Abstract
Obesity has become a universal and major public health problem with increasing prevalence in both adults and children in the 21st century, even in developing countries. Extensive epidemiological studies reveal a strong link between obesity and development and progression of various types of cancers. The connection between obesity and liver cancer is particularly strong and obesity often results in liver diseases such as non-alcoholic fatty liver disease (NAFLD) and the more severe non-alcoholic steatohepatitis (NASH). NASH is characterized by fatty liver inflammation and is believed to cause fibrosis and cirrhosis. The latter is a known liver cancer risk factor. In fact, due to its much higher prevalence, obesity may be a more substantial contributor to overall hepatocellular carcinoma burden than infection with hepatitis viruses. Here we review and discuss recent advances in elucidation of cellular and molecular alterations and signaling pathways associated with obesity and liver inflammation, and their contribution to hepatocarcinogenesis.
Keywords: Obesity, Inflammation, Hepatocellular carcinoma, hepatocarcinogenesis
Introduction
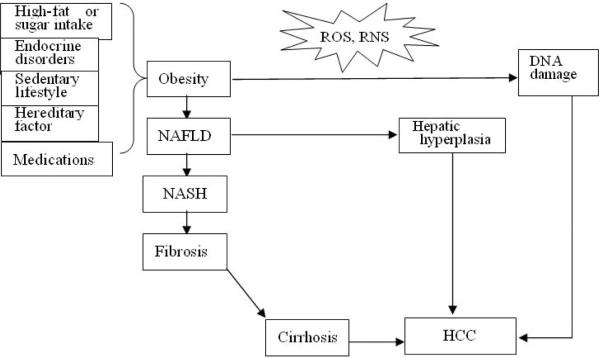
Obesity, an abnormal medical condition, is becoming one of the most serious public health problems worldwide and its prevalence has dramatically increased in the last few decades. Obesity is defined as having a body mass index (BMI) equal to or higher than 30 kg/m2. The marked increase in the worldwide incidence of obesity, particularly in children, has been noted by the World Health Organization (WHO) [1]. Obesity often causes a number of medical disorders, including metabolic syndrome, type 2 diabetes, non-alcoholic fatty liver disease (NAFLD), and the more severe non-alcoholic steatohepatitis (NASH). Recently, however, obesity was recognized as a major risk factor for several common types of cancer of which pancreatic and liver cancer show the highest increase in risk [2], notably, these are two of the most lethal cancers with 5 years survival rate of 4–8%. Several epidemiological and clinical studies have confirmed the importance of obesity as an independent risk factor for hepatocellular carcinoma (HCC), the most common form of liver cancer [3, 4]. Due to its much wider spread prevalence in some parts of the world, obesity makes a larger contribution to overall HCC burden than hepatitis B or C virus (HBV, HCV) infections. The connection between obesity and cancer is likely to be mediated, in part, by a state of chronic low-grade inflammation in the involved tissues [5–10]. Liver inflammation has been shown to be associated with obesity-induced NAFLD, NASH, fibrosis, and cirrhosis, resulting in elevated production of various cytokines and adipokines, which have been implicated in hepatocarcinogenesis. There are additional explanations for the effect of obesity on HCC risk and the process of cirrhosis (Figure 1). This review is focused on the pathogenic role of inflammation and it aims to summarize recent advances in understanding of the obesity-HCC link based on basic mechanistic studies carried out in mouse models that were confirmed in human clinical material.
Figure 1.
Three putative mechanisms for obesity-induced and obesity-promoted hepatocarcinogenesis. ROS, reactive oxygen species; RNS, reactive nitrogen species
Obesity and hepatocellular carcinoma
The steady increase in BMI has become a worldwide pandemic and is currently estimated to cause more than 90,000 cancer-related deaths per year in the US alone [6]. The incidence of obesity in both adults and children during the past three decades has increased drastically, also in other parts of the world, including developing countries such as China and India [11–14]. Obesity has been shown to be an independent risk factor for some malignancies including breast cancer, endometrial cancer, colon cancer, renal cell carcinoma, esophageal adenocarcinoma, pancreatic ductal adenocarcinoma and HCC [3, 15–19]. Furthermore, obesity is associated with poor prognosis of breast cancer and colon cancer [19, 20].
HCC is the dominant form of primary liver carcinoma (PLC), ranking sixth in incidence and third in mortality amongst all cancers. HCC accounts for 85% to 90% of PLC worldwide and constitutes 70% to 75% of PLC cases in the US [21–23]. Although HBV and HCV infections are considered as major HCC risk factors worldwide, at least in the US, obesity is likely to be the primary risk factor along with other non-viral factors, such as type 2 diabetes mellitus, alcohol, tobacco and oral contraceptives [23, 24]. Obesity also represents an independent HCC risk factor in patients with alcoholic cirrhosis and cryptogenic cirrhosis [3]. A follow-up study in Taiwan has implicated synergistic effects between metabolic disorders (obesity and diabetes) and viral hepatitis, with HCC risk increasing by more than 100-fold in HBV or HCV carriers with obesity and diabetes [25].
Obesity has been implicated in the genesis of metabolic syndromes including insulin resistance and type 2 diabetes, and a spectrum of non-cancerous liver diseases, such as NAFLD and NASH, hepatic fibrosis and cirrhosis [26]. On the other hand, some “metabolic benign obesity” with only abdominal adiposity and without insulin resistance does not appear to play a determining role in steatohepatitis [27], suggesting that the obesity-induced metabolic disorder may be a major cause of fatty liver. Indeed, NAFLD is strongly associated with type 2 diabetes mellitus and dyslipidemia [28–30]. Accumulation of fat, because of excess caloric intake, genetic factors or other diseases, can result in liver dysfunction as the liver synthesizes more triglycerides but fails to export them. Consequently, triglycerides accumulate in parenchymal liver cells (hepatocytes), leading to hepatosteatosis. As such, obesity is the main risk factor for NAFLD, but NAFLD is a reversible disorder, whose underlying causes can be treated and inhibited in its early stages [29]. For example, obesity-induced fatty liver can be treated by weight loss through exercise and dietary control. However, without proper management, NAFLD may progress to chronic liver inflammation, termed as steatohepatitis (NASH), which is a severe condition of inflamed fatty liver that can further progress to liver fibrosis and cirrhosis causing serious complications, including liver failure and HCC [8, 31]. PLC, including both HCC and intrahepatic cholangiocarcinoma, often occur in patients with NASH, especially in those with advanced fibrosis and cirrhosis, and the occurrence of HCC is the strongest predictor of mortality in patients with old age and advanced fibrosis [26, 32]. It should also be noted that obesity and NAFLD can induce proliferation and decrease apoptosis of hepatocytes in a mouse model, resulting in hepatic hyperplasia, in the absence of inflammation, fibrosis and cirrhosis [33].
Cytokines and adipokines in obesity-induced liver inflammation
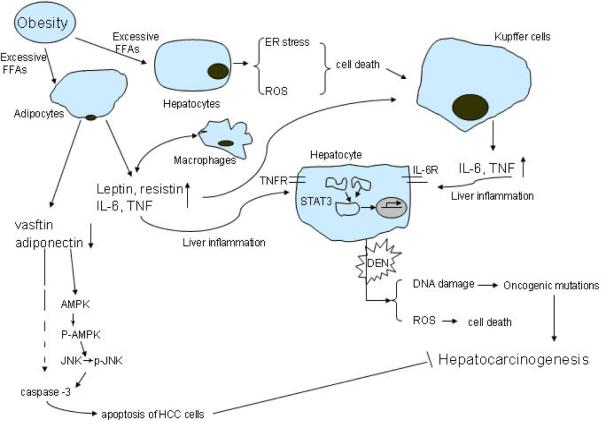
There is substantial evidence that obesity is associated with chronic low-grade systemic inflammation, which is believed to contribute to metabolic disorders, and the progression from hepatic steatosis to NASH, fibrosis, cirrhosis and finally to HCC. Although the entire process of progression has not been fully elucidated, within this proces, the switch for hepatosteatosis to steatohepatitis is key, as without inflammation, none of the other pathologies will ensure. We will therefore discuss the cytokines involved in liver inflammation and its associated metabolic disorders. Obesity and inflammation associated metabolic disorders are often manifested by insulin resistance, resulting in elevated plasma concentrations of insulin and insulin-like growth factor 1 (IGF-1), and can lead to increased secretion of cytokines (known as adipokines) by adipose tissue [34], as well as inflammatory cells, which include resident liver macrophages or Kupffer cells (KCs) [10, 34]. Adipocytes in obese individuals undergo hypertrophy due to deposition and accumulation of excess lipids. Hypertrophic adipocytes secrete free fatty acids (FFAs), and together with various immune cells they release various pro-inflammatory cytokines including tumor necrosis factor (TNF), inerleukin-6 (IL-6), IL-1β, IL-8, IL-10, IL-18 and IL-17, as well as more specialized adipokines, such as leptin and adiponectin [8, 34–40] (Figure. 2). Furthermore, saturated FFAs lead to activation of Jun kinases (JNK) and the production of inflammatory cytokines by different cell types [41, 42]. We have recently found that one of the first steps in cell signaling elicited by saturated FFA is the clustering and eventual activation of c-Src within specific membrane sub-domains (Holzer et al, Cell in Press). Notably, it has been demonstrated that a 19% weight loss in obese women led to reduced plasma TNF, IL-6 and leptin, and increased plasma adiponectin [43]. Among all of these cytokines, IL-6 is both pro-inflammatory and a useful marker for obesity-associated inflammation. In the liver, IL-6 is mainly secreted by KC and hepatic stellate cells (HSC), and to a lesser extent by stimulated hepatocytes [44, 45]. Circulating IL-6 is elevated in obese individuals and type 2 diabetics [46–48]. On the other hand, reduced caloric intake and increased physical activity result in reduced plasma IL-6 in obese children and adolescents [49]. Earlier studies have also revealed that both IL-6 and TNF increase hepatic production of C-reactive protein (CRP), a major acute phase protein, which is a nonspecific but sensitive marker of infection and tissue inflammation that is increased in obesity [50–53]. Other cytokines, including IL-1β, oncostatin M (OSM) or leukaemia inhibitory factor (LIF), can drive hepatic inflammation by inducing production of CRP, independently of IL-6 [50–52]. Concerning the cellular source of these cytokines, besides KC and adipocytes, infiltration of CD8+ T cells into obese epididymal adipose tissue was found to precede accumulation of macrophages and a CD8-specific antibody treatment lowered the mRNA expression of both TNF and IL-6 in adipose tissue, suggesting that CD8+ T cells may be key regulators of adipose inflammation [54, 55]. As cytokines produced by adipocytes and macrophages reach the portal venous system, KC and hepatocytes are stimulated to produce more cytokines, resulting in an inflammatory cascade in the liver [56].
Figure 2.
Adipokines, cytokines and hepatocarcinogenesis. Excessive free fatty acids (FFAs) can activate various immune cells and cause hepatocytes cell death. Moreover, cell debris, pro-inflammatory cytokines and adipokines can further enhance TNF and IL-6 secretion from Kupffer cells, leading to activation of downstream signaling molecules, such as STAT3 in hepatocytes which contribute to hepatocarcinogenesis.
Several cytokines have strong influence on the regulation of insulin resistance in the context of hepatic inflammation. TNF is primarily produced by macrophages, but also by adipose tissue of obese mice and men [57]. Furthermore, TNF was demonstrated to play a significant role in insulin resistance at least in mice[58]. Elevated expression of TNF mRNA and protein was detected in obese rodents and humans. Loss of TNF or its receptors (TNFR1 and TNFR2) improves insulin sensitivity in obese mice [59]. However, neutralization of TNF was found ineffective in restoring insulin sensitivity in diabetic patients [60, 61]. Insulin sensitivity in leptin-deficient ob/ob mice is improved by IL-6 depletion using a neutralizing antibody [62], moreover, a recent study has shown that IL-6 can inhibit insulin signaling in hepatocytes [63]. However, so far no clinical studies of the ability of anti-IL-6 drugs to improve insulin sensitivity and liver metabolism have been reported. Furthermore, administration of an inhibitory anti-IL-6 receptor antibody was found to cause a transient increase in serum lipoproteins [64]
Leptin, whose effects were discovered in the 1950s [65], but was not identified until 1994 [66], is the product of the obese (ob) gene and is mainly produced by adipocytes of white adipose tissue (WAT), and to a lesser extent by brown adipose tissues, placenta, ovaries, skeletal muscle, stomach, bone marrow, and liver [67–70]. Leptin can regulate energy intake and expenditure by binding to receptors expressed by CNS neurons [71, 72]. Leptin signaling prevents weight gain under physiological conditions and the serum concentration and mRNA amounts of leptin are positively associated with the amount of energy stored in adipose tissue, and total adipose tissue mass, in both humans and mice [73–75]. Thus, leptin production is a key negative feedback mechanism in BMI regulation. Leptin expression is stimulated by many acute phase factors, such as TNF, IL-1 and IL-6, and during bacterial infection, or lipopolysaccharide (LPS) challenge [76]. Leptin-deficient (ob/ob) or leptin receptor-deficient (db/db) mice spontaneously develop obesity even on normal chow [77–79].
Adiponectin is a protein which is encoded by the Ad/Poq gene [27, 80]. Like leptin, it is also secreted by adipocytes, but unlike leptin, adiponectin is inversely associated with high BMI in adults and the circulating concentrations of adiponectin are reduced in diabetics compared to non-diabetics [81]. Adiponectin is an anti-inflammatory hormone and its circulating concentration is inversely correlated with those of inflammatory markers, and positively associated with the anti-inflammatory cytokine IL-10 [82, 83]. Moreover, there is a significant increase in circulating adiponectin in obese individuals undergoing weight loss [84, 85]. Circulating adiponectin is also increased in children after short-term weight loss, which also ameliorates insulin sensitivity [85, 86]. In ob/ob mice, acute treatment with adiponectin stimulated phosphorylation of AMP-activated protein kinase (AMPK) in liver tissue and improved insulin sensitivity [87]. Additionally, adiponectin-deficient mice on high fat diet developed early-stage NASH with increased TNF expression and fibrosis [88, 89]. Knockout of one of the two adiponectin receptors (adipoR1 and R2) in mice increased insulin resistance, whereas knockout of both adipoR1 and R2 caused increased tissue triglyceride content, inflammation and oxidative stress, leading to insulin resistance and marked glucose tolerance [90, 91]. Moreover, adiponectin protects against liver tumorigenesis directly by increasing phosphorylation of AMPK and tumor suppressor tuberous sclerosis complex 2 (TSC2) protein and inhibiting the phosphorylation of mammalian target of rapamycin (mTOR), reduced adiponectin expression is associated with poor prognosis in obese patients with HCC[92]. Taken together, adiponectin is a negative regulator of obesity-induced inflammation and other pathologies.
Other cytokines and adipokines
IL-1β is another inflammation cytokine that can induce insulin resistance in Fao and HepG2 cell lines, and in primary rat hepatocytes, whereas cells treated with IL-1 receptor antagonist (IL-1RA) were protected against insulin resistance induced by conditioned medium from 3T3-L1 adipocytes treated with TNF [38]. IL-17 secreted by T helper 17 (Th17) cells was also reported to have a pivotal role in obesity-induced inflammation [35]. Another adipokine suggested to provide a potential link between obesity and diabetes is resistin, as its circulating amounts were decreased by treatment with anti-diabetic drugs and its administration impaired insulin function in normal mice [93]. Recently, a new adipokine, chemerin, whose concentrations are elevated in morbidly obese patients, was described. Chemerin is involved in adipogenesis and is positively associated with insulin resistance, increased CRP and IL-6, and negatively associated with high-density lipoprotein [94]. By contrast, IL-33 was suggested to protect obese individuals from development of adipose tissue inflammation [95]. Secreted frizzled-related protein (Sfrp) 5 was identified as a new anti-inflammatory adipokine, whose expression is reduced in ob/ob mice and Zucker diabetic fatty rats (91). It was proposed that Sfrp5 neutralized noncanonical JNK activation by Wnt5a in macrophages and adipocytes to improve metabolic function and reduce adipose tissue inflammation [96]. JNK activation by inflammatory cytokines and FFA was shown to be a major contributor to obesity-induced insulin resistance and metabolic inflammation [42, 97].
Cytokine signaling pathways associated with obesity-induced inflammation
Although many cytokines were shown to modulate and mediate obesity-induced inflammation and progression of NAFLD, the central mechanism that mediates the effects of these cytokines on obesity-induced metabolic disorders associated with chronic steatohepatitis such as insulin resistance, NAFLD, and NASH, are not fully clear. Nonetheless, several specific intracellular signaling pathways, including nuclear factor (NF)-κB, JNK, activating protein-1 (AP-1), and STAT3 have emerged as potential targets for many of these cytokines and chemokines. Another important signaling pathway – the AMPK-TORC1 pathway will be discussed separately below.
NF-κB is a collection of protein dimers that control the transcription of a host of target genes [98]. Abnormal regulation of NF-κB has been linked to cancer and inflammatory disease [99]. In non-stimulated cells, NF-κB dimers are mainly kept inactive in the cytoplasm, through binding to inhibitory proteins called IκB [98]. The IκB kinase (IKK) complex, which is responsive to many inflammatory stimuli, phosphorylates the IκBs, thereby triggering their degradation, and causing NF-κB activation [100]. Activated NF-κB dimers translocate to the nucleus where they bind to specific DNA sequences and regulate transcription of distinct target genes. Mice lacking IKKβ in hepatocytes (IkkβΔhep) or in myeloid cells (IkkβΔmye) were generated and fed either normal chow or high fat diet (92). IkkβΔhep mice retained liver insulin sensitivity, but developed insulin resistance in muscle and fat in response to high fat diet. IkkβΔmye mice, however, retained global insulin responsiveness and were protected from obesity-induced insulin resistance. It was suggested based on these results that inhibition of IKKβ, especially in myeloid cells, is useful for the treatment of insulin resistance [101]. However, conditional disruption of IKKβ in skeletal muscle failed to prevent obesity-induced insulin resistance [102]. It was also found that high fat diet increased NF-κB activation, which results in a sustained elevation of the IKK-related kinase IKKε in liver, adipocytes, and adipose tissue macrophages [103]. IKKε ablation reduced expression of inflammatory cytokines and protected mice from high-fat diet-induced obesity, chronic inflammation in liver and adipose tissue, and hepatic steatosis [103]. As liver specific ablation of IKKβ increases sensitivity to inflammatory and toxic challenges [104, 105] and systemic IKKβ inhibition can lead to neutrophilia [106, 107], IKKε inhibition may be preferable to IKKβ inhibition. In addition to IKKs and NF-κB, Jun kinases (JNK) are activated by almost all signaling pathways proposed to cause insulin resistance or β-cell failure, and their inhibition provides protection from obesity and glucose intolerance in rodents [10]. The two main isoforms of JNK, JNK1 and JNK2, appear to have distinct specific effects on murine steatohepatitis and insulin resistance. Singh et al demonstrated that both JNK1 and JNK2 are involved in insulin resistance in mice fed with high-fat diet mice through genetic ablation of JNK1 or JNK2; but whereas JNK1 promotes steatosis and hepatitis, JNK2 inhibits hepatocyte cell death [108]. Interestingly, obesity also leads to JNK activation in humans, whereas reduced JNK activity was seen upon weight loss [109]. JNK activation can lead to increased production of inflammatory cytokines capable of causing insulin resistance [42]. IL-6 and TNF expression in liver is strongly induced in response to high fat diet, but inhibition of TNF signaling through TNFR1 or ablation of IL-6 prevented hepatosteatosis without a considerable effect on weight gain [8]. Fas (CD95), a receptor related to TNFR1, can also activate inflammatory pathways in several cell lines and tissues, and its deficiency either in all cells or specifically in adipocytes protected mice from insulin resistance induced by high-fat diet [110].
Although several pathways have been implicated in metabolic inflammation, the IKK and JNK signaling pathways in adipocytes, macrophages and hepatocytes have emerged as the pivotal mediators of obesity-induced inflammation and even systemic metabolic disorders [8, 42, 97, 101, 111, 112]. As discussed below, these pathways are also involved in liver tumorigenesis.
TORC1 signaling, autophagy and hepatosteatosis
In addition to the inflammatory signaling pathways listed above, a major signaling pathway involved in hepatosteatosis and hepatocarcinogenesis is the AMPK-TORC1 pathway. AMPK is a protein kinase complex composed of alpha (catalytic subunit), beta and gamma (regulatory subunits), whose activity is stimulated upon binding of AMP [113]. Since AMP concentrations in the cell are much higher when the conversion of AMP to ADP and eventually ATP is inhibited, AMPK is activated upon starvation, caloric restriction, exercise or drugs that act as mitochondrial uncouplers. AMPK has many important substrates involved in metabolic regulation, including ACC (acetyl CoA carboxylase) and HMGCR (HMG CoA reductase), rate limiting enzymes that control biosynthesis of fatty acids and cholesterol, respectively [113]. One of the main AMPK substrates is the TSC1:TSC2 tumor suppressor complex, whose activity is inhibited upon AMPK-mediated phosphorylation [114]. Inhibition of TSC1:TSC2 activity decreases the GTP loading of the Ras-related GTPase Rab, which serves as the activator of the TORC1 protein kinase complex [115–117]. TORC1 contains the catalytic subunit mTOR (mammalian target of rapamycin) in complex with the adaptor protein raptor and several other subunits [115]. TORC1 also has a number of substrates, including the translational inhibitor 4EBP1 and p70S6 kinase, through which TORC1 activation stimulates the translation of certain mRNAs and ribosome biosynthesis [118]. Activation of TORC1 also leads to phosphorylation and inhibition of the ULK1 protein kinase complex composed of ATG1, ATG13 and FIP200, whose activity is required for the initiation of autophagy [119]. Curiously, AMPK-mediated phosphorylation was recently found to have the opposite effect on ULK1 activity [120]. Thus, inhibition of AMPK1 in response to hypernutrition and the activation of TORC1 result in inhibition of autophagy, which is a major catabolic pathway and quality control process. In addition to degradation and eventual recycling of abnormal proteins and damaged organelles [121], autophagy was recently found to be a major pathway for the removal of lipid droplets from hepatocytes [122], and is likely to have tumor suppressive and auto-inflammatory activities[123, 124]. Thus, by inhibition of AMPK and activation of TORC1, hypernutrition and excessive caloric intake lead to inhibition of autophagy, thereby stimulating the development of hepatosteatosis and all of its sequella, including NASH and increased HCC risk. Histological studies have revealed the accumulation of p62, a hallmark of autophagy, during steatohepatitis [125, 126].
One way to reactivate autophagy in the hepatosteatotic liver is through the use of the anti-diabetic drug metformin. Metformin is known to cause activation of AMPK through a poorly defined mechanism and thereby it leads to inhibition of TORC1 and stimulation of autophagy [127, 128]. Another way to inhibit TORC1 and stimulate autophagy is through the use of rapamycin and other TORC1 inhibitors [129]. Interestingly, metformin use was found to be associated with reduced cancer risk [130]. In particular, metformin treatment was found to be associated with a strong and statistically significant reduction in HCC risk amongst diabetics and it also seems to slow down HCC development [131, 132]. Thus, metformin use by type 2 diabetes may reverse the increase in HCC risk associated with insulin resistance and obesity. Rapamycin use may also reduce HCC risk and clinical trials using rapamycin and other TORC1 inhibitors in the treatment of HCC were recently conducted [133, 134].
Genetic instability associated with obesity
Although the progression from inflammation to fibrosis, and then cirrhosis is widely accepted as the main etiology of obesity-associated cancers including HCC, other mechanisms such as DNA damage and oncogenic mutations remain relatively underexplored in obesity-related tumorigenesis. Recently, Scarpato et al compared DNA damage lesions and chromosome mutations in the peripheral lymphocytes from normal, overweight, and obese Italian children [9]. As expected, they found that obesity was associated with chronic inflammation as marked by higher serum levels of IL-6 and CRP in obese and overweight children than in normal-weight children. They also found that both DNA strand breaks, detected with a γ-H2AX focus assay, and micronucleus frequency, detected by staining for broken chromosomes, were elevated in peripheral lymphocytes from obese and overweight children in comparison to those from normal-weight children. These results suggest that a constitutively high frequency of DNA lesions and unrepaired DNA damage in micronuclei may contribute to increased risk of cancer, including HCC, later in life of obese children. Thus, while inflammation plays an important role in tumor initiation, promotion, and metastasis [135], the contribution of genetic instability to obesity-enhanced cancer needs further investigation.
Murine models for obesity-promoted liver cancer
Although epidemiological and retrospective studies have provided considerable insights to the effects of obesity on liver inflammation and the development of HCC, a full mechanistic understanding of obesity-promoted liver tumorigenesis depends on the use of appropriate animal models that replicate the human pathology and are amenable to genetic analysis. Several such models were recently developed. One particularly interesting model is based on the conditional deletion of the gene encoding NEMO/IKKγ, the IKK regulatory subunit in hepatocytes. IkkγΔhep mice develop spontaneous liver damage, hepatosteatosis, fibrosis and eventually HCC [136]. However, just like IkkβΔhep mice [101], IkkγΔhep mice are protected from obesity-induced insulin resistance, although their hepatosteatosis becomes worse when kept on HFD [137]. In addition to augmenting hepatosteatosis, feeding HFD to IkkγΔhep mice accelerates and enhances HCC development. Since in addition to insulin resistance, IkkγΔhep mice are also protected from peripheral obesity in response to HFD [137]. Similar findings were observed in Tak1Δhep mice, enhancement of hepatocarcinogenesis was due to a downstream consequence of sustained apoptosis and the emergence of regenerative clones that acquire a dedifferentiated phenotype[138]. Furthermore, TAK1-deficient mice were also resistant to the development of HFD-induced metabolic syndrome and protected from development of glucose intolerance and insulin resistance through decreased infiltration of inflammatory cells and expression of inflammatory genes in white adipose tissue[139]. However, TAK1 has been reported to repress transcription of the telomerase reverse-transcriptase gene, suggesting a direct effect of TAK1 in cancer promotion, which was different to IkkγΔhep mice [140]. These results strongly suggest that increased BMI and elevated blood glucose or blood insulin are not directly responsible for obesity-promoted liver tumorigenesis.
A more commonly used model of HCC induction in rodents is based on administration of the chemical pro-carcinogen diethyl nitrosamine (DEN). It was found that even a short time of HFD (6 weeks) led to a marked increase in induction of pre-neoplastic liver lesions in DEN-administered rats [141]. This was accompanied by enhanced infiltration of inflammatory cells and higher ERK activity in livers of HFD-fed rats, but lower amounts of p38 phosphorylation and activity [141]. A more thorough mechanistic analysis of obesity-promoted chemically-induced hepatocarcinogenesis was conducted by Park et al., who injected 2 weeks old mice with DEN and at 4 weeks of age placed the mice either on normal chow or HFD [8]. Tumors were analyzed 8 months later. Consumption of HFD led to a marked increase in HCC incidence, multiplicity and size and as observed in humans, the effect was more pronounced in males than in females [8]. An even more striking enhancement of HCC development was seen in mice that were first fed HFD for 3 months and then given DEN. These mice all developed HCC, whereas mice kept on normal chow did not develop any tumors unless DEN administration was followed by treatment with the hepatic tumor promoter phenobarbitol. Analysis of the mechanism through which HFD may enhance DEN-induced hepatocarcinogenesis revealed elevated ERK and JNK activities in HCCs that evolved in mice on HFD but reduced p38 MAPK activity [8]. Although the basis for the reduction of p38 MAPK activity and its effect on HCC development in mice or rats kept on HFD has not been explored, it should be noted that ablation of p38α strongly enhances DEN-induced HCC development [142, 143]. Thus, reduced p38 activation may be an important pathogenic mechanism.
Another signaling protein whose activity is elevated in both non-tumor liver tissues and HCCs of HFD-fed mice is STAT3 [8]. STAT3 activation in hepatocytes is essential for DEN-induced HCC development [144] and for obesity-stimulated tumor growth [8]. The main cause of STAT3 activation is elevated production of the pro-inflammatory cytokines IL-6, which leads to direct STAT3 activation, and TNF which stimulates the expression of IL-6 [8]. Ablation of IL-6 or TNFR1 blocked obesity-promoted hepatocarcinogenesis. The mechanism responsible for this protective effect was determined to be reduced hepatosteatosis and steatohepatitis [8]. As seen with the ablation of NEMO, the IL-6 or TNFR1 deficiencies had little effect, if any, on fat accumulation in peripheral depots, underscoring the notion that increased BMI is not directly responsible for obesity-promoted hepatocarcinogenesis. In other words, fat accumulation in hepatocytes which can culminate in fatty liver inflammation is far more important than accumulation of subcutaneous fat [145].
HCCs as well as normal liver tissue of mice fed with HFD revealed elevated TORC1 activity manifested by increased phosphorylation of the TORC1 substrate p70S6 kinase and its substrate ribosomal protein S6 [8]. By contrast, phosphorylation of AKT was reduced, most likely reflecting the insulin resistant state of mice kept on HFD. Future studies should be directed at assessing the contribution of elevated TORC1 activity to obesity-promoted hepatocarcinogenesis. Nonetheless, it is well established that TORC1 activation can disrupt autophagy and may be the primary mediator of defective autophagy in the hepatosteatotic livers. Curiously, disruption of autophagy, as occurs in ATG7 or ATG5 knockout mice, leads to spontaneous HCC development[146, 147]. Interestingly, the ablation of p62, a chaperon for ubiquitinated proteins, that accumulates in steatohepatitis[148], protected liver specific ATG7 KO mice from HCC development[147]. Although much more work remains to be done with these mouse models, the conclusions from all of these studies are similar and clear. Hepatosteatosis promotes HCC development through enhancement of liver inflammation and disruption of autophagy, mechanisms that appear to be highly relevant to the pathogenesis of human HCC [149–151]. On the other hand, insulin resistance and diabetes may not be as important and could be unrelated pathogenic processes instigated by hepatosteatosis.
Conclusions
Obesity has become a serious public health problem in the United States and elsewhere due to its effects on human health, resulting in metabolic and cardiovascular disorders and increasing cancer risk. Amongst all cancers, the one that is most strongly enhanced by obesity is HCC. Obesity enhances HCC development through lipid accumulation within hepatocytes, thereby leading to a chronic low-grade liver inflammation, involving various cytokines and adipokines. Extensive research in this field has shed some light on some of the cytokines and adipokines that contribute to the onset of steatohepatitis and the initiation and promotion of HCC. However, there are many remaining questions, including the effect of hepatosteatosis on genetic instability within hepatocytes, the mechanisms that control the progression from hepatosteatosis to steatohepatitis and how chronic steatohepatitis leads to tumor initiation, that remain to be answered. While weight loss by bariatric surgery, diet or exercise has been shown to ameliorate obesity-induced metabolic syndromes, more effective therapeutic interventions are needed to prevent the development of HCC or halt its progression. The basic research reviewed above has revealed several new targets for therapeutic and preventive intervention, but advanced translational research has only begun.
Table 1.
Murine models associated with obesity and HCC
| Gene | KO Phenotype | Advantages and Shortcomings |
|---|---|---|
| IL-6 | Mature onset of obesity and insulin resistance on HFD; reduced obesity-induced HCC promotion | Advantages:
|
| TNFR1 | Rapid weight-gain like WT on HFD; ablation of obesity-enhanced HCC development; reduced obesity-induced steatohepatitis | Shortcomings:
|
| IKKβ | Improved insulin sensitivity; Enhanced DEN-induced HCC development, but protection from LT-induced HCC suggesting that IKKβ and NF-κB activation promote, rather than inhibit, HCC development. | |
| p38α | Enhanced DEN-induced HCC development | |
| NEMO/IK Kγ | Protected from obesity-induced insulin resistance; development of spontaneous liver damage, hepatosteatosis, fibrosis and eventually HCC | |
| TAK-1 | Protected from obesity-induced insulin resistance; development of spontaneous liver damage, hepatosteatosis, fibrosis and eventually HCC. | |
| ATG7 | Spontaneously multiple benign hepatocellular adenoma development accompanied by mitochondria dysfunction and genomic instability. | |
| ATG5 | Spontaneously multiple benign hepatocellular adenoma development accompanied mitochondrial swelling, p62 accumulation, and oxidative stress and genomic damage responses |
Acknowledgement
Grant support: supported by grants from the National Natural Science Foundation (81072029 and 91029721 to B.S.), the New Century Excellent Talents in University, Ministry of Education (NCET-09-0160 to B.S.) and the National Institutes of Health (ES010337, ES006376 and CA118165 to M.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest The authors declare that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript. The authors do not have any relationship with the manufacturers of the drugs mentioned in the review and did not and do not receive funding from any drug manufacturer to carry out their research.
References
- [1].Rocchini AP. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346:854–855. doi: 10.1056/NEJM200203143461112. [DOI] [PubMed] [Google Scholar]
- [2].Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- [3].Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150–155. doi: 10.1053/jhep.2002.33713. [DOI] [PubMed] [Google Scholar]
- [4].Regimbeau JM, Colombat M, Mognol P, Durand F, Abdalla E, Degott C, et al. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transpl. 2004;10:S69–73. doi: 10.1002/lt.20033. [DOI] [PubMed] [Google Scholar]
- [5].Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- [6].Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- [7].Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5:70–75. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- [8].Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Scarpato R, Verola C, Fabiani B, Bianchi V, Saggese G, Federico G. Nuclear damage in peripheral lymphocytes of obese and overweight Italian children as evaluated by the gamma-H2AX focus assay and micronucleus test. FASEB J. 2011;25:685–693. doi: 10.1096/fj.10-168427. [DOI] [PubMed] [Google Scholar]
- [10].Solinas G, Karin M. JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. FASEB J. 2010;24:2596–2611. doi: 10.1096/fj.09-151340. [DOI] [PubMed] [Google Scholar]
- [11].Adams JP, Murphy PG. Obesity in anaesthesia and intensive care. Br J Anaesth. 2000;85:91–108. doi: 10.1093/bja/85.1.91. [DOI] [PubMed] [Google Scholar]
- [12].Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- [13].Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- [14].Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- [15].Chow WH, Gridley G, Fraumeni JF, Jr., Jarvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med. 2000;343:1305–1311. doi: 10.1056/NEJM200011023431804. [DOI] [PubMed] [Google Scholar]
- [16].Richardson LC, Thomas C, Bowman BA. Obesity and endometrial cancer: challenges for public health action. Womens Health (Lond Engl) 2009;5:595–597. doi: 10.2217/whe.09.62. [DOI] [PubMed] [Google Scholar]
- [17].Schapira DV, Kumar NB, Lyman GH, Cox CE. Abdominal obesity and breast cancer risk. Ann Intern Med. 1990;112:182–186. doi: 10.7326/0003-4819-112-3-182. [DOI] [PubMed] [Google Scholar]
- [18].Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: weight of the evidence. J Clin Oncol. 2011;29:4–7. doi: 10.1200/JCO.2010.32.1752. [DOI] [PubMed] [Google Scholar]
- [19].Sinicrope FA, Foster NR, Sargent DJ, O'Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16:1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111:329–342. doi: 10.1007/s10549-007-9785-3. [DOI] [PubMed] [Google Scholar]
- [21].El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- [22].Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- [23].Yu MC, Yuan JM. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology. 2004;127:S72–78. doi: 10.1016/j.gastro.2004.09.018. [DOI] [PubMed] [Google Scholar]
- [24].Blonski W, Kotlyar DS, Forde KA. Non-viral causes of hepatocellular carcinoma. World J Gastroenterol. 2010;16:3603–3615. doi: 10.3748/wjg.v16.i29.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- [26].Diehl AM. Hepatic complications of obesity. Gastroenterol Clin North Am. 2010;39:57–68. doi: 10.1016/j.gtc.2009.12.001. [DOI] [PubMed] [Google Scholar]
- [27].Tarantino G, Colicchio P, Conca P, Finelli C, Di Minno MN, Tarantino M, et al. Young adult obese subjects with and without insulin resistance: what is the role of chronic inflammation and how to weigh it non-invasively? J Inflamm (Lond) 2009;6:6. doi: 10.1186/1476-9255-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48(Suppl 1):S104–112. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- [29].Eriksson S, Eriksson KF, Bondesson L. Nonalcoholic steatohepatitis in obesity: a reversible condition. Acta Med Scand. 1986;220:83–88. doi: 10.1111/j.0954-6820.1986.tb02733.x. [DOI] [PubMed] [Google Scholar]
- [30].Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- [31].Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- [32].Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Tokushige K, et al. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44(Suppl 19):89–95. doi: 10.1007/s00535-008-2262-x. [DOI] [PubMed] [Google Scholar]
- [33].Yang S, Lin HZ, Hwang J, Chacko VP, Diehl AM. Hepatic hyperplasia in noncirrhotic fatty livers: is obesity-related hepatic steatosis a premalignant condition? Cancer Res. 2001;61:5016–5023. [PubMed] [Google Scholar]
- [34].Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- [35].Ahmed M, Gaffen SL. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 2010;21:449–453. doi: 10.1016/j.cytogfr.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bruun JM, Pedersen SB, Richelsen B. Regulation of interleukin 8 production and gene expression in human adipose tissue in vitro. J Clin Endocrinol Metab. 2001;86:1267–1273. doi: 10.1210/jcem.86.3.7264. [DOI] [PubMed] [Google Scholar]
- [37].Nawrocki AR, Scherer PE. Keynote review: the adipocyte as a drug discovery target. Drug Discov Today. 2005;10:1219–1230. doi: 10.1016/S1359-6446(05)03569-5. [DOI] [PubMed] [Google Scholar]
- [38].Nov O, Kohl A, Lewis EC, Bashan N, Dvir I, Ben-Shlomo S, et al. Interleukin-1beta may mediate insulin resistance in liver-derived cells in response to adipocyte inflammation. Endocrinology. 2010;151:4247–4256. doi: 10.1210/en.2010-0340. [DOI] [PubMed] [Google Scholar]
- [39].Patton JS, Shepard HM, Wilking H, Lewis G, Aggarwal BB, Eessalu TE, et al. Interferons and tumor necrosis factors have similar catabolic effects on 3T3 L1 cells. Proc Natl Acad Sci U S A. 1986;83:8313–8317. doi: 10.1073/pnas.83.21.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- [41].Solinas G, Naugler W, Galimi F, Lee MS, Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc Natl Acad Sci U S A. 2006;103:16454–16459. doi: 10.1073/pnas.0607626103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- [43].Bougoulia M, Triantos A, Koliakos G. Effect of weight loss with or without orlistat treatment on adipocytokines, inflammation, and oxidative markers in obese women. Hormones (Athens) 2006;5:259–269. doi: 10.14310/horm.2002.11190. [DOI] [PubMed] [Google Scholar]
- [44].Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- [45].Jarrar MH, Baranova A, Collantes R, Ranard B, Stepanova M, Bennett C, et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412–421. doi: 10.1111/j.1365-2036.2007.03586.x. [DOI] [PubMed] [Google Scholar]
- [46].Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003;52:2784–2789. doi: 10.2337/diabetes.52.11.2784. [DOI] [PubMed] [Google Scholar]
- [47].Muller S, Martin S, Koenig W, Hanifi-Moghaddam P, Rathmann W, Haastert B, et al. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-alpha or its receptors. Diabetologia. 2002;45:805–812. doi: 10.1007/s00125-002-0829-2. [DOI] [PubMed] [Google Scholar]
- [48].Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–1292. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- [49].Gallistl S, Sudi KM, Aigner R, Borkenstein M. Changes in serum interleukin-6 concentrations in obese children and adolescents during a weight reduction program. Int J Obes Relat Metab Disord. 2001;25:1640–1643. doi: 10.1038/sj.ijo.0801808. [DOI] [PubMed] [Google Scholar]
- [50].Castell JV, Gomez-Lechon MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12:1179–1186. doi: 10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- [51].Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- [52].Weinhold B, Ruther U. Interleukin-6-dependent and -independent regulation of the human C-reactive protein gene. Biochem J. 1997;327(Pt 2):425–429. doi: 10.1042/bj3270425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Weyer C, Yudkin JS, Stehouwer CD, Schalkwijk CG, Pratley RE, Tataranni PA. Humoral markers of inflammation and endothelial dysfunction in relation to adiposity and in vivo insulin action in Pima Indians. Atherosclerosis. 2002;161:233–242. doi: 10.1016/s0021-9150(01)00626-8. [DOI] [PubMed] [Google Scholar]
- [54].Nishimura S, Manabe I, Nagai R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov Med. 2009;8:55–60. [PubMed] [Google Scholar]
- [55].Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- [56].Schwarzenberg SJ, Sinaiko AR. Obesity and inflammation in children. Paediatr Respir Rev. 2006;7:239–246. doi: 10.1016/j.prrv.2006.08.002. [DOI] [PubMed] [Google Scholar]
- [57].Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- [58].Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- [60].Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med. 2006;166:902–908. doi: 10.1001/archinte.166.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes. 1996;45:881–885. doi: 10.2337/diab.45.7.881. [DOI] [PubMed] [Google Scholar]
- [62].Klover PJ, Clementi AH, Mooney RA. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology. 2005;146:3417–3427. doi: 10.1210/en.2004-1468. [DOI] [PubMed] [Google Scholar]
- [63].Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–3399. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- [64].Kawashiri SY, Kawakami A, Yamasaki S, Imazato T, Iwamoto N, Fujikawa K, et al. Effects of the anti-interleukin-6 receptor antibody, tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol Int. 2011;31:451–456. doi: 10.1007/s00296-009-1303-y. [DOI] [PubMed] [Google Scholar]
- [65].Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- [66].Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- [67].Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- [68].Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- [69].Rayner DV, Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J Mol Med. 2001;79:8–20. doi: 10.1007/s001090100198. [DOI] [PubMed] [Google Scholar]
- [70].Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–339. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- [71].Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–388. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- [72].Williams KW, Scott MM, Elmquist JK. From observation to experimentation: leptin action in the mediobasal hypothalamus. Am J Clin Nutr. 2009;89:985S–990S. doi: 10.3945/ajcn.2008.26788D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- [74].Iikuni N, Lam QL, Lu L, Matarese G, La Cava A. Leptin and Inflammation. Curr Immunol Rev. 2008;4:70–79. doi: 10.2174/157339508784325046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Vidal H, Auboeuf D, De Vos P, Staels B, Riou JP, Auwerx J, et al. The expression of ob gene is not acutely regulated by insulin and fasting in human abdominal subcutaneous adipose tissue. J Clin Invest. 1996;98:251–255. doi: 10.1172/JCI118786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Faggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15:2565–2571. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- [77].Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- [78].Friedman JM, Leibel RL, Siegel DS, Walsh J, Bahary N. Molecular mapping of the mouse ob mutation. Genomics. 1991;11:1054–1062. doi: 10.1016/0888-7543(91)90032-a. [DOI] [PubMed] [Google Scholar]
- [79].Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- [80].Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- [81].Gannage-Yared MH, Khalife S, Semaan M, Fares F, Jambart S, Halaby G. Serum adiponectin and leptin levels in relation to the metabolic syndrome, androgenic profile and somatotropic axis in healthy non-diabetic elderly men. Eur J Endocrinol. 2006;155:167–176. doi: 10.1530/eje.1.02175. [DOI] [PubMed] [Google Scholar]
- [82].Choi KM, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, et al. Serum adiponectin, interleukin-10 levels and inflammatory markers in the metabolic syndrome. Diabetes Res Clin Pract. 2007;75:235–240. doi: 10.1016/j.diabres.2006.06.019. [DOI] [PubMed] [Google Scholar]
- [83].Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, et al. Association between adiponectin and mediators of inflammation in obese women. Diabetes. 2003;52:942–947. doi: 10.2337/diabetes.52.4.942. [DOI] [PubMed] [Google Scholar]
- [84].Coppola A, Marfella R, Coppola L, Tagliamonte E, Fontana D, Liguori E, et al. Effect of weight loss on coronary circulation and adiponectin levels in obese women. Int J Cardiol. 2009;134:414–416. doi: 10.1016/j.ijcard.2007.12.087. [DOI] [PubMed] [Google Scholar]
- [85].Reinehr T, Roth C, Menke T, Andler W. Adiponectin before and after weight loss in obese children. J Clin Endocrinol Metab. 2004;89:3790–3794. doi: 10.1210/jc.2003-031925. [DOI] [PubMed] [Google Scholar]
- [86].Asayama K, Hayashibe H, Dobashi K, Uchida N, Nakane T, Kodera K, et al. Decrease in serum adiponectin level due to obesity and visceral fat accumulation in children. Obes Res. 2003;11:1072–1079. doi: 10.1038/oby.2003.147. [DOI] [PubMed] [Google Scholar]
- [87].Wang Y, Lam KS, Chan L, Chan KW, Lam JB, Lam MC, et al. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem. 2006;281:16391–16400. doi: 10.1074/jbc.M513907200. [DOI] [PubMed] [Google Scholar]
- [88].Fu JF, Fang YL, Liang L, Wang CL, Hong F, Dong GP. A rabbit model of pediatric nonalcoholic steatohepatitis: the role of adiponectin. World J Gastroenterol. 2009;15:912–918. doi: 10.3748/wjg.15.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Krawczyk K, Szczesniak P, Kumor A, Jasinska A, Omulecka A, Pietruczuk M, et al. Adipohormones as prognostric markers in patients with nonalcoholic steatohepatitis (NASH) J Physiol Pharmacol. 2009;60(Suppl 3):71–75. [PubMed] [Google Scholar]
- [90].Asano T, Watanabe K, Kubota N, Gunji T, Omata M, Kadowaki T, et al. Adiponectin knockout mice on high fat diet develop fibrosing steatohepatitis. J Gastroenterol Hepatol. 2009;24:1669–1676. doi: 10.1111/j.1440-1746.2009.06039.x. [DOI] [PubMed] [Google Scholar]
- [91].Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- [92].Saxena NK, Fu PP, Nagalingam A, Wang J, Handy J, Cohen C, et al. Adiponectin modulates C-jun N-terminal kinase and mammalian target of rapamycin and inhibits hepatocellular carcinoma. Gastroenterology. 2010;139:1762–1773. 1773, e1761–1765. doi: 10.1053/j.gastro.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- [94].Sell H, Divoux A, Poitou C, Basdevant A, Bouillot JL, Bedossa P, et al. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2010;95:2892–2896. doi: 10.1210/jc.2009-2374. [DOI] [PubMed] [Google Scholar]
- [95].Miller AM, Asquith DL, Hueber AJ, Anderson LA, Holmes WM, McKenzie AN, et al. Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res. 2010;107:650–658. doi: 10.1161/CIRCRESAHA.110.218867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- [98].Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- [99].Ben Neriah Y, Karin M. Inflammation meets cancer - NF-κB as the matchmaker. Nat Immunol. 2011 doi: 10.1038/ni.2060. In Press. [DOI] [PubMed] [Google Scholar]
- [100].Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- [101].Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- [102].Rohl M, Pasparakis M, Baudler S, Baumgartl J, Gautam D, Huth M, et al. Conditional disruption of IkappaB kinase 2 fails to prevent obesity-induced insulin resistance. J Clin Invest. 2004;113:474–481. doi: 10.1172/JCI18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003;19:725–737. doi: 10.1016/s1074-7613(03)00301-7. [DOI] [PubMed] [Google Scholar]
- [105].Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- [106].Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hsu LC, Enzler T, Seita J, Timmer AM, Lee CY, Lai TY, et al. IL-1beta-driven neutrophilia preserves antibacterial defense in the absence of the kinase IKKbeta. Nat Immunol. 2011;12:144–150. doi: 10.1038/ni.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Cho H, Black SC, Looper D, Shi M, Kelly-Sullivan D, Timofeevski S, et al. Pharmacological characterization of a small molecule inhibitor of c-Jun kinase. Am J Physiol Endocrinol Metab. 2008;295:E1142–1151. doi: 10.1152/ajpendo.90298.2008. [DOI] [PubMed] [Google Scholar]
- [110].Wueest S, Rapold RA, Schumann DM, Rytka JM, Schildknecht A, Nov O, et al. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J Clin Invest. 2010;120:191–202. doi: 10.1172/JCI38388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, et al. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- [113].Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- [114].Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- [115].Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Nobukini T, Thomas G. The mTOR/S6K signalling pathway: the role of the TSC1/2 tumour suppressor complex and the proto-oncogene Rheb. Novartis Found Symp. 2004;262:148–154. discussion 154–149, 265–148. [PubMed] [Google Scholar]
- [117].Li Y, Corradetti MN, Inoki K, Guan KL. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. 2004;29:32–38. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- [118].Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- [119].Chan EY. mTORC1 phosphorylates the ULK1-mAtg13-FIP200 autophagy regulatory complex. Sci Signal. 2009;2:pe51. doi: 10.1126/scisignal.284pe51. [DOI] [PubMed] [Google Scholar]
- [120].Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Yen WL, Klionsky DJ. How to live long and prosper: autophagy, mitochondria, and aging. Physiology (Bethesda) 2008;23:248–262. doi: 10.1152/physiol.00013.2008. [DOI] [PubMed] [Google Scholar]
- [122].Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Dikic I, Johansen T, Kirkin V. Selective autophagy in cancer development and therapy. Cancer Res. 2010;70:3431–3434. doi: 10.1158/0008-5472.CAN-09-4027. [DOI] [PubMed] [Google Scholar]
- [124].Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- [126].Kitamura T, Koshino Y, Shibata F, Oki T, Nakajima H, Nosaka T, et al. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol. 2003;31:1007–1014. [PubMed] [Google Scholar]
- [127].Hardie DG. Neither LKB1 nor AMPK are the direct targets of metformin. Gastroenterology. 2006;131:973. doi: 10.1053/j.gastro.2006.07.032. author reply 974–975. [DOI] [PubMed] [Google Scholar]
- [128].Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- [129].Liu Q, Thoreen C, Wang J, Sabatini D, Gray NS. mTOR Mediated Anti-Cancer Drug Discovery. Drug Discov Today Ther Strateg. 2009;6:47–55. doi: 10.1016/j.ddstr.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kourelis TV, Siegel RD. Metformin and cancer: new applications for an old drug. Med Oncol. 2011 doi: 10.1007/s12032-011-9846-7. [DOI] [PubMed] [Google Scholar]
- [131].Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- [132].Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Piguet AC, Semela D, Keogh A, Wilkens L, Stroka D, Stoupis C, et al. Inhibition of mTOR in combination with doxorubicin in an experimental model of hepatocellular carcinoma. J Hepatol. 2008;49:78–87. doi: 10.1016/j.jhep.2008.03.024. [DOI] [PubMed] [Google Scholar]
- [134].Treiber G. mTOR inhibitors for hepatocellular cancer: a forward-moving target. Expert Rev Anticancer Ther. 2009;9:247–261. doi: 10.1586/14737140.9.2.247. [DOI] [PubMed] [Google Scholar]
- [135].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- [137].Wunderlich FT, Luedde T, Singer S, Schmidt-Supprian M, Baumgartl J, Schirmacher P, et al. Hepatic NF-kappa B essential modulator deficiency prevents obesity-induced insulin resistance but synergizes with high-fat feeding in tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:1297–1302. doi: 10.1073/pnas.0707849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Inokuchi S, Aoyama T, Miura K, Osterreicher CH, Kodama Y, Miyai K, et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci U S A. 2010;107:844–849. doi: 10.1073/pnas.0909781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Kang HS, Okamoto K, Kim YS, Takeda Y, Bortner CD, Dang H, et al. Nuclear orphan receptor TAK1/TR4-deficient mice are protected against obesity-linked inflammation, hepatic steatosis, and insulin resistance. Diabetes. 2011;60:177–188. doi: 10.2337/db10-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Fujiki T, Miura T, Maura M, Shiraishi H, Nishimura S, Imada Y, et al. TAK1 represses transcription of the human telomerase reverse transcriptase gene. Oncogene. 2007;26:5258–5266. doi: 10.1038/sj.onc.1210331. [DOI] [PubMed] [Google Scholar]
- [141].Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Nonalcoholic steatohepatitis induced by a high-fat diet promotes diethylnitrosamine-initiated early hepatocarcinogenesis in rats. Int J Cancer. 2009;124:540–546. doi: 10.1002/ijc.23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Hui L, Bakiri L, Mairhorfer A, Schweifer N, Haslinger C, Kenner L, et al. p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat Genet. 2007;39:741–749. doi: 10.1038/ng2033. [DOI] [PubMed] [Google Scholar]
- [143].Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, et al. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–297. doi: 10.1016/j.ccr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Sakaguchi S, Takahashi S, Sasaki T, Kumagai T, Nagata K. Progression of alcoholic and non-alcoholic steatohepatitis: common metabolic aspects of innate immune system and oxidative stress. Drug Metab Pharmacokinet. 2011;26:30–46. doi: 10.2133/dmpk.dmpk-10-rv-087. [DOI] [PubMed] [Google Scholar]
- [146].Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol. 2011;193:275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–1381. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Toffanin S, Friedman SL, Llovet JM. Obesity, inflammatory signaling, and hepatocellular carcinoma-an enlarging link. Cancer Cell. 2010;17:115–117. doi: 10.1016/j.ccr.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Amir M, Czaja MJ. Autophagy in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5:159–166. doi: 10.1586/egh.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R. Autophagy in liver diseases. J Hepatol. 2010;53:1123–1134. doi: 10.1016/j.jhep.2010.07.006. [DOI] [PubMed] [Google Scholar]