Abstract
Deletion of the long arm of chromosome 5, del(5q), is the most prevalent cytogenetic abnormality in patients with myelodysplastic syndromes (MDS). In isolation, it is traditionally associated with favorable prognosis compared with other subtypes of MDS. However, owing to the inherent heterogeneity of the disease, prognosis for patients with del(5q) MDS is highly variable depending on the presence of factors such as additional chromosomal abnormalities, >5 % blasts in the bone marrow (BM), or transfusion dependence. Over recent years, the immunomodulatory drug lenalidomide has demonstrated remarkable efficacy in patients with del(5q) MDS. Advances in the understanding of the pathogenesis of the disease have suggested that lenalidomide targets aberrant signaling pathways caused by haplosufficiency of specific genes in a commonly deleted region on chromosome 5 (e.g., SPARC, RPS14, Cdc25C, and PP2A). As a result, the agent specifically targets del(5q) clones while also promoting erythropoiesis and repopulation of the bone marrow in normal cells. This review discusses recent developments in the understanding of the mechanism of action of lenalidomide, and how this underlies favorable outcomes in patients with del(5q) MDS. In addition, we discuss how improved understanding of the mechanism of disease will facilitate clinicians’ ability to predict/monitor response and identify patients at risk of relapse.
Keywords: Erythropoiesis, Immunomodulatory, Lenalidomide, Myelodysplastic syndromes
Introduction
Myelodysplastic syndromes (MDS) constitute a heterogeneous group of clonal hematopoietic disorders characterized by bone marrow (BM) failure, dysplasia, and an increased risk of developing acute myeloid leukemia (AML) [1]. About 50 % of cases of MDS are characterized by the presence of cytogenetic abnormalities in the BM [2]. The most prevalent cytogenetic abnormality is a partial deletion of the long arm of chromosome 5, del(5q), which is present in about 15 % of cases [2]. It is becoming increasingly evident that del(5q) MDS is a highly heterogeneous disease, and prognosis varies widely depending on other factors such as presence of additional chromosomal abnormalities, >5 % blasts in the BM, and transfusion requirement [3, 4]. Therefore, it is important that multiple risk factors are considered when deciding upon optimal management strategies for patients with del(5q) MDS.
Most patients with del(5q) MDS become red blood cell transfusion-dependent (RBC-TD) over the course of the disease. This has a major negative impact on survival, disease-related morbidity, and quality of life [5–7]. Therefore, a major goal of the clinical management of del(5q) MDS is to help patients become red blood cell transfusion independent (RBC-TI); this is complicated by the fact that patients with del(5q) MDS tend to respond poorly to erythropoiesis-stimulating agents [8]. However, over the past decade, clinical trials have demonstrated that patients with del(5q) MDS respond very well to lenalidomide, an oral antineoplastic and immunomodulatory agent [9–11]. Lenalidomide has direct effects on the del(5q) clone as well as pleiotropic effects on erythropoiesis and BM function, though the molecular basis of these phenomena is only just beginning to be unravelled. As such, it is important that the mechanism of action (MoA) of lenalidomide is fully delineated in order to understand why some patients are refractory, or become resistant, to its effects. Furthermore, precise tools are required to predict loss of response, or disease progression, in patients treated with lenalidomide.
In this review, we discuss how the clinical activity and tolerability profile of lenalidomide are linked to its MoA at the cellular and molecular level. We also discuss how emerging insights into the “natural history” of del(5q) MDS necessitate routine monitoring during lenalidomide therapy in order to identify early signs of disease progression.
An overview of the pathogenesis of del(5q) MDS
By comparing overlapping chromosome 5 deletions among patients with del(5q) MDS, two commonly deleted regions (CDRs) have been identified. Horrigan et al. identified a 1–1.5 megabase region at chromosome 5q31 that was consistently deleted in a cohort of patients with del(5q) MDS or AML (not including patients with “5q– syndrome”—a subclass of disease characterized by <5 % BM blasts and specific morphology and blood counts) [12]. This is known as the proximal CDR and contains tumor suppressor genes associated with advanced MDS. Similarly, Boultwood et al. identified a 1.5-Mb region at 5q32–33 (the distal CDR) that underlies the characteristic “5q– syndrome” phenotype [13]. Owing to the proximity of the two CDRs, many patients with del(5q) MDS have interstitial deletions that overlap both regions [14], and this at least partially explains why most patients with del(5q) MDS have a more severe disease phenotype, and poorer prognosis, than that associated with “5q– syndrome.” Indeed, in a recent study of patients with del(5q) MDS that utilized single nucleotide polymorphism microarrays, Jerez et al. demonstrated that severity of disease largely correlated with the size of the 5q deletion [15].
Over the past decade, intensive analysis of the CDRs has been undertaken to try to identify the pathogenetic determinants of del(5q) MDS. Boultwood et al. systematically sequenced 40 genes within the distal CDR [16]. Despite exhaustive efforts, no mutations were detected in these genes, suggesting that qualitative changes in, or complete loss of, gene expression are unlikely to underlie the“5q– syndrome” phenotype. Conversely, gene expression analysis in hematopoietic stem cells (CD34+ cells) indicated that most genes within the distal CDR were downregulated in patients with “5q– syndrome,” suggesting that haploinsufficiency (reduced expression to a level that does not support a wild-type phenotype, caused by the presence of only one copy of a gene rather than two) could at least partially explain the disease phenotype [16].
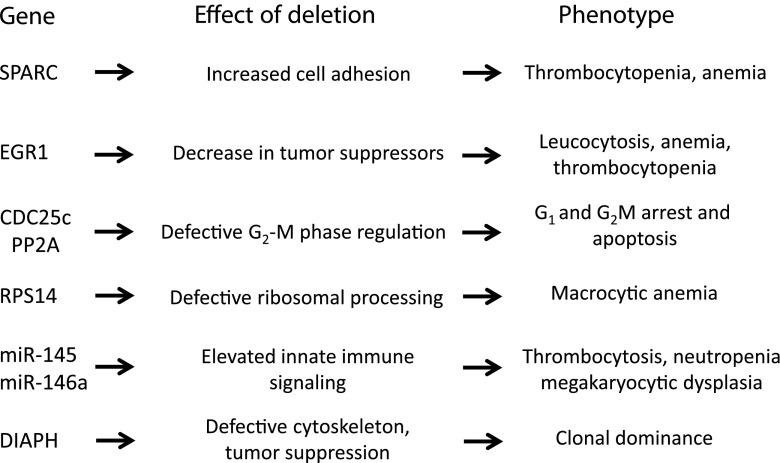
As discussed below, emerging data indicate that haploinsufficiency of distinct genes underlie different characteristic features of the “5q– syndrome” including hypoplastic anemia, megakaryocytic abnormalities, and clonal dominance of del(5q) cells in the BM (Fig. 1). Notably, other chromosome 5q genes outside of the distal CDR seem to underlie the heterogeneity of del(5q) MDS in terms of prognosis and clinical outcomes [17].
Fig. 1.
Genes in the commonly deleted region of chromosome 5q that are implicated in the pathogenesis of del(5q) MDS [19, 20, 25, 26, 66–68]. del(5q) deletion of the long arm of chromosome 5, MDS myelodysplastic syndromes
The molecular basis for impaired erythropoiesis in del(5q) MDS: haploinsufficiency of RPS14 and elevated p53 signaling
The ribosomal subunit 14 gene (RPS14), located within the distal CDR, is essential for the assembly of ribosomal complexes that translate mRNA into proteins. Several lines of direct and indirect evidence strongly implicate RPS14 in the pathogenesis of del(5q) MDS: (1) inactivating mutations of other ribosomal proteins have been linked with congenital BM failure syndromes such as Diamond–Blackfan anemia [18]; (2) systematic experimental knockdown of CDR genes in normal human CD34+ cells by RNA interference (RNAi) indicated that only the knockdown of RPS14 suppressed erythroblast proliferation and viability, without a significant impact on thrombopoiesis [19]; (3) forced expression of RPS14 in CD34+ cells from patients with del(5q) MDS restored normal erythropoiesis [19];(4) haploinsufficiency of the RPS14 region in a mouse model led to a “5q– syndrome” MDS-like phenotype with macrocytic anemia and monolobulated megakaryocytes; [20] however, these mice did not demonstrate thrombocytosis or neutropenia, suggesting the involvement of distinct haploinsufficient genes in the myeloid and megakaryocyte abnormalities that are characteristic of del(5q) MDS. Experimental evidence indicates that haploinsufficiency of RPS14 upregulates the p53 pathway, a key pathway that induces cell cycle arrest and apoptosis, specifically in erythroid cells, leading to hypoplastic anemia [17, 21]. Dutt et al. demonstrated that RNAi downregulation of RPS14 led to an accumulation of p53 in erythroid progenitor cells, but not myeloid cells or megakaryoctes, leading to apoptosis [21]. Pharmacological inhibition of p53 blocked apoptosis in these cells [21]. Furthermore, in a series of intricate in vivo experiments involving the cross breeding of RPS14 haploinsufficient mice with p53 knockout mice, Barlow et al. demonstrated that absence of p53 completely blocked the development of a “5q–syndrome” MDS-like phenotype [20]. There is also evidence that the p53 pathway plays a key role in the development of del(5q) MDS in patients; gene expression analysis of CD34+ cells indicated that several genes in the p53 pathway are upregulated in patients with del(5q) MDS compared with healthy controls [22].
Progress has been made in understanding the molecular mechanism by which RPS14 haploinsufficiency leads to an accumulation of p53 in del(5q) erythroid progenitors [21]. It is thought that reduced expression of RPS14 interferes with normal ribosomal biosynthesis and leads to high levels of free ribosomal proteins in the cell. Free ribosomal proteins block the binding of human homolog of murine double minute 2 (MDM2) to p53, thereby preventing ubiquitination and degradation [23]. Consequently, p53 accumulates in erythroid progenitor cells and drives apoptosis [23]. Interestingly, the proximal CDR includes two genes that are complementary coregulators of MDM2 and the cell cycle, the protein phosphatase 2a (PP2A) gene and the cell division cycle 25 C (CDC25C) gene. PP2A inactivates MDM2 by dephosphorylating it; in its inactive state, MDM2 cannot bind and inactivate p53 [24]. CDC25C is a phosphatase that positively regulates the G2-M checkpoint in the cell cycle and is also regulated by the activity of PP2A [25]. It follows, therefore, that MDM2, PP2A, and CDC25C constitute therapeutic targets for the correction of defective erythropoiesis in patients with del(5q) MDS.
The molecular basis for thrombocytosis and neutropenia in del(5q) MDS: haploinsufficiency of microRNA genes
Two important microRNA (miRNA) genes, miRNA-145 and -146a, are located within or close to the distal CDR. These encode RNA sequences that downregulate genes involved in the regulation of the innate immune system. Both genes are downregulated in CD34+ cells from patients with del(5q) MDS compared with CD34+ cells from patients with non-del(5q) MDS and healthy controls [26]. To assess the biological consequences of their reduced expression, Starczynowski et al. stably knocked down miRNA-145 and 146a in mouse CD34+ cells, which were subsequently transplanted into mice that had been myeloablated by irradiation, along with normal stem/progenitor cells to mimic BM mosaicism [26]. After 8 weeks, mice exhibited variable neutropenia, hypolobulated megakaryocytes, and suppressed myeloid progenitor activity in the BM. A similar phenotype was observed in irradiated mice transplanted with CD34+ cells with forced overexpression of TNF receptor-associated factor-6 (TRAF-6), a downstream target of miRNA-146a [26]. The phenotype was dependent on IL-6 (a pro-inflammatory cytokine); a near normal phenotype was observed when irradiated mice were transplanted with CD34+ cells derived from IL-6-deficient mice that were manipulated to overexpress TRAF-6 [26]. Interestingly, these mouse models mimicked the natural progression of del(5q) MDS with approximately a third of mice developing BM failure or AML. Disease progression was associated with clonal dominance; mice that progressed had much higher levels of the manipulated clone in the BM than mice that did not progress.
The molecular basis for clonal dominance of del(5q) progenitors: haploinsufficiency of tumor suppressor genes
Del(5q) MDS is characterized by its clonal nature. In most patients, >99 % of CD34+ cells carry the del(5q) abnormality and the BM is generally hypercellular or normocellular [27, 28]. Clearly, therefore, the presence of a del(5q) aberration exerts a proliferative advantage.
Several haploinsufficient genes may contribute to the proliferative advantage of del(5q) clones (Fig. 1). Specific genes that may be involved include: EGR1, a zinc finger transcription factor gene that regulates the expression of several known tumor suppressor genes (e.g., TP53, CDKN1A/p21, TGFB, and PTEN) and reduces proliferation [29]; APC, a negative regulator of beta catenin function which itself is closely involved in stem cell self-renewal and hematologic malignancy [30, 31]; and SPARC, a tumor suppressor gene that regulates extracellular interactions and is known to have anti-angiogenic, antiproliferative, and anti-adhesive properties [32].
In addition, mice that are haploinsufficient for DIAPH1 display myelodysplastic features with hypercellular bone marrow [33]. DIAPH1 is located just outside the CDR, has been shown to regulate the dynamics of the actin cytoskeleton, which is essential for the coordination of cell division and other cellular processes. Another notable gene candidate that is likely to be involved in del(5q) clonal dominance is NPM-1, a tumor suppressor gene that is frequently mutated in patients with AML and high-risk MDS [34].
The molecular basis for heterogeneity of del(5q) MDS: molecular defects outside of chromosome 5q
Despite common pathophysiological features due to haploinsufficiency of chromosome 5q, del(5q) MDS is actually a highly heterogeneous disease with variable prognosis. Several retrospective analyses of patients with del(5q) MDS have shown that the existence of additional karyotypic abnormalities is strongly associated with poorer overall survival (OS) and greater risk of progression to AML [3, 35]. In the largest study to date, in 541 patients with del(5q) MDS, Mallo et al. demonstrated that OS was significantly reduced in patients with ≥2 additional karyotypic abnormalities vs patients with ≤1 additional karyotypic abnormality [3]. Furthermore, the presence of any additional karyotypic abnormality increased the risk of progression to AML compared with isolated del(5q) MDS. The most common single additional aberrations observed were del(12p), trisomy 21, trisomy 8 and del(20q). These observations highlight the importance of full karyotypic analysis for patients with suspected del(5q) MDS, both in terms of accurate prognostication and selection of appropriate treatment strategy.
In addition to karyotypic abnormalities, the existence of certain point mutations can also have a profound impact on prognosis in patients with del(5q) MDS. Notably, mutations in TP53 (the gene that encodes p53) are frequently observed in patients with complex karyotypes involving del(5q) [36–38]. It has long been recognized that the presence of TP53 mutations has a negative impact on prognosis that is independent of International Prognostic Scoring System (IPSS) score [39]. Moreover, recent technological advances have facilitated the screening of patients for TP53 mutations. By using next-generation sequencing, a technique that can detect mutations present in a small proportion of cells, Jadersten et al. detected TP53 mutations in ∼20 % of patients with lower-risk del(5q) MDS, although notably, the majority of patients did not have a complex karyotype. The presence of TP53 mutations predicted progression to AML and the proportion of cells carrying mutations increased with disease progression [40]. As TP53 mutations were generally detectable at an early stage of disease, the authors concluded that TP53 should be routinely analyzed, either by immunohistochemistry or next-generation sequencing during initial patient assessments [40]. Indeed, as the molecular pathogenesis of MDS is further dissected and emerging genomic technologies such as next-generation sequencing and gene expression microarrays become more accessible, it is likely that they will become increasingly important in assessing prognosis in patients with del(5q) MDS.
Other prognostic indicators that must be taken into consideration include bone marrow blast count ≥5 % [3], dysgranulopoiesis [4], transfusion need at presentation [4], and platelet count [3]. Another factor that confers poor prognosis in patients with del(5q) MDS is a BM blast count of ≥5 %. The World Health Organization (WHO) definition of del(5q) MDS specifies a blast count of <5 % [41]. However, ∼40 % of patients with del(5q) MDS have a higher blast count than this [3]. Multivariate analysis has indicated that a blast count of ≥5 % is associated with poor survival and increased risk of progression to AML [3]. It is essential, therefore, that BM biopsies are taken from patients with del(5q) MDS in order to allow an accurate diagnosis and prognosis.
Molecular and cellular mechanisms of sensitivity to lenalidomide in del(5q) MDS patients
Early clinical trial data demonstrated that patients with del(5q) MDS were particularly sensitive to lenalidomide; in the MDS-001 trial, a phase I–II trial of lenalidomide in 43 patients with both del(5q) and non-del(5q) MDS, 10/12 (83 %) patients with a del(5q) aberration achieved an erythroid response compared with 13/23 (57 %) patients with a normal karyotype and 1/8 (12 %) patients with other cytogenetic abnormalities [10]. Erythroid responses were also high in subsequent trials; 67 % of del(5q) MDS patients treated with lenalidomide became RBC-TI in the single-arm phase II MDS-003 study; in the placebo-controlled phase III MDS-004 study 56 % of patients who received lenalidomide 10 mg became RBC-TI [9, 11].
In support of these results, emerging data have provided insights into why lenalidomide displays such remarkable efficacy in patients with del(5q) MDS.
Evidence that lenalidomide directly targets the del(5q) clone
The sensitivity of del(5q) MDS cells to lenalidomide has been demonstrated in vitro. Pellagatti et al. isolated del(5q) and normal CD34+ cells from patients with del(5q) MDS and cultured them in the presence or absence of lenalidomide. After 14 days, lenalidomide inhibited del(5q) cell growth but had no effect on the growth of normal cells [32]. In a separate study, Wei et al. demonstrated that lenalidomide induced a concentration-dependent increase in apoptosis in del(5q) CD34+ cells isolated from patients with AML that evolved from MDS. In contrast, lenalidomide had no effect on apoptosis in CD34+ cells with a normal karyotype [25]. The sensitivity of del(5q) cells to lenalidomide has also been demonstrated in vivo. As part of the MDS-001 trial, List et al. analyzed trephine BM biopsy specimens from 18 patients for changes in BM microvessel density and apoptotic index following treatment with lenalidomide. Patients with del(5q) MDS who achieved a major erythroid response displayed increased apoptosis and reduced microvessel density compared with patients who did not respond [42]. In a study by Tehranchi et al., the cytogenetics of hematologic stem and progenitor cell populations in eight RBC-TD del(5q) MDS patients was evaluated, before and during treatment with lenalidomide [43]. All seven patients that became transfusion-independent during treatment showed cytogenetic responses by conventional cytogenetics, and a dramatic reduction in the proportion of CD34+/CD38+ progenitor cells with the 5q deletion. Notably, in three patients, no CD34+/CD38+ progenitors with the 5q deletion were identified at remission. In contrast, the one patient who did not become transfusion-independent did not achieve a cytogenetic response in either the hematologic stem cell or progenitor cell populations. Together, these findings strongly suggest that lenalidomide is particularly cytotoxic to del(5q) progenitor cells, while having negligible effects on normal progenitors.
Although lenalidomide can target and reduce del(5q) clones, it is not a curative treatment; approximately 50 % of patients have clinical and cytogenetic relapse within 2–3 years of treatment [10, 11]. The Tehranchi et al. study demonstrated that although the majority of del(5q) clones are removed from the BM progenitor compartment, some lenalidomide-resistant del(5q) cells may persist in the stem cell compartment due to their quiescent state and/or high expression of multidrug resistance genes [43]. In patients who relapse, it is thought that these resistant stem cells expand to populate the progenitor compartment. The precise molecular mechanism(s) underlying acquired resistance to lenalidomide has not been fully elucidated, but seems to be associated with the acquisition of additional cytogenetic aberrations in re-emergent del(5q) clones (clonal evolution) [43]. It is very important, therefore, to monitor cytogenetic response in patients treated with lenalidomide in order to detect early evidence of disease progression [44]. At the moment, it is recommended that follow-up karyotyping should be undertaken by standard metaphase cytogenetics [44]. However, emerging techniques, such as fluorescence in situ hybridization on CD34+ peripheral blood cells [45], may provide supplementary and more convenient approaches for monitoring of patients in the future.
Impact of lenalidomide on haploinsufficient genes and their pathways in the del(5q) clone
As discussed above, a key effector of hypoplastic anemia in patients with del(5q) MDS is thought to be impaired ribosome biosynthesis that leads to p53-mediated apoptosis of erythroid cells carrying the del(5q) aberration. Central to this phenomenon is the p53 downregulator, MDM2, whose degradation is triggered by free ribosomal proteins [24]. Recent data have demonstrated that lenalidomide stabilizes MDM2, thereby accelerating p53 degradation. The molecular basis of this phenomenon has been elucidated based on an elaborate series of experiments undertaken by Wei et al. In an in vitro assay, it was demonstrated that lenalidomide dose-dependently inhibited CDC25C and PP2A which, in turn, suppressed MDM2 auto-ubiquitinylation and subsequent degradation [25]. Further experiments demonstrated that the haploinsufficiency of CDC25C and PP2A in del(5q) cells is important to confer sensitivity to lenalidomide. In cultured non-del(5q) BM cells, lenalidomide induced statistically significant apoptotic activity. However, when CDC25C or PP2A expression was reduced by RNAi (mimicking their haploinsufficiency) lenalidomide had a greater apoptotic effect [25]. Therefore, reduced expression of key negative regulators of MDM2 in del(5q) MDS cells leads to increased sensitivity to lenalidomide.
As well as mediating sensitivity to lenalidomide in del(5q) cells, experimental evidence suggests that PP2A and CDC25C are integral to acquired resistance to lenalidomide therapy. For instance, forced overexpression of PP2A in cells promotes drug resistance [24]. Moreover, sequential BM specimens from patients who become resistant to lenalidomide indicate that resistance coincides with overexpression of PP2A and restored accumulation of p53 in erythroid precursors [24].
Experiments in primary del(5q) CD34+ cells have demonstrated that lenalidomide can also upregulate haploinsufficient miRNAs implicated in the pathogenesis of the disease, such as miRNA-143 and miRNA-145 [46] Venner et al. examined the effect of lenalidomide on pre-treatment CD34+ cells from ten patients with del(5q) MDS. Statistically significant increases in miRNA-143 (1.8-fold; P = 0.02) and miRNA-145 (1.9-fold; P = 0.01) expression were observed in del(5q) cells compared with wild-type CD34+ cells; changes in expression correlated with sensitivity to lenalidomide [46]. Furthermore, RNAi knockdown of miRNA-143 and miRNA-145 in healthy cord blood CD34+ cells (thus mimicking haploinsufficiency) conferred sensitivity to lenalidomide. These data suggest that the sensitivity of del(5q) cells to lenalidomide is at least partially explained by normalization of expression of haploinsufficient miRNAs.
Lenalidomide also appears to normalize the expression of SPARC. Pellagati et al. assessed global gene expression in CD34+ cells isolated from patients with del(5q) MDS that were cultured for 5 days with or without lenalidomide [32]. After incubation with lenalidomide, 98 % of cells were still del(5q) positive; therefore, any changes in mRNA levels were likely to reflect a direct effect of lenalidomide on gene expression, rather than an indirect effect due to clonal suppression of del(5q) cells. Out of the 41 genes within the distal CDR that were analyzed, only SPARC was upregulated by lenalidomide (demonstrating a fourfold increase in expression), although a number of genes outside the CDR were also upregulated, including several genes involved in extracellular matrix interactions. Venner et al. also observed an increase in SPARC mRNA levels when CD34+ cells from ten del(5q) MDS patients were treated ex vivo with lenalidomide [46]. These observations suggest that lenalidomide may normalize SPARC expression in patients with del(5q) MDS [46]; increased SPARC would reduce cell–cell adhesion and proliferation of del(5q) clones by normalizing extracellular matrix interactions.
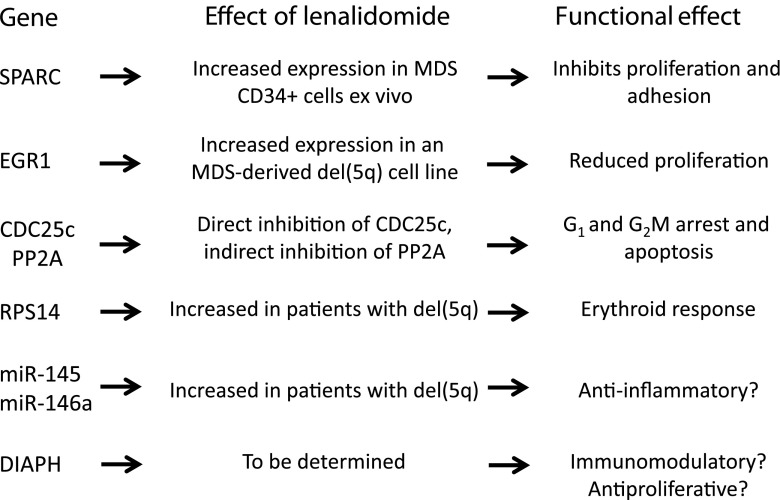
Collectively, these studies demonstrate that lenalidomide can effectively reduce del(5q) progenitors in the BM by specifically targeting haploinsufficient genes and their pathways (Fig. 2). This MoA is reflected in the high rates of cytogenetic response (50–83 %) in patients with del(5q) MDS treated with lenalidomide [9–11].
Fig. 2.
The effect of lenalidomide on haploinsufficient genes and their pathways in del(5q) clones [25, 29, 32, 69–71] del(5q) deletion of the long arm of chromosome 5
Impact of lenalidomide on erythropoiesis and BM function in patients with del(5q) MDS
Clinical data have demonstrated that lenalidomide often has a potent myeloablative effect during the early stages of treatment which coincides with the effective elimination of del(5q) clonal myeloid progenitors [9]. Consequently, cytopenic adverse events (AEs) are to be expected in patients during early cycles (discussed below). Following reduction of the del(5q) clone, it is thought that a small pre-existing pool of normal hematopoietic stem cells repopulate the myeloid and erythroid progenitor compartments [43]. Along with reducing the del(5q) clone, there is evidence to suggest that lenalidomide can accelerate this repopulation process owing to a range of beneficial effects on BM function.
In a recent study, Ximeri et al. collected BM cells from ten patients with del(5q) MDS before and after treatment with lenalidomide and assessed changes in erythropoiesis and clonogenic potential [47]. Purified CD34+ cells, early erythroid precursors, and mature erythroid precursors were sorted and quantified by flow cytometric analysis. Unsurprisingly, the percentage of CD34+ cells in the total progenitor population was significantly decreased following lenalidomide treatment, reflecting specific targeting of del(5q) progenitor cells. In contrast, the percentage of early and mature precursors was significantly increased following lenalidomide treatment; these normal cells were less resistant to apoptosis than del(5q) cells. Lenalidomide improved the clonogenic potential of normal BM progenitor cells in erythroid, myeloid, and megakaryocytic lineages. In a different study of cultured non-del(5q) CD34+ cells, lenalidomide induced expression of a number of genes known to promote erythroid differentiation including IL1R2, FLT3, and CD163 [48]. These studies suggest that lenalidomide not only selectively destroys del(5q) cells but also promotes repopulation of the BM with normal erythroid precursors. Moreover, lenalidomide seems to correct hypercellularity of the BM by restoring normal apoptotic potential of erythroid precursor cells [47].
Lenalidomide also seems to improve the hematopoiesis-supporting capacity of BM stroma. Ximeri et al. assessed the impact of lenalidomide on the BM microenviroment in long-term cultures of BM cells (LTBMCs) isolated from patients with del(5q) MDS treated with lenalidomide [47]. LTBMCs are an established in vitro model of BM function which involves growing hematopoietic and mesenchymal-derived cells in layers; clonogenicity of the LTBMCs is reflected by the number of colony-forming cells (CFCs) released into the media. In this study, the number of CFCs in the media was significantly greater in LTBMCs from lenalidomide-treated patients than untreated patients [47]. Improved BM function was reflected by an increase in the adhesive properties of CD34+ cells and stromal cells. Lenalidomide increased cell surface expression of adhesion molecules on CD34+ cells including CD11a, CD48, and CxCR4; lenalidomide also increased release of pro-adhesive cytokines from stromal cells in LTBMCs including ICAM-1 and SDF-1α (the natural ligands for CD11a and CXCR4, respectively).
Impact of lenalidomide on immune function and angiogenesis in patients with del(5q) MDS
In many cancers, including myeloid malignancies, impaired immune function is thought to contribute to disease progression by allowing tumor cells to evade the immune system [49]. Specific immune abnormalities implicated in the pathogenesis of MDS include defective synapse interactions between antigen-presenting cells and T cells, diminished natural killer cell activity, and alteration of cytokines in the BM microenvironment [49].
Experimental evidence suggests that lenalidomide corrects immune defects in patients with MDS. For example, in cultured CD34+ cells from patients with del(5q) MDS, treatment with lenalidomide increased the proportion of activated peripheral blood T cells compared with untreated cells [47]. Furthermore, although not specifically assessed in MDS, lenalidomide can correct defective synapse interactions between antigen-presenting cells and T cells in chronic lymphocytic leukemia cells by modifying T cell actin dynamics [50]. Lenalidomide also reduces the expression of proinflammatory cytokines (e.g., TNF-α, IL-1β, and IL-6) and increases anti-inflammatory cytokines (e.g., IL-10) in peripheral blood mononuclear cells [51]. These observations are of interest because IL-6, in particular, is a key effector of dysregulated innate immune pathways in del(5q) MDS, due to haploinsufficiency of miRNA-145 and miRNA-146a [26].
Angiogenesis also plays an important role in the progression of hematopoietic malignancies. Lenalidomide is known to have an anti-angiogenic effect in patients with del(5q) MDS [52–55]. Reduced BM vascularity in del(5q) MDS patients treated with lenalidomide correlated with clinical response and reduced risk of disease progression [42]. Loss of the anti-angiogenic effect predicted disease progression.
Linking the mechanism of action of lenalidomide to observed clinical outcomes
Clinical experience with lenalidomide in del(5q) MDS has recently been reviewed in detail elsewhere [14, 56]. In this section, we briefly discuss key clinical findings from the MDS-003 and MDS-004 trials in the context of recent developments in the understanding of the MoA of lenalidomide and suggest a model linking its molecular and cellular actions with clinical outcomes (Fig. 3). MDS-003 was a single-arm phase II study and MDS-004 was a placebo-controlled phase III study. Both trials assessed the safety and efficacy of lenalidomide in patients with lower-risk RBC-TD del(5q) MDS [9, 11].
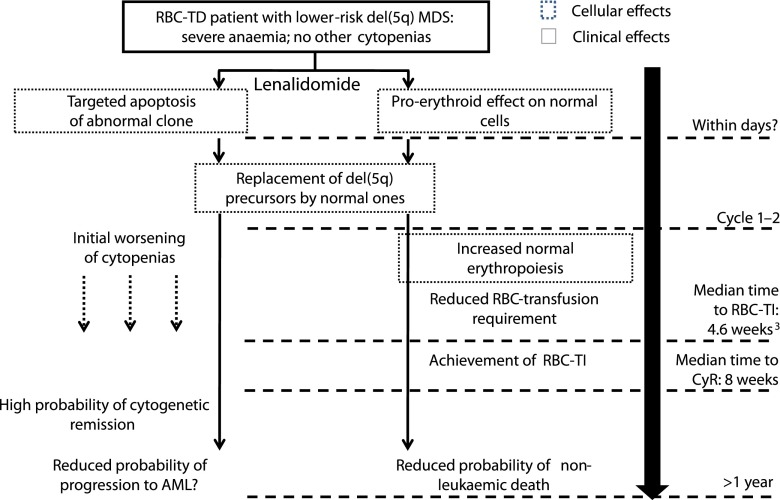
Fig. 3.
A model linking MoA of lenalidomide with clinical data. In patients with del(5q) MDS, lenalidomide has a direct cytotoxic effect on abnormal del(5q) clones, by targeting haploinsufficient genes and their pathways. The high probability of cytogenetic remission in patients given lenalidomide reflects this potent cytotoxic effect on del(5q) cells. Because of the clonal nature of the disease, lenalidomide often has a myeloablative effect during early cycles of treatment resulting in high frequencies of cytopenic AEs [9, 11]. These AEs are transient and usually manageable on-treatment by dose reductions, delays, or concomitant myeloid growth factors. Once the del(5q) clone has effectively been eliminated, the BM is repopulated with lineages derived from a small number of normal CD34+ that are not eliminated by lenalidomide. A rapid return to normal hematopoiesis is facilitated by pleiotropic beneficial effects of lenalidomide on BM function [11, 47, 49]. The replacement of del(5q) precursors with normal ones leads to high rates of durable RBC-TI, usually with 4–5 weeks [9, 11]. Achievement of RBC-TI is associated with significant benefits in terms of improved overall survival, reduced probability of non-leukemic death, and improved QoL [9, 11]. Cytogenetic response is associated with reduced progression to AML [14]. However, del(5q) MDS is a heterogeneous disease, and some patients, including those treated with lenalidomide, remain at risk of AML progression due to clonal evolution [3, 4]. Therefore, patients must be carefully assessed for their probability of disease progression prior to initiating treatment. Also, regular monitoring of cytogenetic response in patients treated with lenalidomide is essential [44]. MoA mechanism of action, del(5q) deletion of the long arm of chromosome 5, MDS myelodysplastic syndromes, AE adverse event, BM bone marrow, RBC-TI red blood cell transfusion independent, QoL quality of life, AML acute myeloid leukemia
Erythroid and cytogenetic responses in patients with del(5q) MDS treated with lenalidomide
In clinical trials, treatment with lenalidomide has been associated with high erythroid response rates in patients with RBC-TD del(5q) MDS. In MDS-003 (N = 148), 67 % of patients became RBC-TI by week 24 of treatment [11]. In MDS-004 (N = 205), 56 % of the 41 patients who initially received 10 mg/day of lenalidomide became RBC-TI for ≥26 weeks [9]. The observed high response rates in patients with del(5q) MDS are of major clinical relevance because chronic anemia impacts quality of life (QoL) and is associated with hypertrophic cardiac remodeling over time [11]. Moreover, anemia and RBC-TD are both independently correlated with reduced survival [57]. Erythroid responses with lenalidomide tended to be durable; in MDS-003, responses lasted for >1 year in 62 % of patients [14], in MDS-004, median duration of response had not been reached after a median follow-up of 1.5 years [9]. Responses were generally rapid, with median times to response of 4.6 and 4.1 weeks in MDS-003 and MDS-004, respectively. Erythroid responses correlated with cytogenetic responses, which occurred in 73 % of 85 patients in MDS-003 evaluable for cytogenetic response and 50 % of 69 patients receiving lenalidomide 10 mg in MDS-004 [9, 11]. Response to lenalidomide in MDS-004 was also associated with clinically meaningful improvements in health-related QoL [9].
The observed rapid and durable responses to lenalidomide in patients with del(5q) MDS in clinical trials reflect: (1) direct cytotoxicity against the del(5q) clone, leading to high cytogenetic response rates; (2) the ability of lenalidomide to stimulate normal erythropoiesis and BM function, leading to a repopulation of BM with lineages derived from normal CD34+ cells.
Characteristic AE profile in patients with del(5q) MDS treated with lenalidomide
In both MDS-003 and MDS-004, there was a high incidence of cytopenias that occurred soon after initiation of lenalidomide treatment. In MDS-003, moderate-to-severe thrombocytopenia and neutropenia occurred in 44 and 55 % of patients, respectively. Approximately two thirds of hematologic grade 3–4 AEs occurred in the first 8 weeks of treatment. Importantly, most cases could be effectively managed with dose adjustments, dose delays, and/or myeloid growth factors; 20 % of patients discontinued lenalidomide due to AEs. In MDS-004, thrombocytopenia and neutropenia occurred in 41 and 75 % of patients treated with 10 mg lenalidomide, respectively. Dose reduction was required in 58 % of patients, and discontinuation was necessary in 9 % of patients. Apart from hematologic AEs, lenalidomide was generally well tolerated.
The high incidence of early hematologic AEs likely reflects rapid elimination of del(5q) progenitors, which disrupts hematopoiesis before the BM has a chance to recover and repopulate with normal progenitor cells and derived lineages. Indeed, the development of early hematologic AEs seems to predict subsequent clinical response, probably because they signify the effective elimination of the oncogenic clone. For example, in MDS-003, development of thrombocytopenia or neutropenia correlated with attainment of RBC-TI [58]. Notably, patients with del(5q) MDS have higher rates of cytopenias than non-del(5q) patients, suggesting that effective clonal suppression may be the predominant explanation for hematologic AEs during early treatment cycles.
The impact of hematologic and cytogenetic responses on survival and disease progression in patients with del(5q) MDS treated with lenalidomide
Response to lenalidomide is associated with enduring improvements in survival and reduced risk of progression to AML. In MDS-004, patients treated with lenalidomide who achieved RBC-TI had significantly improved OS, and reduced risk of progression to AML compared with patients who did not respond [9]. There was no significant difference in OS or progression to AML in patients treated with lenalidomide and those originally assigned placebo; however, these observations may be confounded by the fact that patients given placebo could cross over to receive lenalidomide after 16 weeks. Furthermore, in MDS-004, higher doses of lenalidomide were associated with more hematologic and cytogenetic responses. RBC-TI was achieved by 56.1, 42.6, and 5.9 % of patients treated with lenalidomide 10 mg, 5 mg, and placebo, respectively (P < 0.001). Cytogenetic response rates were 50.0 and 25.0 % in the lenalidomide 10 and 5 mg cohorts, respectively (P = 0.066). In addition, median OS was 42.4, ≥35.5, and 44.5 months in the placebo, lenalidomide 5 and 10 mg groups, respectively. After a recent follow-up study, the 4-year OS rates were 50 % in patients treated with lenalidomide (10 mg) and 44 % in patients who were originally assigned to placebo but crossed over to lenalidomide (median follow-up, 3.1 and 3.8 years, respectively) [59]. In MDS-003, the predicted 10-year OS was 78 % for cytogenetic responders and 4 % for non-responders; the predicted AML progression rate in cytogenetic responders and non-responders was 15 and 67 %, respectively [14].
The risk of AML progression in patients with del(5q) MDS
There has been some concern that patients with del(5q) MDS who do not respond to lenalidomide treatment may have an enhanced risk of progression to AML. Gohring et al. undertook a long-term follow-up (median follow-up of 40 months) of 42 patients treated with lenalidomide [60]. A total of 36 % of patients progressed to AML. Strikingly, 87 % of patients who progressed acquired additional chromosomal aberrations. The risk of progression was higher in patients who did not achieve a cytogenetic response; cumulative incidence of AML after 5 years was 21 and 60 % in cytogenetic responders and non-responders, respectively [60]. These observations further reinforce the hypothesis that clonal evolution is the driving force of leukemic transformation in patients with del(5q) MDS. Regular cytogenetic assessment of patients treated with lenalidomide is a prudent approach to monitor for clonal evolution [60].
Concerns about the possibility of leukemic progression with lenalidomide have prompted studies of AML progression rates in del(5q) MDS patients in general. For example, in a retrospective multicenter analysis of 318 untreated lower-risk del(5q) MDS patients from the Dusseldorf registry, Germing et al. reported that 12.6 % of patients progressed to AML, and cumulative progression rates at 2 and 5 years were 4.9 and 17.6 %, respectively [61]. However, the risk of progression was very variable reflecting the heterogeneity of the disease. Risk factors that were independently associated with disease progression included blast count >5 %, RBC-TD at diagnosis, and a WHO Prognostic Scoring System score of intermediate, high, or very high. RBC-TD at diagnosis was a particularly important parameter that indicated increased risk of progression. Progression within the first 2 years was mainly observed in RBC-TD patients; the risk of progression at 2 years was 11 and 2 % in RBC-TD and RBC-TI patients, respectively. It seems, therefore, that some patients with del(5q) MDS may be at high risk of AML progression, reflecting the complexity of the biology of the disease.
As a result of this finding, several retrospective studies of AML progression rates have been undertaken in treated vs untreated patients. Ades et al. assessed AML progression rates in 95 patients with RBC-TD del(5q) MDS treated with lenalidomide [62]. Progression rates were compared with a historical cohort of 99 patients who had never received lenalidomide using a propensity score approach, a statistical methodology that can compare treatment effects from independent observational studies in an unbiased manner [63]. In this study, the 4-year cumulative incidence of AML was 9.0 % in the lenalidomide group and 15.8 % in the untreated group (P = 0.16). Median survival from diagnosis was 150 and 78 months in the two groups, respectively (P = 0.06). Similar results were reported in a separate analysis by Kuendgen et al. [64]. In this study, del(5q) MDS patients treated with lenalidomide in the MDS-003 and MDS-004 trials were compared with del(5q) patients from the Dusseldorf MDS registry who were never exposed to the drug, After a median follow-up of 4.3 years (lenalidomide-treated patients) and 4.6 years (untreated patients), there was no significant difference in 2-year cumulative AML progression risk between the two groups (6.9 vs 12.1 %, P = 0.930). Conversely, treatment with lenalidomide was associated with improved survival; 2-year OS probabilities were 89.9 and 74.4 %, respectively [64]. Based on these data, lenalidomide does not appear to increase the risk of progression to AML in patients with del(5q) MDS.
Emerging data indicate that AML progression rates in patients treated with lenalidomide are not drug-related, but are associated with additional risk factors [65]. In a combined analysis of MDS-003 and MDS-004, both transfusion burden and karyotype complexity were associated with increased risk of AML progression. For instance, patients with del(5q) MDS and ≥2 additional cytogenetic abnormalities were at increased risk of progression to AML vs patients with isolated del(5q) MDS [65].
Summary and conclusions
In patients with del(5q) MDS, lenalidomide induces durable hematologic responses through a direct cytotoxic effect to abnormal del(5q) clones, by targeting haploinsufficient genes and their pathways. The high probability of cytogenetic remission in patients given lenalidomide reflects this potent cytotoxic effect on del(5q) cells. It also has pleiotropic effects on hematopoiesis and the BM microenvironment. Myelosuppression during early treatment cycles should be expected with lenalidomide, which reflects the time between elimination of the del(5q) clone and repopulation of the BM with normal precursors. Effective reduction of the del(5q) clone has a positive impact on clinical outcome; achievement of cytogenetic response and RBC-TI are associated with reduced risk of AML progression and improved survival. Del(5q) MDS is a highly heterogeneous disease, and a number of additional risk factors have been identified that predict disease progression. Patients treated with lenalidomide should be routinely monitored, with particular attention given to possible clonal evolution, so that changes in treatment strategy may be considered prior to clinical progression.
Acknowledgments
All authors (AG, GJM, PF, UG, AL, and KJM) were responsible for all content and editorial decisions throughout the development of this manuscript. The authors would like to thank Andrew Brittain of FireKite for medical writing assistance, which was financially supported by Celgene.
Conflict of interest
Aristoteles Giagounidis has received lecture honoraria from Celgene.
Ghulam J. Mufti has received lecture honoraria and research support from Celgene.
Pierre Fenaux has received lecture honoraria from Celgene.
Ulrich Germing has received lecture honoraria from Celgene.
Alan List has received lecture honoraria and research support from Celgene.
Kyle J. MacBeth is a full-time employee of Celgene.
References
- 1.Malcovati L, Nimer SD. Myelodysplastic syndromes: diagnosis and staging. Cancer Control. 2008;15(Suppl):4–13. doi: 10.1177/107327480801504s02. [DOI] [PubMed] [Google Scholar]
- 2.Haase D, Germing U, Schanz J, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110:4385–4395. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 3.Mallo M, Cervera J, Schanz J, et al. Impact of adjunct cytogenetic abnormalities for prognostic stratification in patients with myelodysplastic syndrome and deletion 5q. Leukemia. 2011;25:110–120. doi: 10.1038/leu.2010.231. [DOI] [PubMed] [Google Scholar]
- 4.Patnaik MM, Lasho TL, Finke CM, et al. WHO-defined ‘myelodysplastic syndrome with isolated del(5q)’ in 88 consecutive patients: survival data, leukemic transformation rates and prevalence of JAK2, MPL and IDH mutations. Leukemia. 2010;24:1283–1289. doi: 10.1038/leu.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg SL, Chen E, Corral M, et al. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. J Clin Oncol. 2010;28:2847–2852. doi: 10.1200/JCO.2009.25.2395. [DOI] [PubMed] [Google Scholar]
- 6.Malcovati L, la Porta MG, Cazzola M. Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica. 2006;91:1588–1590. [PubMed] [Google Scholar]
- 7.Oliva EN, Dimitrov BD, Benedetto F, D’Angelo A, Nobile F. Hemoglobin level threshold for cardiac remodeling and quality of life in myelodysplastic syndrome. Leuk Res. 2005;29:1217–1219. doi: 10.1016/j.leukres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Kelaidi C, Park S, Brechignac S, et al. Treatment of myelodysplastic syndromes with 5q deletion before the lenalidomide era; the GFM experience with EPO and thalidomide. Leuk Res. 2008;32:1049–1053. doi: 10.1016/j.leukres.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 9.Fenaux P, Giagounidis A, Selleslag D, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with low-/intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118:3765–3776. doi: 10.1182/blood-2011-01-330126. [DOI] [PubMed] [Google Scholar]
- 10.List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 11.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 12.Horrigan SK, Westbrook CA, Kim AH, Banerjee M, Stock W, Larson RA. Polymerase chain reaction-based diagnosis of del (5q) in acute myeloid leukemia and myelodysplastic syndrome identifies a minimal deletion interval. Blood. 1996;88:2665–2670. [PubMed] [Google Scholar]
- 13.Boultwood J, Fidler C, Strickson AJ, et al. Narrowing and genomic annotation of the commonly deleted region of the 5q- syndrome. Blood. 2002;99:4638–4641. doi: 10.1182/blood.V99.12.4638. [DOI] [PubMed] [Google Scholar]
- 14.Padron E, Komrokji R, List AF. Biology and treatment of the 5q- syndrome. Expert Rev Hematol. 2011;4:61–69. doi: 10.1586/ehm.11.2. [DOI] [PubMed] [Google Scholar]
- 15.Jerez A, Gondek LP, Jankowska AM, et al. Topography, clinical, and genomic correlates of 5q myeloid malignancies revisited. J Clin Oncol. 2012;30:1343–1349. doi: 10.1200/JCO.2011.36.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boultwood J, Pellagatti A, Cattan H, et al. Gene expression profiling of CD34+ cells in patients with the 5q- syndrome. Br J Haematol. 2007;139:578–589. doi: 10.1111/j.1365-2141.2007.06833.x. [DOI] [PubMed] [Google Scholar]
- 17.Boultwood J, Pellagatti A, McKenzie AN, Wainscoat JS. Advances in the 5q- syndrome. Blood. 2010;116:5803–5811. doi: 10.1182/blood-2010-04-273771. [DOI] [PubMed] [Google Scholar]
- 18.Gazda HT, Grabowska A, Merida-Long LB, et al. Ribosomal protein S24 gene is mutated in Diamond-Blackfan anemia. Am J Hum Genet. 2006;79:1110–1118. doi: 10.1086/510020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebert BL, Pretz J, Bosco J, et al. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature. 2008;451:335–339. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barlow JL, Drynan LF, Hewett DR, et al. A p53-dependent mechanism underlies macrocytic anemia in a mouse model of human 5q- syndrome. Nat Med. 2010;16:59–66. doi: 10.1038/nm.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutt S, Narla A, Lin K, et al. Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood. 2011;117:2567–2576. doi: 10.1182/blood-2010-07-295238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellagatti A, Marafioti T, Paterson JC, et al. Induction of p53 and up-regulation of the p53 pathway in the human 5q- syndrome. Blood. 2010;115:2721–2723. doi: 10.1182/blood-2009-12-259705. [DOI] [PubMed] [Google Scholar]
- 23.Narla A, Ebert BL. Ribosomopathies: human disorders of ribosome dysfunction. Blood. 2010;115:3196–3205. doi: 10.1182/blood-2009-10-178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei S, Chen X, McGraw K, et al. Lenalidomide promotes p53 degradation by inhibiting MDM2 auto-ubiquitination in myelodysplastic syndrome with chromosome 5q deletion. Oncogene. 2012;32(9):1110–1120. doi: 10.1038/onc.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei S, Chen X, Rocha K, et al. A critical role for phosphatase haplodeficiency in the selective suppression of deletion 5q MDS by lenalidomide. Proc Natl Acad Sci USA. 2009;106:12974–12979. doi: 10.1073/pnas.0811267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starczynowski DT, Kuchenbauer F, Argiropoulos B, et al. Identification of miR-145 and miR-146a as mediators of the 5q- syndrome phenotype. Nat Med. 2010;16:49–58. doi: 10.1038/nm.2054. [DOI] [PubMed] [Google Scholar]
- 27.Jadersten M. Pathophysiology and treatment of the myelodysplastic syndrome with isolated 5q deletion. Haematologica. 2010;95:348–351. doi: 10.3324/haematol.2009.019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson L, strand-Grundstrom I, Arvidsson I, et al. Isolation and characterization of hematopoietic progenitor/stem cells in 5q-deleted myelodysplastic syndromes: evidence for involvement at the hematopoietic stem cell level. Blood. 2000;96:2012–2021. [PubMed] [Google Scholar]
- 29.Matsuoka A, Tochigi A, Kishimoto M, et al. Lenalidomide induces cell death in an MDS-derived cell line with deletion of chromosome 5q by inhibition of cytokinesis. Leukemia. 2010;24:748–755. doi: 10.1038/leu.2009.296. [DOI] [PubMed] [Google Scholar]
- 30.Lane SW, Sykes SM, Al-Shahrour F, et al. The Apc(min) mouse has altered hematopoietic stem cell function and provides a model for MPD/MDS. Blood. 2010;115:3489–3497. doi: 10.1182/blood-2009-11-251728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Fernald AA, Anastasi J, Le Beau MM, Qian Z. Haploinsufficiency of Apc leads to ineffective hematopoiesis. Blood. 2010;115:3481–3488. doi: 10.1182/blood-2009-11-251835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellagatti A, Jadersten M, Forsblom AM, et al. Lenalidomide inhibits the malignant clone and up-regulates the SPARC gene mapping to the commonly deleted region in 5q- syndrome patients. Proc Natl Acad Sci USA. 2007;104:11406–11411. doi: 10.1073/pnas.0610477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng J, Kitchen SM, West RA, Sigler R, Eisenmann KM, Alberts AS. Myeloproliferative defects following targeting of the Drf1 gene encoding the mammalian diaphanous related formin mDia1. Cancer Res. 2007;67:7565–7571. doi: 10.1158/0008-5472.CAN-07-1467. [DOI] [PubMed] [Google Scholar]
- 34.Sportoletti P, Grisendi S, Majid SM, et al. Npm1 is a haploinsufficient suppressor of myeloid and lymphoid malignancies in the mouse. Blood. 2008;111:3859–3862. doi: 10.1182/blood-2007-06-098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kantarjian H, O’Brien S, Ravandi F, et al. The heterogeneous prognosis of patients with myelodysplastic syndrome and chromosome 5 abnormalities: how does it relate to the original lenalidomide experience in MDS? Cancer. 2009;115:5202–5209. doi: 10.1002/cncr.24575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christiansen DH, Andersen MK, Pedersen-Bjergaard J. Mutations with loss of heterozygosity of p53 are common in therapy-related myelodysplasia and acute myeloid leukemia after exposure to alkylating agents and significantly associated with deletion or loss of 5q, a complex karyotype, and a poor prognosis. J Clin Oncol. 2001;19:1405–1413. doi: 10.1200/JCO.2001.19.5.1405. [DOI] [PubMed] [Google Scholar]
- 37.Kulasekararaj AG, Smith AE, Mian SA, et al. TP53 mutations in myelodysplastic syndrome are strongly correlated with aberrations of chromosome 5, and correlate with adverse prognosis. Br J Haematol. 2013;160:660–672. doi: 10.1111/bjh.12203. [DOI] [PubMed] [Google Scholar]
- 38.Kaneko H, Misawa S, Horiike S, Nakai H, Kashima K. TP53 mutations emerge at early phase of myelodysplastic syndrome and are associated with complex chromosomal abnormalities. Blood. 1995;85:2189–2193. [PubMed] [Google Scholar]
- 39.Kita-Sasai Y, Horiike S, Misawa S, et al. International prognostic scoring system and TP53 mutations are independent prognostic indicators for patients with myelodysplastic syndrome. Br J Haematol. 2001;115:309–312. doi: 10.1046/j.1365-2141.2001.03073.x. [DOI] [PubMed] [Google Scholar]
- 40.Jadersten M, Saft L, Smith A, et al. TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol. 2011;29:1971–1979. doi: 10.1200/JCO.2010.31.8576. [DOI] [PubMed] [Google Scholar]
- 41.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 42.List AF, Baker AF, Green S, Bellamy W. Lenalidomide: targeted anemia therapy for myelodysplastic syndromes. Cancer Control. 2006;13(Suppl):4–11. doi: 10.1177/107327480601304s02. [DOI] [PubMed] [Google Scholar]
- 43.Tehranchi R, Woll PS, Anderson K, et al. Persistent malignant stem cells in del(5q) myelodysplasia in remission. N Engl J Med. 2010;363:1025–1037. doi: 10.1056/NEJMoa0912228. [DOI] [PubMed] [Google Scholar]
- 44.Gohring G, Giagounidis A, Busche G, et al. Cytogenetic follow-up by karyotyping and fluorescence in situ hybridization: implications for monitoring patients with myelodysplastic syndrome and deletion 5q treated with lenalidomide. Haematologica. 2011;96:319–322. doi: 10.3324/haematol.2010.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braulke F, Schanz J, Jung K, et al. FISH analysis of circulating CD34+ cells as a new tool for genetic monitoring in MDS: verification of the method and application to 27 MDS patients. Leuk Res. 2010;34:1296–1301. doi: 10.1016/j.leukres.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 46.Venner CP, Wegrzyn WJ, Nevill TJ, et al. Correlation of clinical response and response duration with miR-145 induction by lenalidomide in CD34+ cells from patients with del(5q) myelodysplastic syndrome. Haematologica. 2012;98(3):409–413. doi: 10.3324/haematol.2012.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ximeri M, Galanopoulos A, Klaus M, et al. Effect of lenalidomide therapy on hematopoiesis of patients with myelodysplastic syndrome associated with chromosome 5q deletion. Haematologica. 2010;95:406–414. doi: 10.3324/haematol.2009.010876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narla A, Dutt S, McAuley JR, et al. Dexamethasone and lenalidomide have distinct functional effects on erythropoiesis. Blood. 2011;118:2296–2304. doi: 10.1182/blood-2010-11-318543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heise C, Carter T, Schafer P, Chopra R. Pleiotropic mechanisms of action of lenalidomide efficacy in del(5q) myelodysplastic syndromes. Expert Rev Anticancer Ther. 2010;10:1663–1672. doi: 10.1586/era.10.135. [DOI] [PubMed] [Google Scholar]
- 50.Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T cell immunological synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide. Blood. 2012;120(7):1412–1421. doi: 10.1182/blood-2012-02-411678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163:380–386. [PubMed] [Google Scholar]
- 52.Buesche G, Dieck S, Giagounidis A (2005) Anti-angiogenic in vivo effect of lenalidomide (CC-5013) in myelodysplastic syndrome with del(5q) chromosome abnormality and its relation to the course of disease. Blood 106:(Abstract 372)
- 53.Buesche G, Dieck S, Giagounidis A (2009) Anti-angiogenic in-vivo effect of lenalidomide and its impact on neoplastic and non-neoplastic hematopoiesis in MDS with del(5q) chromosome abnormality. Blood 114:(Abstract 3800)
- 54.Dredge K, Marriott JB, Macdonald CD, et al. Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. Br J Cancer. 2002;87:1166–1172. doi: 10.1038/sj.bjc.6600607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu L, Payvandi F, Wu L, et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res. 2009;77:78–86. doi: 10.1016/j.mvr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Komrokji RS, List AF. Lenalidomide for treatment of myelodysplastic syndromes. Curr Pharm Des. 2012;18:3198–3203. doi: 10.2174/1381612811209023198. [DOI] [PubMed] [Google Scholar]
- 57.Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–7603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 58.Sekeres MA, Maciejewski JP, Giagounidis AA, et al. Relationship of treatment-related cytopenias and response to lenalidomide in patients with lower-risk myelodysplastic syndromes. J Clin Oncol. 2008;26:5943–5949. doi: 10.1200/JCO.2007.15.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hellstrom-Lindberg E, Giagounidis A, Selleslag D, Mittelman M, Muus P, Benettaib B, Fu T, Fenaux P. Update on the safety and long-term outcomes in lenalidomide-treated patients with red blood cell transfusion-dependent low-/int-1-risk myelodysplastic syndromes and del(5q) Haematologica. 2012;97(Suppl 1):358–359. [Google Scholar]
- 60.Gohring G, Giagounidis A, Busche G, et al. Patients with del(5q) MDS who fail to achieve sustained erythroid or cytogenetic remission after treatment with lenalidomide have an increased risk for clonal evolution and AML progression. Ann Hematol. 2010;89:365–374. doi: 10.1007/s00277-009-0846-z. [DOI] [PubMed] [Google Scholar]
- 61.Germing U, Lauseker M, Hildebrandt B, et al. Survival, prognostic factors and rates of leukemic transformation in 381 untreated patients with MDS and del(5q): a multicenter study. Leukemia. 2012;26:1286–1292. doi: 10.1038/leu.2011.391. [DOI] [PubMed] [Google Scholar]
- 62.Ades L, Le BF, Sebert M, et al. Treatment with lenalidomide does not appear to increase the risk of progression in lower risk myelodysplastic syndromes with 5q deletion. A comparative analysis by the Groupe Francophone des Myelodysplasies. Haematologica. 2012;97:213–218. doi: 10.3324/haematol.2011.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(SICI)1097-0258(19981015)17:19<2265::AID-SIM918>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 64.Kuendgen A, Lauseker M, List AF, et al. Lenalidomide does not increase AML progression risk in RBC transfusion-dependent patients with low- or intermediate-1-risk MDS with del(5q): a comparative analysis. Leukemia. 2012;27(5):1072–1079. doi: 10.1038/leu.2012.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giagounidis A, Hellstrom-Lindberg E, Fenaux P, Backstrom J, Fu T, List A (2011) Interaction of karyotype complexity and response on overall survival and AML progression in lenalidomide-treated low-/int-1-risk del(5q) MDS patients. Leuk Res 35 (Suppl 1):s21 (Abstract 0058)
- 66.DeWard AD, Leali K, West RA, Prendergast GC, Alberts AS. Loss of RhoB expression enhances the myelodysplastic phenotype of mammalian diaphanous-related Formin mDia1 knockout mice. PLoS One. 2009;4:e7102. doi: 10.1371/journal.pone.0007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eisenmann KM, Dykema KJ, Matheson SF, et al. 5q- myelodysplastic syndromes: chromosome 5q genes direct a tumor-suppression network sensing actin dynamics. Oncogene. 2009;28:3429–3441. doi: 10.1038/onc.2009.207. [DOI] [PubMed] [Google Scholar]
- 68.Lehmann S, O’Kelly J, Raynaud S, Funk SE, Sage EH, Koeffler HP. Common deleted genes in the 5q- syndrome: thrombocytopenia and reduced erythroid colony formation in SPARC null mice. Leukemia. 2007;21:1931–1936. doi: 10.1038/sj.leu.2404852. [DOI] [PubMed] [Google Scholar]
- 69.Oliva E, Nobile F, Iacopino P, Alimena G, Di Raimondo F, Palumbo G, Lagana C, D’Errigo MD, Latagliata R, Cuzzola M (2010) Increases in miRNA-145 and miRNA-146a expression in patients with IPSS lower-risk MDS and del(5q) treated with lenalidomide. Blood 116:(Abstract 3631)
- 70.Oliva EN, Cuzzola M, Nobile F, et al. Changes in RPS14 expression levels during lenalidomide treatment in low- and intermediate-1-risk myelodysplastic syndromes with chromosome 5q deletion. Eur J Haematol. 2010;85:231–235. doi: 10.1111/j.1600-0609.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 71.Zhang L, Schafer PH, Muller G, Stirling D, Bartlett B (2008) Direct inhibitory effects of lenalidomide on the proliferation and VEGF production of non-Hodgkin lymphoma cells are associated with increased SPARC expression. Blood 112 (Suppl):(Abstract 2612)