Abstract
Objective
To determine whether granulosa cells contribute to excess androgen production, inhibin B (Inh B) responses to hCG were assessed in women with polycystic ovary syndrome (PCOS) and normal women.
Design
A prospective study.
Setting
An academic medical center.
Patients
20 women with PCOS and 16 normal women.
Interventions
Blood samples obtained before and 24 hr after injection of recombinant hCG (r-hCG), 25 µg.
Main Outcome Measures
Basal and stimulated Inh B, estradiol (E2), androstenedione (A4), and testosterone (T) responses after r-hCG administration.
Results
In normal and PCOS women, r-hCG induced a significant reduction of Inh B levels. Lowered Inh B responses were not related to BMI, PCOS status and age by multivariate regression. r-hCG significantly increased serum A4 and E2 in both normal and PCOS women.
Conclusions
In normal and PCOS women, Inh B production was deceased following r-hCG administration. These findings strongly suggest that in PCOS women androgen excess is not enhanced by LH-stimulated Inh B production.
Keywords: Inhibin B, hCG, Polycystic Ovary Syndrome
Introduction
In women with polycystic ovary syndrome (PCOS), excessive ovarian androgen production is a major pathophysiological feature. The basis for androgen overproduction has been attributed to altered theca cell responsiveness to gonadotropin stimulation in association with increased pituitary LH secretion (1–3). In particular, hyperandrogenemic women with PCOS have exhibited exaggerated 17-hydroxyprogesterone (17–OHP) production in response to hCG compared with those observed in normal women (4). In addition, studies have shown that women with PCOS exhibit significant increases in circulating androgens after an acute injection of FSH, which suggests that ovarian androgen production may also be subject to paracrine regulation by factors derived from granulosa cells (GCs) (5). Early in vivo and in vitro animal reports have suggested an interaction between adjacent granulosa and theca cells because reduction of androgen production was observed after removal of GCs from theca tissue cultures (6, 7). Subsequently, it was shown that ovine theca cells co-incubated with conditioned media from FSH-stimulated GC cultures produced significantly more LH-induced androgen than theca cells incubated with untreated media (8). In addition, LH-stimulated androgen production from cultured rat theca cells of animals pretreated with FSH was substantially greater than that produced by theca cells of animals treated with vehicle (9).
Among GC-derived proteins, inhibin appears to enhance LH-mediated androgen production. In cultured human ovarian theca cells, the presence of inhibin was clearly associated with greater production of androgen compared with that observed in the absence of inhibin (10, 11). In addition, inhibin was dose-dependently able to negate the inhibitory effect of activin on human theca cell androgen production (12). In women with PCOS, significant increases in ovarian androgens stimulated by FSH were accompanied by similar significant increments in FSH-stimulated inhibin B (Inh B) levels compared with those of normal women (5).
Granulosa cells are also known to possess LH receptors. During normal follicular development, acquisition of LH receptors by GCs occurs with advanced stages of growth and antrum formation (13–15). However, in GCs obtained from ovaries of anovulatory PCOS women, LH receptor mRNA expression was abundant in small antral follicles between 4–8 mm (16). This suggests that inhibin production may be enhanced by increased LH secretion in women with PCOS, which may provide an indirect mechanism of androgen production beyond that of direct theca cell stimulation by LH. We have previously demonstrated that women with PCOS exhibit a marked androgen production in response to hCG administered intravenously (4). To further explore whether excess androgen production may be coupled to corresponding inhibin responses to hCG, Inh B, E2 and androgen levels were assessed prior to and following intravenous administration of hCG to women with PCOS and normal women.
Materials and Methods
Participants
Twenty women with PCOS and 16 normal women were recruited. The diagnosis of PCOS was based on 1992 NIH criteria: clinical and/or biochemical evidence of hyperandrogenism and irregular menstrual bleeding, either oligomenorrhea or amenorrhea (17). Oligomenorrhea was defined as irregular menstrual bleeding occurring less than six times a year. Each PCOS subject had enlarged polycystic ovaries by ultrasound. The antral follicle count per ovary was greater than 12 in all subjects. None of the follicles exceeded 9 mm in diameter and the vast majority were 2–5 mm in size. Normal women did not exhibit enlarged ovaries, had antral follicle counts of 7–10 per ovary, and no follicles greater than 10 mm in diameter. PCOS and normal women had comparable mean ages (± SE) of 27.5 ± 0.9 and 27.9 ± 1.4 yr, respectively. Mean body mass index (BMI) was higher in PCOS subjects (34.7 ± 16 vs. 29.3 ± 2.2 kg/m2, respectively; P<0.05). Late-onset congenital adrenal hyperplasia was excluded by serum 17-OHP less than 2 ng/ml. Circulating TSH and prolactin were normal among all subjects. No subject had received hormone medication for 2 months before study. The study was approved by the Human Research Protection Program at the University of California, San Diego (UCSD), and written informed consent was obtained from each participant.
Procedures
Subjects were admitted to the General Clinical Research Center at UCSD on the day of testing. Each subject received recombinant hCG (r-hCG), 25 µg, as an iv bolus. In normal subjects, r-hCG was given during the midfollicular phase of the menstrual cycle. In PCOS women, r-hCG was administered on a random day. Blood samples were obtained at 0 and 24 h after r-hCG administration. None of the PCOS subjects had experienced recent ovulation, as evidenced by absence of recent menstrual bleeding for 2 months before study and serum progesterone (P4) less than 2.0 ng/ml.
Assays
Serum Inh B levels were measured using a commercially available Gen II ELISA (Beckman Coulter, Inc., Brea, CA), with a sensitivity of 2.6 pg/ml, intraassay CV of 2.2–3.8%. Serum estradiol (E2), androstenedione (A4), and testosterone (T) were measured by well established RIA with intra-assay CV less than 7% (18, 19). Briefly, radioimmunoassay for E2, A4 and T were developed in-house. The labeled antigen is commercially available. The antibodies were raised in rabbits and checked for cross reactivity to other steroid hormones. Standards are made from reagents which are also commercially available. To ensure a specific assay the samples were purified in a two step process. Initially, to separate hydrophilic from hydrophobic hormones 7.0 ml of solvent (hexane:ethyl acetate) was added to 0.8 ml of serum and vortexed. The solvent was decanted and chromatographed on a microcelite column. Chromatography columns utilized ethylene glycol:propylene glycol as the stationary phase. The chromatography system was checked for separation by comparing radioactive peaks to immunoreactive peaks. Each sample was chromatographed on a celite column separating the steroids based on their polarity. Individual purified steroid fractions were then used in their respective radioimmunoassay. Serum 17-OHP was measured by RIA with intra-assay CV less than 7% (Diagnostic Systems Laboratories, Inc., Webster, TX). Serum concentrations of LH and FSH were measured by radioimmunoassay (RIA) with intra- and inter-assay coefficients of variation (CV) of 5.4% and 8.0%, respectively, for LH and 3.0% and 4.6%, respectively, for FSH (Diagnostic Products Corp., Los Angeles, CA).
Statistical analysis
STATA software (Release 12, College Station, TX) was used for analysis. Summary statistics were performed for all variables. Graphic displays of continuous variables were explored to determine data distributions. As the distribution of hormone levels did not meet the assumptions of normality, non-parametric tests were used. Baseline hormone values were compared by PCOS status by using the Wilcoxon rank-sum test. The Wilcoxon sign-rank test was used to compare hormone responses over 24 hours (hr 0 versus hr 24) after r-hCG injection. To determine variables associated with the change in Inh B, linear regression methods were used to model the association between the percent change in Inh B levels and covariates. For all analyses, P values less than 0.05 were considered statistically significant.
Results
Baseline hormone concentrations in PCOS and normal women
Baseline circulating hormone levels are shown in Table 1. In women with PCOS, serum LH, T, A4 and 17-OHP levels were significantly greater than those of normal controls. Serum FSH and E2 were similar between groups.
Table 1.
Mean (SE) basal clinical and serum hormone data in normal women and women with PCOSγ
| Normal (n = 16) |
PCOS (n = 20) |
|
|---|---|---|
| Age (yrs) | 27.9 ± 1.4 | 27.5 ± 1.6 |
| BMI | 29.3 ± 2.2 | 34.7 ± 11.6* |
| LH (mIU/ml) | 3.8 ± 0.5 | 8.0 ± 1.1** |
| FSH (mIU/ml | 5.8 ± 0.4 | 5.3 ± 0.3 |
| E2 (pg/ml) | 52 ± 6 | 46 ± 3 |
| T (ng/ml) | 0.29 ± 0.04 | 0.48 ± 0.04** |
| A4 (ngml) | 0.93 ± 0.08 | 1.56 ± 0.10*** |
| 17-OHP (ng/ml) | 0.66 ± 0.10 | 1.09 ± 0.09** |
| Inh B (pg/ml) | 92.6 ± 7.7 | 100.5 ± 13.5 |
Baseline characteristics compared by Wilcoxon rank sum test
p<0.05;
p<0.01;
p<0.001 PCOS vs Normal
To convert to SI units multiply by the following conversion factor: E2 (3.67); T (3.47); A4 (3.49); 17-OHP (3.03); Inh B (0.00003125)
Inhibin B response to r-hCG administration
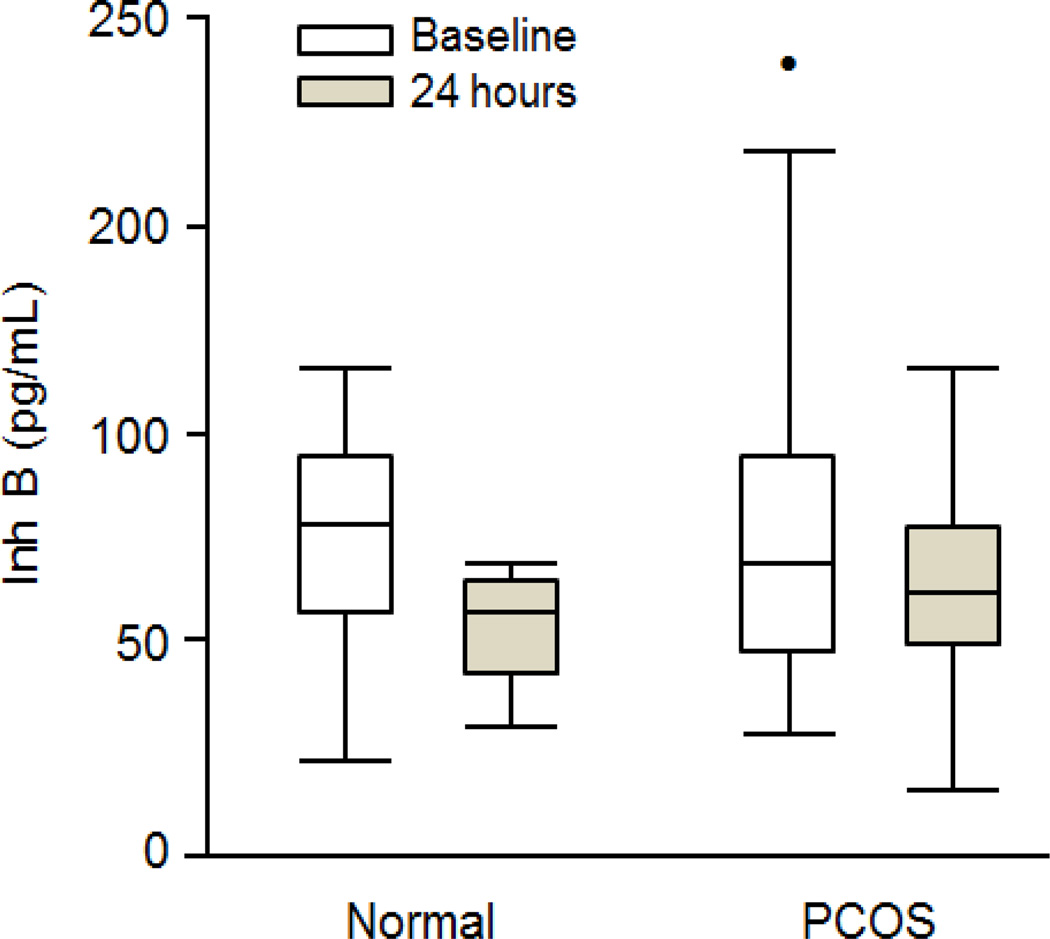
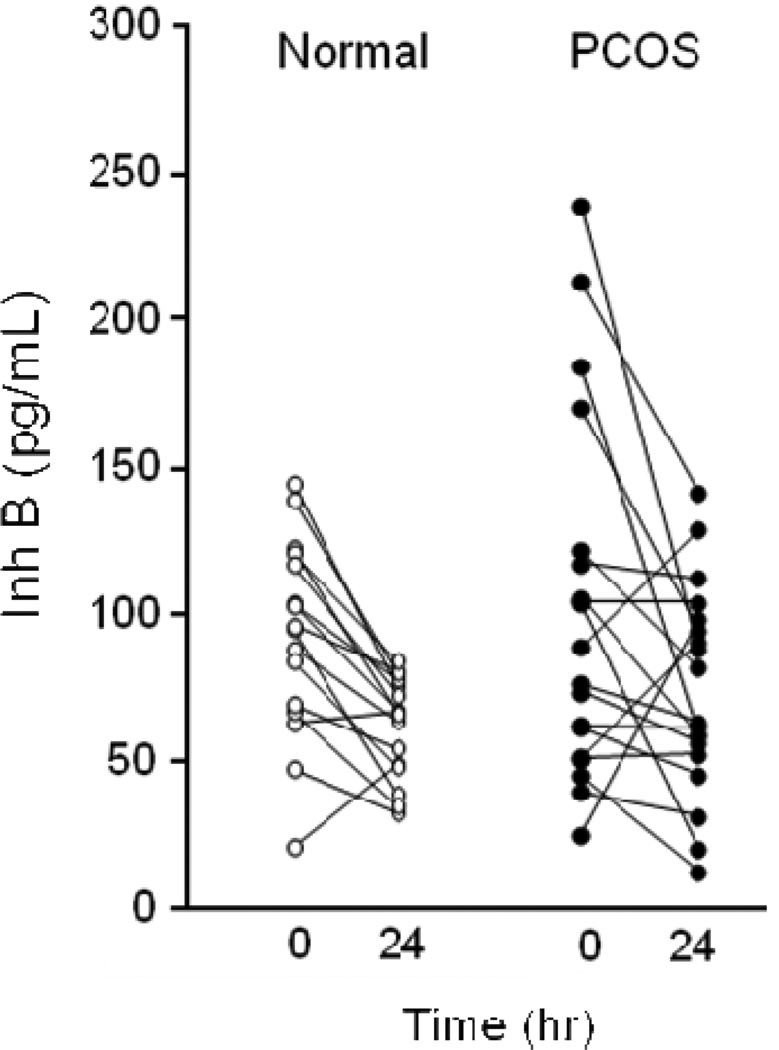
Prior to r-hCG administration baseline Inh B levels were not significantly different between normal women and women with PCOS (Fig. 1 and 2). Following r-hCG injection both groups exhibited decreases in circulating Inh B at 24 hr. In normal women the median serum Inh B level (interquartile range [IQR]) declined from 95.3 (50.9) to 66.6 (28.2) pg/ml (30%) (P = 0.002) as lowered responses were observed in 14 of 16 individuals (Fig. 2). In one subject the Inh B response was increased while in another there was no change. In women with PCOS the reduction of Inh B from 83.7 (37.2) to 73.6 (42.4) pg/ml (12%) (P = 0.05) was less compared to that of the normal group. Decreased responses were observed in 13 of 20 individuals while Inh B rose in 4 and were unchanged in 3.
Figure 1.
Box and whisker plots showing median serum Inh B levels and interquartile ranges at baseline and 24-h after iv administration of 25 ug of r-hCG in normal and PCOS women. The decline in Inh B following r-hCG was significant in normal (P = 0.002) and PCOS (P = 0.05) women. The closed circle in the baseline PCOS box plot denotes an Inh B value outside of the 95 percentile.
Figure 2.
Individual baseline and 24-h serum Inh B levels following iv administration of r-hCG, 25 ug, in normal and PCOS women.
On univariate analysis, the percent change in Inh B decreased with increasing BMI (β=− 0.03 [95% CI −0.06, −0.001], P = 0.05), but was not associated with age (p=0.81) or PCOS status (P = 0.37). This association between the change in Inh B and BMI was no longer statistically significant (P = 0.08) in multivariate linear regression model adjusting for age (p=0.85) and PCOS status (P = 0.71).
E2 responses to r-hCG administration
Significant rises of serum E2 following r-hCG were observed in normal women (P = 0.05) and in women with PCOS (P = 0.003) as shown in Table 2. In women with PCOS E2 responses were higher after r-hCG although the percent change of response was not different between groups.
Table 2.
Mean (±SE) steroid hormone levels before and 24 hrs after r-hCG, 25 µg iv, in normal women and women with PCOSγ
| E2 (pg/ml) |
A4 (ng/ml) |
T (ng/ml) |
|
|---|---|---|---|
| Normal | |||
| Baseline | 51 ± 6 | 0.93 ± 0.08 | 0.29 ± 0.04 |
| 24 hour | 62 ± 6* | 1.25 ± 0.10** | 0.32 ± 0.04 |
| PCOS | |||
| Baseline | 46 ± 3 | 1.56 ± 0.10 | 0.48 ± 0.04 |
| 24 hour | 76 ± 8** | 2.25 ± 0.15** | 0.57 ± 0.05 |
Baseline and 24 hour steroid hormone levels compared by Wilcoxon signed-rank test;
p<0.05;
p<0.01 24 hour vs. Baseline
To convert to SI units multiply by the following conversion factor: E2 (3.67); A4 (3.49); T (3.47)
Androgen responses to r-hCG administration
Both normal and PCOS women demonstrated significant (P =0.001) increases of A4 after receiving r-hCG (Table 2)http://jcem.endojournals.org/content/96/4/1106.long-F2#F2. The percent change in A4 was similar between normal and PCOS women. Increased T responses to r-hCG were not observed in normal women while the incremental response in PCOS women approached statistical significance (P =0.07).
Discussion
The results of this study have demonstrated that in normal women as well as in women with PCOS serum Inh B levels following r-hCG administration were significantly reduced compared to baseline values. The lowered Inh B responses were not related to any covariate in a model that adjusted for BMI, PCOS status and age. In women with PCOS, serum T, A4 and E2 responses after hCG were increased whereas in normal women significant increments were noted for A4 and E2.
The finding of reduced Inh B production in response to hCG in normal women was unexpected as previous clinical studies have demonstrated that serum Inh B was not significantly altered by subcutaneous (sq) or intramuscular (im) administration of r-hLH or hCG, respectively (20, 21). The lack of response to r-hLH in previous studies was attributed to a lack of small antral follicles and absence of LH receptors as ovarian suppression had been induced by GnRH agonist 2 weeks prior to stimulation (22). Another consideration was that the dose of r-hLH administered to normal women resulted in minimal increments of circulating LH levels, about 1 mIU/ml, that were insufficient to increase Inh B or ovarian steroid production. Administration of hCG, 5,000 IU intramuscularly was noted to decrease Inh B levels in normal women; however, the Inh B decrement did not achieve statistical significance despite significant production of E2 (21). Notably, serum androgen levels were not increased by this dose of hCG. These results were consistent with in vitro studies in which cultured GCs from small antral follicles failed to exhibit changes of Inh B when treated with hCG (22). The difference in Inh B responses to r-hCG between our results and those previously reported for normal women, at least in part, may be due to the amount of gonadotropin and route of administration.
Decreased Inh B responses to r-hCG in women with PCOS in the current study were consistent with previously published findings by Welt et al (20). While the inverse relationship of basal serum Inh B levels to age and BMI has been well recognized in normal and PCOS women, responses of Inh B to hCG were not influenced by age or BMI in our subjects as assessed by multivariate linear regression (23–25). Moreover, Inh B suppression by r-hCG was not a unique feature of PCOS status which likely reflected the similarity of response in both groups. Earlier reports have demonstrated in cultured granulosa-luteal cells obtained from aspirated follicles of women with unexplained or male factor infertility undergoing in vitro fertilization that Inh B production was decreased by relatively low doses of LH and hCG (26–28). However, in these studies luteinized granulosa cells were obtained from large follicles (>10 mm) following controlled ovarian hyperstimulation. These findings suggest that with advanced follicle maturity and/or luteinization Inh B production by granulosa cells may become highly responsive to the effects of hCG.
A limitation of this study is the small number of PCOS subjects studied. In contrast to normal subjects in whom decreased Inh B responses were observed in 14 of 16 individuals, similar declines in women with PCOS were noted in only 13 of 20 subjects whereas Inh B rose in 4 and was unchanged in 3. These findings suggest the possibility that our findings in PCOS women may have been due to chance and that a larger sample size may have revealed a reversal of these findings.
In conclusion, our findings do not exclude a possible role for Inh B in excessive theca cell androgen production as we have previously shown that FSH-stimulated increases of ovarian androgens are accompanied by corresponding rises in Inh B (29). However, it appears that androgen production induced by r-hCG arise from a direct action on TC steroidogenesis.
Acknowledgements
This research was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement (U54 HD12303-28) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and in part by NIH grant MO1 RR00827.
We wish to thank Annette Ramos-Haggan, R.N., for her invaluable clinical assistance, Jeff Wong for superb technical expertise, and Andi Hartgrove for formatting the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to disclose.
References
- 1.Yen SSC. The polycystic ovary syndrome. Clin Endocrinol Oxf. 1980;12:177–207. doi: 10.1111/j.1365-2265.1980.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 2.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 3.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 4.Rosencrantz MA, Coffler MS, Haggan A, Duke KB, Donohue MC, Shayya RF, et al. Clinical evidence for predominance of delta-5 steroid production in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:1106–1113. doi: 10.1210/jc.2010-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wachs DS, Coffler MS, Malcom PJ, Shimasaki S, Chang RJ. Increased androgen response to follicle-stimulating hormone administration in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:1827–1833. doi: 10.1210/jc.2007-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortune JE, Armstrong DT. Androgen production by theca and granulosa isolated from proestrous rat follicles. Endocrinology. 1977;100:1341–1347. doi: 10.1210/endo-100-5-1341. [DOI] [PubMed] [Google Scholar]
- 7.Moor RM. Sites of steroid production in ovine graafian follicles in culture. J Endocrinol. 1977;73:143–150. doi: 10.1677/joe.0.0730143. [DOI] [PubMed] [Google Scholar]
- 8.Lischinsky A, Armstrong DT. Granulosa cell stimulation of thecal androgen synthesis. Can J Physiol Pharmacol. 1983;61:472–477. doi: 10.1139/y83-072. [DOI] [PubMed] [Google Scholar]
- 9.Smyth CD, Miro F, Whitelaw PF, Howles CM, Hillier SG. Ovarian thecal/interstitial androgen synthesis is enhanced by a follicle-stimulating hormone-stimulated paracrine mechanism. Endocrinology. 1993;133:1532–1538. doi: 10.1210/endo.133.4.8404591. [DOI] [PubMed] [Google Scholar]
- 10.Hillier SG, Yong EL, Illingworth PJ, Baird DT, Schwall RH, Mason AJ. Effect of recombinant inhibin on androgen synthesis in cultured human thecal cells. Mol Cell Endocrinol. 1991;75:R1–R6. doi: 10.1016/0303-7207(91)90234-j. [DOI] [PubMed] [Google Scholar]
- 11.Hsueh AJ, Dahl KD, Vaughan J, Tucker E, Rivier J, Bardin CW, et al. Heterodimers and homodimers of inhibin subunits have different paracrine action in the modulation of luteinizing hormone-stimulated androgen biosynthesis. Proc Natl Acad Sci U S A. 1987;84:5082–5086. doi: 10.1073/pnas.84.14.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, et al. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404:411–414. doi: 10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M, Nakano R, Ooshima A. Immunohistochemical localization of pituitary gonadotrophins and gonadal steroids confirms the 'two-cell, two-gonadotrophin' hypothesis of steroidogenesis in the human ovary. J Endocrinol. 1990;126:483–488. doi: 10.1677/joe.0.1260483. [DOI] [PubMed] [Google Scholar]
- 14.Yamoto M, Shima K, Nakano R. Gonadotropin receptors in human ovarian follicles and corpora lutea throughout the menstrual cycle. Horm Res. 1992;37(Suppl 1):5–11. doi: 10.1159/000182335. [DOI] [PubMed] [Google Scholar]
- 15.Shaw HJ, Hillier SG, Hodges JK. Developmental changes in luteinizing hormone/human chorionic gonadotropin steroidogenic responsiveness in marmoset granulosa cells: Effects of follicle-stimulating hormone and androgens. Endocrinology. 1989;124:1669–1677. doi: 10.1210/endo-124-4-1669. [DOI] [PubMed] [Google Scholar]
- 16.Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab. 2001;86:1318–1323. doi: 10.1210/jcem.86.3.7318. [DOI] [PubMed] [Google Scholar]
- 17.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 18.Judd HL, Scully RE, Herbst AL, Yen SS, Ingersol FM, Kliman B. Familial hyperthecosis: Comparison of endocrinologic and histologic findings with polycystic ovarian disease. Am J Obstet Gynecol. 1973;117:976–982. doi: 10.1016/0002-9378(73)90071-9. [DOI] [PubMed] [Google Scholar]
- 19.Tsai CC, Yen SS. The effect of ethinyl estradiol administration during early follicular phase of the cycle on the gonadotropin levels and ovarian function. J Clin Endocrinol Metab. 1971;33:917–923. doi: 10.1210/jcem-33-6-917. [DOI] [PubMed] [Google Scholar]
- 20.Welt CK, Smith ZA, Pauler DK, Hall JE. Differential regulation of inhibin a and inhibin b by luteinizing hormone, follicle-stimulating hormone, and stage of follicle development. J Clin Endocrinol Metab. 2001;86:2531–2537. doi: 10.1210/jcem.86.6.7597. [DOI] [PubMed] [Google Scholar]
- 21.Welt CK, Taylor AE, Martin KA, Hall JE. Serum inhibin b in polycystic ovary syndrome: Regulation by insulin and luteinizing hormone. J Clin Endocrinol Metab. 2002;87:5559–5565. doi: 10.1210/jc.2002-020546. [DOI] [PubMed] [Google Scholar]
- 22.Welt CK, Schneyer AL. Differential regulation of inhibin b and inhibin a by follicle-stimulating hormone and local growth factors in human granulosa cells from small antral follicles. J Clin Endocrinol Metab. 2001;86:330–336. doi: 10.1210/jcem.86.1.7107. [DOI] [PubMed] [Google Scholar]
- 23.Pigny P, Cortet-Rudelli C, Decanter C, Deroubaix D, Soudan B, Duhamel A, et al. Serum levels of inhibins are differentially altered in patients with polycystic ovary syndrome: Effects of being overweight and relevance to hyperandrogenism. Fertil Steril. 2000;73:972–977. doi: 10.1016/s0015-0282(00)00421-0. [DOI] [PubMed] [Google Scholar]
- 24.Cortet-Rudelli C, Pigny P, Decanter C, Leroy M, Maunoury-Lefebvre C, Thomas-Desrousseaux P, et al. Obesity and serum luteinizing hormone level have an independent and opposite effect on the serum inhibin b level in patients with polycystic ovary syndrome. Fertil Steril. 2002;77:281–287. doi: 10.1016/s0015-0282(01)02968-5. [DOI] [PubMed] [Google Scholar]
- 25.Laven JS, Imani B, Eijkemans MJ, de Jong FH, Fauser BC. Absent biologically relevant associations between serum inhibin b concentrations and characteristics of polycystic ovary syndrome in normogonadotrophic anovulatory infertility. Hum Reprod. 2001;16:1359–1364. doi: 10.1093/humrep/16.7.1359. [DOI] [PubMed] [Google Scholar]
- 26.Muttukrishna S, Groome N, Ledger W. Gonadotropic control of secretion of dimeric inhibins and activin a by human granulosa-luteal cells in vitro. J Assist Reprod Genet. 1997;14:566–574. doi: 10.1023/A:1022524516824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanttinen T, Liu J, Hyden-Granskog C, Parviainen M, Penttila I, Voutilainen R. Regulation of immunoreactive inhibin a and b secretion in cultured human granulosa-luteal cells by gonadotropins, activin a and insulin-like growth factor type-1 receptor. J Endocrinol. 2000;167:289–294. doi: 10.1677/joe.0.1670289. [DOI] [PubMed] [Google Scholar]
- 28.Wen X, Tozer AJ, Li D, Docherty SM, Al-Shawaf T, Iles RK. Human granulosa-lutein cell in vitro production of progesterone, inhibin a, inhibin b, and activin a are dependent on follicular size and not the presence of the oocyte. Fertil Steril. 2008;89:1406–1413. doi: 10.1016/j.fertnstert.2007.03.086. [DOI] [PubMed] [Google Scholar]
- 29.Wachs DS, Coffler MS, Malcom PJ, Chang RJ. Comparison of follicle-stimulating-hormone-stimulated dimeric inhibin and estradiol responses as indicators of granulosa cell function in polycystic ovary syndrome and normal women. J Clin Endocrinol Metab. 2006;91:2920–2925. doi: 10.1210/jc.2006-0442. [DOI] [PubMed] [Google Scholar]