The surface of the eye is covered by a protective tear film consisting of an aqueous-mucus layer and a superficial lipid layer, which are vital for light refraction and protection of vision. The aqueous component of the tear film contains electrolytes and a large variety of proteins, peptides and glycoproteins secreted by the lacrimal and meibomian glands, and apical cells of the corneal and conjunctival epithelia (Ohashi, Y. et al., 2006; Ruiz-Ederra, J. et al., 2009). Maintenance of a precise osmotic gradient of electrolytes between the tear film and the ocular surface epithelia is of paramount importance in regulating cell function and homeostasis. An imbalance of electrolytes is a hallmark of many pathologies, including dry eye, a disease affecting between 6 and 43 million people in the United States alone (Gilbard, J.P., 1994; Pflugfelder, S.C., 2011).
Tear film hyperosmolarity results primarily from an increased electrolyte concentration and is regarded as the central mechanism causing ocular surface damage and inflammation, as well as the initiation of compensatory events in dry eye (Lemp, M. et al., 2007). In normal human tear fluid, the primary cationic electrolytes are sodium (120-170 mM), potassium (6-42 mM), calcium (0.3-2 mM), and magnesium (0.3-1.1 mM) (Gilbard, J.P. and Rossi, S.R., 1994; Stahl, U. et al., 2012). In dry eye disorders, the concentration of all electrolytes and tear osmolarity rises as a result of (i) water evaporation from the exposed ocular surface in conditions of low aqueous tear flow, (ii) excessive evaporation or, (iii) a combination of these events (Lemp, M. et al., 2007). In meibomian gland dysfunction, there is a proportional increase in tear electrolytes and osmolarity, consistent with an evaporative effect at the ocular surface. In lacrimal gland disease, however, the increase in electrolytes is not uniform, and sodium appears to increase disproportionately in the tear film as its secretion by the lacrimal gland increases (Gilbard, J.P. and Rossi, S.R., 1994).
Due to its high concentration in tears, elevated tear osmolarity is primarily attributed to increased levels of sodium chloride (Pflugfelder, S.C., 2011). Consequently, the vast majority of in vitro and in vivo models of hyperosmolarity use hypertonic solutions of sodium cations to evaluate ocular surface disease and corneal barrier disruption (Blalock, T.D. et al., 2008; Chen, Z. et al., 2008; Li, D.Q. et al., 2004; Liu, H. et al., 2009). However, less attention has been paid to the contribution of other tear electrolytes under hyperosmotic conditions to ocular surface disease. Previous evidence suggests that individual cations play unique and varying roles in epithelial cells. For example, potassium ions appear to contribute to the maintenance of epithelial thickness as compared to iso-osmotic solutions containing sodium ions (Green, K. et al., 1992). Therefore, we elected to examine the contribution of different hypertonic electrolytes to corneal epithelial dysfunction.
We determined the contribution of individual cations to barrier function in a model of long-term hyperosmotic stress using the rose bengal penetration assay (Argueso, P. and Gipson, I.K., 2012). This assay was selected based on previous data indicating that hyperosmolarity-induced changes in apical epithelial cells in dry eye can be correlated with the amount and distribution of rose bengal staining (Gilbard, J.P., 1985; Gilbard, J.P. and Farris, R.L., 1979; Tomlinson, A. et al., 2006). For these experiments, epithelial cells were cultured in serum-free medium until confluence, then in serum-containing medium, with or without hypertonic electrolytes, for 7 days as previously described (Xiong, L. et al., 2011). As shown in Figure 1, addition of 50 mM calcium chloride to cultures for 7 days increased rose bengal uptake by 2fold compared to control. More strikingly, dye uptake increased 3-fold after addition of magnesium chloride for the final 3 days of cell culture, and incubation for 5 and 7 days induced cell death, as shown by phase contrast microscopy. These results are consistent with previous data from our laboratory indicating that divalent cations have a harmful effect on the glycocalyx barrier function (Argueso, P. et al., 2006). On the other hand, addition of sodium—which is commonly used in corneal epithelial models of hyperosmolar stress—and potassium chloride did not affect rose bengal uptake as compared to divalent cations. It is important to note that, in our model, incubation with serum-containing medium for 7 days promotes stratification and optimal biosynthesis of cell surface-associated mucins (Gipson, I.K. et al., 2003; Xiong, L. et al., 2011).
Figure 1.
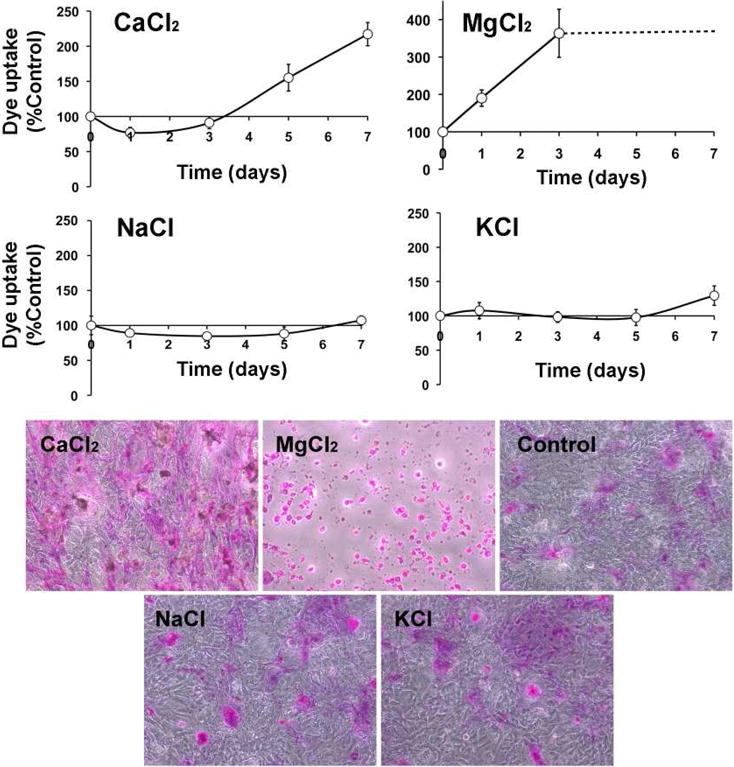
Rose bengal staining in stratified human corneal epithelial cells incubated with 50 mM of individual electrolytes for different time periods. Dashed line in the magnesium chloride graph indicates uniform cell death with longer periods of incubation. Phase contrast images shown below correspond to representative areas following 7-day incubations with or without (control) hypertonic electrolytes.
The long-term effect of hypertonic calcium and sodium cations on cell viability and paracellular barrier function was tested using the colorimetric MTT assay and transepithelial electrical resistance (TEER), respectively. Human corneal epithelial cells were grown for 7 days in media containing increasing concentrations of electrolytes (300-550 mOsm) on the apical portion of Transwell® permeable supports. Osmolarity values were selected based on data indicating that osmolarity in areas of breakup of the precorneal tear layer can reach up to 560 mOsm (Pflugfelder, S.C., 2011). As shown in Figure 2A, incubation with calcium chloride, but not with sodium, decreased cell viability in a concentration-dependent manner, resulting in almost complete loss of viable cells above 450 mOsm. Similarly, the paracellular permeability of corneal epithelial cells after addition of calcium was significantly lower as compared to the addition of sodium (Fig. 2B).
Figure 2.
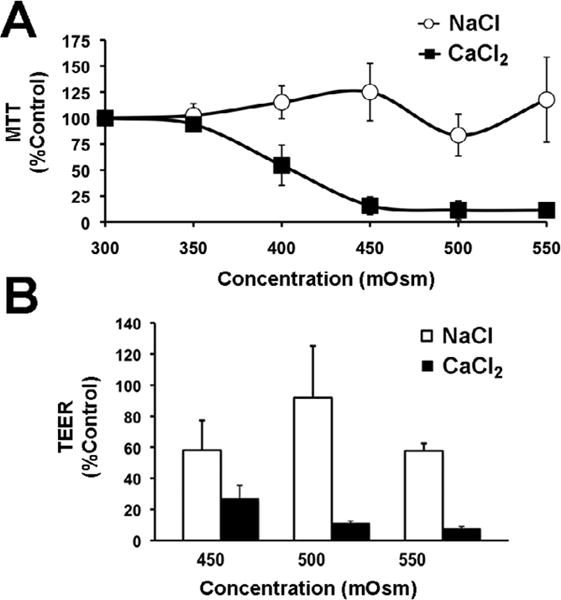
Corneal epithelial cell viability (A) and transepithelial resistance measurements (B) after 7 days' exposure of human corneal epithelial cells to increasing concentrations of calcium and sodium chloride.
It is becoming apparent that both alteration of the physicochemical character of the epithelial glycocalyx and specific hypertonicity-induced signaling events contribute to corneal epithelial dysfunction. Several studies have shown that divalent cations interact noncovalently with mucins and that this interaction can alter the structure and function of these protective molecules (Argueso, P. et al., 2006; Forstner, J.F. and Forstner, G.G., 1975; Marriott, C. et al., 1979; Steiner, C.A. et al., 1984). Similarly, ion channels, such as the vanilloid TRP1 (TRPV1) isoform, can elicit responses to diverse noxious stimuli including hyperosmotic stress (Ciura, S. and Bourque, C.W., 2006). TRPV1 in human corneal epithelium localizes predominantly to superficial cells and exhibits moderate selectivity for calcium and magnesium (Zhang, F. et al., 2007). Interestingly, TRVP1 activation leads to activation of MAPK and increase in proinflammatory cytokine release (Pan, Z. et al., 2011; Zhang, F. et al., 2007), pathways characteristic of dry eye disease (Pflugfelder, S.C., 2011). Further studies are required to understand the function of these and other membrane receptors during exposure to specific hypertonic electrolytes. Elucidating how different tear electrolytes influence ocular surface epithelial responses in cases of dry eye could facilitate the development of better formulations for treatment of the disease.
Acknowledgments
Supported by an SRA from Alcon Research, Ltd., Fort Worth, TX (PA) and National Eye Institute Grant R01 EY014847 (PA).
Footnotes
Conflicts of interest: M.S. was an employee of Alcon Research, Ltd., Fort Worth, TX when work on this project was performed. All other authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argueso P, Gipson IK. Assessing mucin expression and function in human ocular surface epithelia in vivo and in vitro. Methods Mol Biol. 2012;842:313–325. doi: 10.1007/978-1-61779-513-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso P, Tisdale A, Spurr-Michaud S, Sumiyoshi M, Gipson IK. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Invest Ophthalmol Vis Sci. 2006;47:113–119. doi: 10.1167/iovs.05-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock TD, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Release of membrane-associated mucins from ocular surface epithelia. Invest Ophthalmol Vis Sci. 2008;49:1864–1871. doi: 10.1167/iovs.07-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Tong L, Li Z, Yoon KC, Qi H, Farley W, Li DQ, Pflugfelder SC. Hyperosmolarity-induced cornification of human corneal epithelial cells is regulated by JNK MAPK. Invest Ophthalmol Vis Sci. 2008;49:539–549. doi: 10.1167/iovs.07-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci. 2006;26:9069–9075. doi: 10.1523/JNEUROSCI.0877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner JF, Forstner GG. Calcium binding to intestinal goblet cell mucin. Biochim Biophys Acta. 1975;386:283–292. doi: 10.1016/0005-2795(75)90270-6. [DOI] [PubMed] [Google Scholar]
- Gilbard JP. Tear film osmolarity and keratoconjunctivitis sicca. CLAO J. 1985;11:243–250. [PubMed] [Google Scholar]
- Gilbard JP. Human tear film electrolyte concentrations in health and dry-eye disease. Int Ophthalmol Clin. 1994;34:27–36. doi: 10.1097/00004397-199403410-00005. [DOI] [PubMed] [Google Scholar]
- Gilbard JP, Farris RL. Tear osmolarity and ocular surface disease in keratoconjunctivitis sicca. Arch Ophthalmol. 1979;97:1642–1646. doi: 10.1001/archopht.1979.01020020210003. [DOI] [PubMed] [Google Scholar]
- Gilbard JP, Rossi SR. Changes in tear ion concentrations in dry-eye disorders. Adv Exp Med Biol. 1994;350:529–533. doi: 10.1007/978-1-4615-2417-5_89. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- Green K, MacKeen DL, Slagle T, Cheeks L. Tear potassium contributes to maintenance of corneal thickness. Ophthalmic Res. 1992;24:99–102. doi: 10.1159/000267153. [DOI] [PubMed] [Google Scholar]
- Lemp M, Baudouin C, Baum J, Dogru M, Foulks G, Kinoshita S, Laibson P, McCulley J, Murube J, Pflugfelder S, Rolando M, Toda I. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- Li DQ, Chen Z, Song XJ, Luo L, Pflugfelder SC. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:4302–4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- Liu H, Begley C, Chen M, Bradley A, Bonanno J, McNamara NA, Nelson JD, Simpson T. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009;50:3671–3679. doi: 10.1167/iovs.08-2689. [DOI] [PubMed] [Google Scholar]
- Marriott C, Shih CK, Litt M. Changes in the gel properties of tracheal mucus induced by divalent cations. Biorheology. 1979;16:331–337. doi: 10.3233/bir-1979-164-507. [DOI] [PubMed] [Google Scholar]
- Ohashi Y, Dogru M, Tsubota K. Laboratory findings in tear fluid analysis. Clin Chim Acta. 2006;369:17–28. doi: 10.1016/j.cca.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Pan Z, Wang Z, Yang H, Zhang F, Reinach PS. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:485–493. doi: 10.1167/iovs.10-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC. Tear dysfunction and the cornea: LXVIII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2011;152:900–909. e901. doi: 10.1016/j.ajo.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Levin MH, Verkman AS. In situ fluorescence measurement of tear film [Na+], [K+], [Cl−], and pH in mice shows marked hypertonicity in aquaporin-5 deficiency. Invest Ophthalmol Vis Sci. 2009;50:2132–2138. doi: 10.1167/iovs.08-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl U, Willcox M, Stapleton F. Osmolality and tear film dynamics. Clin Exp Optom. 2012;95:3–11. doi: 10.1111/j.1444-0938.2011.00634.x. [DOI] [PubMed] [Google Scholar]
- Steiner CA, Litt M, Nossal R. Effect of Ca++ on the structure and rheology of canine tracheal mucin. Biorheology. 1984;21:235–252. doi: 10.3233/bir-1984-211-226. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Khanal S, Ramaesh K, Diaper C, McFadyen A. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006;47:4309–4315. doi: 10.1167/iovs.05-1504. [DOI] [PubMed] [Google Scholar]
- Xiong L, Woodward AM, Argueso P. Notch signaling modulates MUC16 biosynthesis in an in vitro model of human corneal and conjunctival epithelial cell differentiation. Invest Ophthalmol Vis Sci. 2011;52:5641–5646. doi: 10.1167/iovs.11-7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Yang H, Wang Z, Mergler S, Liu H, Kawakita T, Tachado SD, Pan Z, Capo-Aponte JE, Pleyer U, Koziel H, Kao WW, Reinach PS. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J Cell Physiol. 2007;213:730–739. doi: 10.1002/jcp.21141. [DOI] [PubMed] [Google Scholar]


