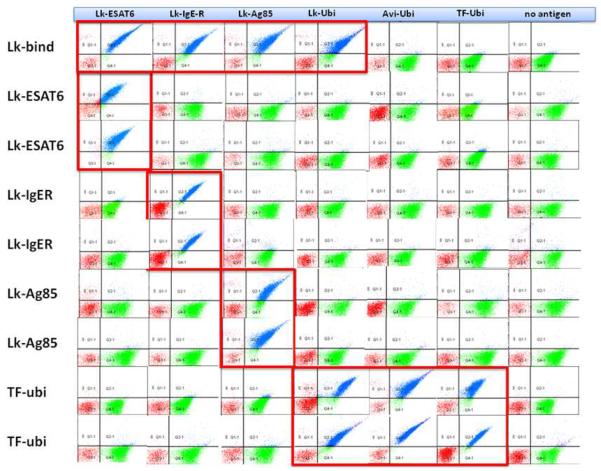
The Lightning-Link® Biotinylation kit was used to biotinylate four different antigens (LL-Ags: ESAT6, Ag85, IgER, ubiquitin). Ubiquitin was also biotinylated using two different methods: i) standard commercial (Thermo Fisher) protocols (TF-Ubi), in which the biotin linking reaction was followed by a dialysis step to remove the excess of biotin and unwanted reagents and ii) biotinylation (Avi-Ubi) was also performed directly in bacteria(7) thanks to the 15 amino acids sequence AvitagTM (8). Three of the LL-Ags (ESAT6, Ag85 and IgER) and TF-Ubi were used for two rounds of phage selection using our naïve single chain (scFv) antibody library (9) to isolate target-specific binders. After the second round of phage selection the output was subcloned into a yeast display vector to allow screening and sorting of the entire phage-selected antibody population by FACS, as previously described (6). After a single sort the populations were enriched for antibodies binding to each of the proteins of interest (Fig 1). When polyclonal populations obtained using LL-Ags were tested for their specificity, they showed essentially no cross-reactivity with the Avi-Ubi, and very low cross-reactivity with the TF-Ubi, or when tested with secondary reagents only, but showed particularly strong cross-reactivity when tested with the other LLAgs, including LL-Ubi. The binders obtained using TF-Ubi show no cross-reaction with the unrelated LL-Ags (Fig. 1) but good binding signal when tested with LL-Ubi and Avi-Ubi .
Figure 1. Specificity test of the polyclonal scFvs populations by flow cytometry.
The polyclonal antibodies (pAb) selected using Lightning-Link® biotin-conjugated antigens (LL-Ags) show cross-reactivity with all the other LL-Ags – including LL-Ubi –, while the pAb obtained with ubiquitin conjugated with a different procedure are specific for their target even when biotinylated using a different procedure.
2.2 Identification of Lightning-Kit® Biotin binder
We have established a pipeline for the generation of polyclonal antibody populations for different targets combining phage and yeast display (Ferrara et al. in preparation). After the enrichment for specific binders we checked the polyclonality of our antibodies by deep sequencing. For this reason all the sorted antibody populations were amplified by PCR and analyzed by Ion-torrent sequencing. To identify different antibodies our analysis was based on the HCDR3 sequence (D'Angelo et al. submitted), and clones were ranked based on their relative abundance (Table 1). By focusing our analysis on the top 10 most abundant antibodies obtained against each antigen, we were able to clearly identify a single potential dominant cross-reactive clone (termed LL-Bind) which was present as the most abundant clone in all the selections performed with LL-Ags but absent from the one where TF-Ubi was used. The abundance of this dominant clone ranged from 11.6% to 31.9%, a value that appeared sufficient to affect the analysis of the polyclonal population when tested by flow cytometry.
Table 1. HCDR3 amino acid sequence of top 10 most abundant clusters in deep sequencing.
The deep sequencing analysis allowed the identification of LL-bind – the most abundant clone in all selections performed with LL-Ags - and the other target specific antibodies present in the 10 most abundant antibodies.
| antigen | Rank | CDR3 AA sequence | % |
|---|---|---|---|
| Lk-ESAT6 | 1 | CARRLRIAVDHRYGFDIW | 31.9 |
| 2 | CAKDPVGRWLQLPGSYW | 4.9 | |
| 3 | CARLRTGYSSSWPYWYFDLW | 4.2 | |
| 4 | CARWDKYYWYFDLW | 3.9 | |
| 5 | CAKGRIAAAGRYYYYMDVW | 2.1 | |
| 6 | CARLRHGYSYGSYYGMGVW | 1.5 | |
| 7 | CARLSRYYYYGMDVW | 1.4 | |
| 8 | CARGSYSSAWSW | 1.4 | |
| 9 | CAKGSGRPARYYYYGMDVW | 1.3 | |
| 10 | CAKGSRYYYGSGSYPTYW | 1.2 | |
| Lk-Ag85 | 1 | CARRLRIAVDHRYGFDIW | 11.6 |
| 2 | CARGGLHPYSYYGMDVW | 5.0 | |
| 3 | CARIRYRTGGIDYW | 3.4 | |
| 4 | CAVRRRGVINGMDVW | 1.7 | |
| 5 | CAKLGRIAAAGKVHW | 1.7 | |
| 6 | CAKWGGRNGAFDIW | 1.5 | |
| 7 | CAKAKSYYDSSGYYPW | 1.4 | |
| 8 | CAGRKPYSSSWYKAGDYW | 1.4 | |
| 9 | CARFGVTARRGYW | 1.3 | |
| 10 | CAKFGSGWYSGHYGMDVW | 1.0 | |
| Lk-lgE-R | 1 | CARRLRIAVDHRYGFDIW | 23.0 |
| 2 | CAKKYDSSGYSPRWYFDLW | 13.1 | |
| 3 | CARGPRWGSSTIIW | 2.4 | |
| 4 | CARVSTWRPFNYYMDVW | 2.4 | |
| 5 | CVRPRGVVAGPPRYW | 2.0 | |
| 6 | CVRGASWGKGWYFDLW | 2.0 | |
| 7 | CARGWVAKRTWYFDLW | 1.5 | |
| 8 | CARGQRFNPYYYYYYMDVW | 1.4 | |
| 9 | CARGRRWLQFDYW | 1.3 | |
| 10 | CARGDTAMDTRSYNWFDPW | 1.3 | |
| St-Ubq | 1 | CAKGIAAVDYW | 25.8 |
| 2 | CARYYYDSSGYYADAFDIW | 15.4 | |
| 3 | CASLRSAYYYDSSGRDAFDIW | 13.5 | |
| 4 | CARGGQLSSGYYFDAFDIW | 9.1 | |
| 5 | CAKVRGGDW | 8.9 | |
| 6 | CAKGVSGMDVW | 4.4 | |
| 7 | CTNKGGAFDIW | 2.8 | |
| 8 | CAKGLGYGMDVW | 2.3 | |
| 9 | CAKPGNGAFDIW | 1.1 | |
| 10 | CARAYSSSWYPFDYW | 1.1 |
2.3 Binding characteristics of the top ranked clones
To determine the binding activity of LL-Bind, we first sequenced (using Sanger sequencing) 96 randomly picked clones for each of the selections. The LL-Bind clone was easily identified, as were some of the other top 10 ranking clones for the LL-Ags and TF-Ubi. The LL-Bind and two clones from the top ten ranked scFvs for each polyclonal population were tested as monoclonal scFvs by flow cytometry to address their binding activities. The results (Fig. 2) show that LL-Bind shows strong reactivity for all the LL-Ags –including LL-Ubi – but almost none for TF-Ubi and Avi-Ubi, indicating the preference for binding to biotin added to proteins by the Lightning-Link® kit. At the same time the other clones in the top 10 ranking for all the LL-Ags selections show specific reactivity for their target proteins, indicating that the presence of one cross-reactive clone can be the cause of the apparent total cross-reactivity of the entire selected yeast population. The average affinity of LL-Bind for the different LL-Ag was calculated by yeast display (6, 10) obtaining an affinity Kd of 9.6 nM.
Figure 2. Monoclonal scFvs analysis by flow cytometry.
The isolated monoclonal LL-Bind antibody shows cross-reactivity for all the LL-Ags but not for the antigen biotinylated with a different procedure (TF-Ubi, Avi-Ubi). The other clones in the top 10 ranking for all the LL-Ags selections show reactivity for their specific target, indicating that the cause of the apparent total cross-reactivity of the entire selected yeast population can be the presence of a single cross-reactive clone.
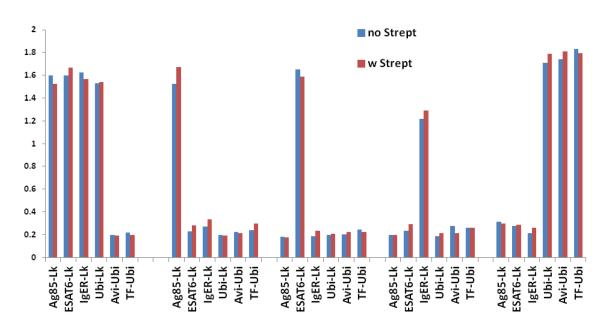
To confirm that the binding activity was independent of the assay format used (yeast display) we recloned LL-Bind and one scFv specific for anti-Ag85, ESAT6, IgER and TF-Ubi into a yeast expression vector as scFv-Fc-rabbit fusions (11). In these constructs the scFv is fused directly to the rabbit immunoglobulin Fc domain, and for all practical purposes performs as well as a full length IgG. When the binding activity of LL-bind and the other selected scFvs were tested by ELISA, using antigens coated to microtiter plates with or without streptavidin (fig 3), we found that LL-Bind bound to all the proteins conjugated with the Lightning-Link®, in the presence or absence of streptavidin, while the target-specific scFvs retained their specificity in the scFv-Fc format (Fig. 3).
Figure 3. Monoclonal scFvs analysis by ELISA.
The binding activity of LL-Bind and the other selected scFvs was tested by ELISA, with or without the use of streptavidin for antigen binding on the plastic surface. All the LL-Ags were bound by the LL-Bind in the presence or absence of streptavidin, while the target-specific scFvs retained their specificity in the scFv-Fc format.
3 Conclusions
We describe a scFv obtained from our naïve phage display library that specifically binds to an epitope created when proteins are biotinylated using the commercial Lightning-Link® kit. Protein biotinylation is a widely used procedure applied in several applications. This particular kit is rapid and easy-to-use, making the use of this particular procedure appealing, especially when the amount of the protein available is limiting. As the use of biotinylation is particularly popular in phage and yeast display systems, these results should be of general interest in the field of in vitro selection of antibodies. Furthermore, it is clear that this antibody can serve as an alternative secondary reagent for LL-Ags, allowing the detection of targets biotinylated with Lightening link, whether they are bound to streptavidin or not.
The approach we used here to identify LL-Bind, deep sequencing of selection outputs followed by the further analysis of highly ranked clones, was extremely effective in the identification of this abundant and cross-reactive clone. It also allowed the identification of target specific antibodies by the testing of the other most abundant clones, indicating that the presence of such cross-reactive clones does not preclude the use of Lightning-Link® biotinylation when antigen is limiting. It does, however, indicate that caution should be used when using such modifying reagents, and that the possibility of selecting antibodies against the modification, rather than the target protein, is always a possibility.
Acknowledgements
This work was supported by Los Alamos National Laboratory and New Mexico Consortium through a NIH U54 Grant “Technology Development for New Affinity Reagents Against the Human Proteome (U54) RFA-RM-10-018”, grant number 1-U54-DK093500-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5 References
- 1.Ashraf SS, Benson RE, Payne ES, Halbleib CM, Gron H. Protein Expr Purif. 2004;33:238–45. doi: 10.1016/j.pep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Bradbury AR, Sidhu S, Dubel S, McCafferty J. Nat Biotechnol. 2011;29:245–54. doi: 10.1038/nbt.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler JE, Navarro P, Sun J. Immunol Invest. 1997;26:39–54. doi: 10.3109/08820139709048914. [DOI] [PubMed] [Google Scholar]
- 4.Butler JE, Ni L, Nessler R, Joshi KS, Suter M. J Immunol Methods. 1992;150:77–90. doi: 10.1016/0022-1759(92)90066-3. [DOI] [PubMed] [Google Scholar]
- 5.Chames P, Baty D. Antibody Engineering. Springer; 2010. [Google Scholar]
- 6.Ferrara F, Naranjo LA, Kumar S, Gaiotto T, Mukundan H, et al. PLoS One. 2012;7:e49535. doi: 10.1371/journal.pone.0049535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scholle MD, Collart FR, Kay BK. Protein Expr Purif. 2004;37:243–52. doi: 10.1016/j.pep.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Beckett D, Kovaleva E, Schatz PJ. Protein Sci. 1999;8:921–9. doi: 10.1110/ps.8.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sblattero D, Bradbury A. Nat Biotechnol. 2000;18:75–80. doi: 10.1038/71958. [DOI] [PubMed] [Google Scholar]
- 10.Gai SA, Wittrup KD. Curr Opin Struct Biol. 2007;17:467–73. doi: 10.1016/j.sbi.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powers DB, Amersdorfer P, Poul M, Nielsen UB, Shalaby MR, et al. J. Immunol. Methods. 2001;251:123–35. doi: 10.1016/s0022-1759(00)00290-8. [DOI] [PubMed] [Google Scholar]