Abstract
Background & Aims
Infantile hypertrophic pyloric stenosis (IHPS) is a common birth anomaly characterized by obstruction of the pyloric lumen. A genome-wide association study implicated NKX2-5, which encodes a transcription factor that is expressed in embryonic heart and pylorus, in the pathogenesis of IHPS. However, the function of the NKX2-5 in pyloric smooth muscle development has not been directly examined. We investigated the pattern of Nkx2-5 during the course of murine pyloric sphincter development and examined co-expression of Nkx2-5 with Gata3 and Sox9—other transcription factors with pyloric-specific mesenchymal expression. We also assessed pyloric sphincter development in mice with disruption of Nkx2-5 or Gata3.
Methods
We used immunofluorescence analysis to compare levels of NKX2-5, GATA3, and SOX9 in different regions of smooth muscle cells. Pyloric development was assessed in mice with conditional or germline deletion of Nkx2-5 or Gata3, respectively.
Results
Gata3, Nkx2-5, and Sox9 were co-expressed in differentiating smooth muscle cells of a distinct fascicle of the pyloric outer longitudinal muscle (OLM). Expansion of this fascicle coincided with development of the pyloric sphincter. Disruption of Nkx2-5 or Gata3 caused severe hypoplasia of this fascicle and alters pyloric muscle shape. Although expression of Sox9 required Nkx2-5 and Gata3, there was no apparent hierarchical relationship between Nkx2-5 and Gata3 during pyloric OLM development.
Conclusions
Nkx2-5 and Gata3 are independently required for the development of a pyloric OLM fascicle, which required for pyloric sphincter morphogenesis, in mice. These data indicate that regulatory changes that alter Nkx2-5 or Gata3 expression could contribute to pathogenesis of IHPS.
Keywords: Infantile hypertrophic pyloric stenosis, primary duodenogastric reflux, Sox9, smooth muscle development
Introduction
The pyloric sphincter integrates neuronal and hormonal signals to control the movement of food from the stomach to the small intestine1. This sphincter is clinically significant in the context of the common human congenital pathology, infantile hypertrophic pyloric stenosis (IHPS), in which both the structure and function of the sphincter are abnormal2–4. Infants with IHPS classically present three to six weeks after birth with projectile vomiting, as well as physical and radiographic findings of gastric outlet obstruction. The etiology of IHPS appears to be complex and may involve both environmental and genetic factors; changes in musculature, mucosa, extracellular matrix, nerve conduction, and nitric oxide signaling have all been implicated in IHPS pathogenesis2–4.
A recent genome-wide association study (GWAS) in humans identified several IHPS susceptibility loci, including the homeodomain transcription factor NKX2-55. This is of interest because Nkx2-5 is expressed in pyloric mesenchyme during embryogenesis in frog, chick, and mouse6, although the precise identity of the expressing cells is unknown. Despite its evolutionarily conserved pyloric expression and association with IHPS, the role of Nkx2-5 in pyloric development has not been examined in vertebrate models, in part because Nkx2-5 null mice die of cardiac abnormalities at E107, well before the pyloric region is fully developed.
Work in the chick model suggests that BMP signaling controls the expression of both Nkx2-5 and the SRY-related, HMG-box gene Sox98–11. Functionally, loss of either Nkx2-5 or Sox9 expression in the chick affects the character of the pyloric epithelium but has no effect on the pyloric musculature8–11, suggesting that these mesenchymal factors act indirectly to control the expression of an unknown modulator of epithelial phenotype.
In the mouse, direct functional analysis of Nkx2-5 or Sox9 at the pylorus has not been reported; however, other genetic models of pyloric sphincter dysmorphogenesis have been described12–16. For example, germline deficiency of Six2, a homeodomain transcription factor expressed in the posterior stomach, abrogates Sox9 expression and temporarily reduces Nkx2-5 expression at the pylorus, though this expression is later recovered16. Importantly, in Six2 mutant mice, the pyloric musculature and its corresponding luminal constriction are highly attenuated, indicating that Six2 and/or one or more of its downstream targets is important for pyloric sphincter development.
Though a role for Nkx2-5 in the formation of the pyloric sphincter might be inferred from the phenotype of Six2 null mice, a direct connection between Nkx2-5 and sphincter muscle development has not been demonstrated in either the mouse or chick models. In fact, while it is clear from previous studies that Nkx2-5 is expressed in pyloric mesenchyme, we present here the first analysis of its expression at the cellular level during pyloric sphincter development and correlate this expression pattern with development of the sphincter muscles.
We find that NKX2-5 protein is expressed in myofibroblasts and smooth muscle cells of the pylorus. NKX2-5 expression is most robust in a dorsal fascicle of outer longitudinal muscle (OLM) that matures between embryonic days (E) 14.5 and 16.5. Interestingly, the cells of this OLM fascicle also express SOX9, as well as GATA3, a zinc finger transcription factor that we previously identified as a pylorus-specific gene17. After germline deletion of Gata3 or conditional deletion of Nkx2-5, the dorsal pyloric OLM fascicle is hypoplastic, the shape of the inner circular muscle (ICM) is altered and constriction of the pyloric sphincter is attenuated. Together, these data reveal a distinct transcriptional regulatory cascade that is used for development of the dorsal pyloric OLM; correct development of this fascicle is required to generate the proper morphology of the pyloric sphincter. These findings have implications for the potential role of NKX2-5 in the pathogenesis of IHPS in humans.
Materials and methods
Mice
All protocols for mouse experiments were approved by and carried out in accordance with the policies of the University of Michigan University Committee of Use and Care of Animals and Unit for Laboratory Animal Medicine. C57BL/6J inbred (“wild type”; WT) mice were obtained from Charles River Laboratories (Wilmington, MA). The generation of Gata3lacZ/+, Nkx2-5lacZ/+ and CAGGCre-ER™ mice has been described previously18–20.
Gata3 null embryos were generated via Gata3lacZ/+ intercrosses. To escape early embryonic lethality, Gata3 null embryos were pharmacologically rescued in utero by treating timed-pregnant dams with α- and β-adrenergic agonists, as previously described21, 22. The rescue solution was administered once daily via a water bottle, beginning at E7.5, and all other drinking water was withheld. Rescue solution was prepared fresh, as follows: 15 mg each of isoproterenol (Sigma-Aldrich, St. Louis, MO, I-5627) and phenylephrine (Sigma-Aldrich, P-6126) was added to 50 mL of water and supplemented with 100 mg of ascorbic acid and 2 g of sucrose.
Nkx2-5flox/+ mice were generated by targeted homologous recombination in embryonic stem cells, as described previously23, 24. The targeting construct was created by cloning a neomycin resistance cassette, flanked by FLP recognition target (FRT) sites, into intron 1 of Nkx2-5; loxP sites were then cloned upstream of the neomycin resistance cassette and downstream of the homeodomain-containing exon 2 (Supplemental Figure 1B). The neomycin resistance cassette was excised via FLP-mediated FRT site recombination, resulting in loxP sites flanking exon 2 (Supplemental Figure 1C). Nkx2-5flox/+ mice were crossed to CAGGCre-ER™ (The Jackson Laboratory, Bar Harbor, ME, 004682) and bred to homozygosity for the conditional allele (CAGGCre-ER™;Nkx2-5flox/flox). Inactivation of Nkx2-5 in timed-pregnant dams was accomplished via intraperitoneal injections of tamoxifen (Sigma-Aldrich, T5648), as described previously19. Briefly, pregnant dams were injected with 150 μL of tamoxifen-corn oil solution (20 mg tamoxifen per mL of corn oil) once daily for up to two days prior to embryo harvest.
Protocols for genotyping, BrdU labeling, whole mount X-gal staining, routine tissue fixation and processing, and immunostaining and quantitation are provided in Supplemental Materials.
Results
Development of pyloric muscular components
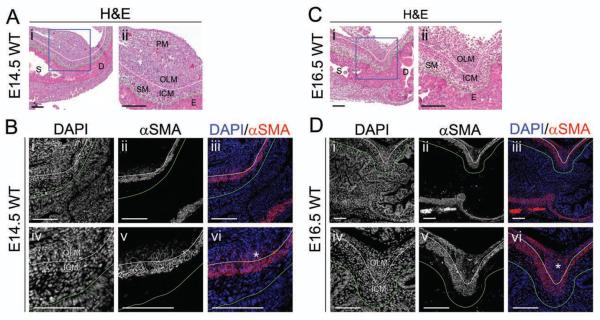
Despite the important function of pyloric sphincter, development of its smooth muscle components has not been assessed at the cellular level. We therefore examined sectioned pyloric tissue using H&E staining and immunofluorescence for alpha smooth muscle actin (αSMA), a marker of differentiated smooth muscle cells and myofibroblasts. At E14.5, the ICM at the pylorus is contiguous with that of the surrounding stomach and intestine and strongly expresses αSMA (Figure 1A,B). In contrast, the nascent OLM contains a thin layer of weakly αSMA positive cells (Figure 1Bv,vi, asterisk). These cells are not yet tightly organized into muscular bundles. Dorsally, these cells bridge directly to and intermingle with a prominent collection of αSMA negative pancreatic mesenchymal cells (Figure 1Aii, PM).
Figure 1. Development of the pylorus between E14.5 and E16.5 involves differentiation of the dorsal OLM and changes in ICM shape.
H&E staining of WT pylorus at (A) E14.5 or (C) E16.5; the dorsal pylorus, within the boxed region in (i) is enlarged in (ii). Immunofluorescence of the dorsal WT pylorus at (B) E14.5 or (D) E16.5: (i,iv) DAPI; (ii,v) αSMA; or (iii,vi) merged. (A–B) Though the ICM is well-differentiated and strongly αSMA positive at E14.5, cells of the OLM stain weakly for αSMA (asterisk in Bvi). (C–D) Expansion and differentiation of the OLM (asterisk in Dvi) is associated with an increase in αSMA expression and inward displacement of the ICM, resulting in pyloric sphincter constriction. For all images, stomach (S) is left; duodenum (D) is right; and, dorsal is top. Green lines mark the epithelial basement membrane, and white lines separate ICM and OLM. E = epithelium; SM = sub-epithelial mesenchyme; and, PM = pancreatic mesenchyme. Scale bars represent 100 μm.
By E16.5, the pyloric OLM is compacted and robustly expresses αSMA, indicative of smooth muscle differentiation (Figure 1C,D). Dorsally, a thickened fascicle of OLM appears to displace the ICM internally (Figure 1Dv,vi, asterisk), thereby narrowing the pyloric lumen to generate the characteristic constriction of the mature pyloric sphincter.
Nkx2-5 and Gata3 are expressed in similar domains at the pylorus
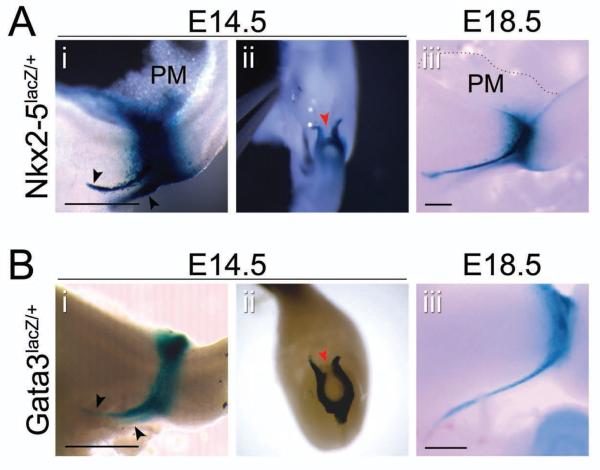
Previous studies have shown that, as early as E9.5, a mesenchymal Nkx2-5 expression domain surrounds the nascent distal stomach and proximal duodenal endoderm25. By E12.5, some cells within this mesenchymal domain have migrated anteriorly along the dorsal left side of the stomach to give rise to the spleen, while others remain at the pylorus25. Since the late embryonic expression pattern of Nkx2-5 at the pylorus has not been carefully described, we examined whole mount X-gal staining of dissected tissue from Nkx2-5lacZ/+ embryos. At E14.5, Nkx2-5-expressing cells encircle the pylorus, and staining on the dorsal side of the pylorus extends into adjoining pancreatic mesenchyme (Figure 2Ai). Bilateral cellular cords, emanating from the pylorus and reaching across the lesser curvature of the antrum, also express Nkx2-5 (Figure 2Ai, black arrowheads); these structures likely correspond to the previously described gastric ligaments26. Continuity between the X-gal positive pyloric band and gastric ligaments is obvious ventrally (Figure 2Aii). Between E14.5 and E18.5, the gastric ligaments lengthen to reach the gastroesophageal junction, but the Nkx2-5 expression pattern is otherwise unchanged (Figure 2Aiii).
Figure 2. Gata3 and Nkx2-5 are expressed in similar domains at the pylorus.
Whole mount X-gal staining of pylorus from (A) Nkx2-5lacZ/+ or (B) Gata3lacZ/+ mice at (i–ii) E14.5 and (iii) E18.5. (i,iii) Lateral view: stomach is left; duodenum is right; dorsal is top. (ii) Ventral view: stomach is top; duodenum is bottom. (Ai,Bi) Nkx2-5 and Gata3 have similar expression patterns, with extension dorsally into the pancreatic mesenchyme (PM in Aii) and ventrally into the gastric ligaments (black arrowheads in Ai and Bi), however, the width of the Gata3 expression domain is narrower than Nkx2-5. (Aii,Bii) Nkx2-5 expression is completely circumferential (red arrowhead in Aii), while Gata3 expression is discontinuous ventrally (red arrowhead in Bii). (Aiii,Biii) By E18.5, the gastric ligaments have lengthened to reach the esophagus (pancreatic mesenchyme outlined by dashed line in Aiii). Scale bars = 100 μm.
In a previous study, we found that Gata3 exhibits a pyloric-specific expression domain in the mesenchyme, similar to that of Nkx2-517. To further characterize Gata3 expression at the pylorus, we examined dissected whole mount X-gal stained tissue from Gata3lacZ/+ embryos. Similar to Nkx2-5, Gata3 is expressed in a discrete band at the pylorus, as well as in the gastric ligaments (Figure 2Bi, black arrowheads). Notably, the pyloric Gata3 expression domain is narrower than Nkx2-5, and on the lesser curvature (ventral) side, there is a small gap in the Gata3 expression domain (Figure 2Bii, red arrowhead). The nature of this gap is further explored below.
Co-expression of pyloric transcription factors during pyloric sphincter maturation
Self et al. previously showed that, between E12.5 and E14.5, Nkx2-5 and Sox9 are expressed in similar domains at the murine pylorus16. This is important since both of these genes have been implicated in pyloric development8–11. However, we were interested to: a) determine whether Nkx2-5 and Sox9 are expressed in the same cells; b) compare these domains with the expression pattern of Gata3; and, c) examine expression of these genes during establishment of the complete pyloric musculature (from E14.5 to E16.5).
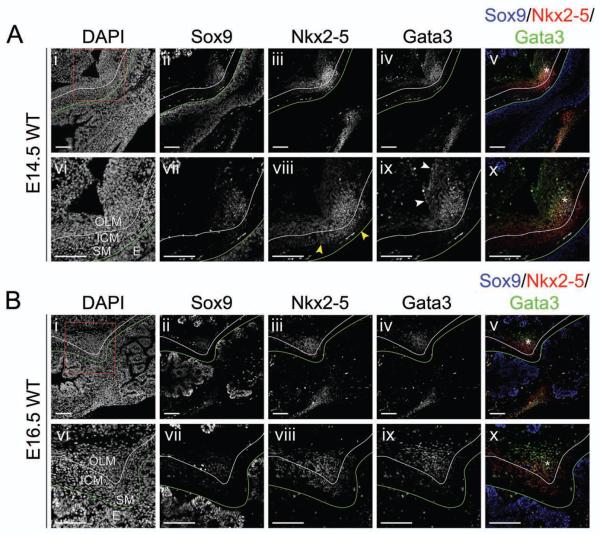
The results of this expression analysis reveal distinct pyloric cell populations that are single, double, or triple positive for NKX2-5, SOX9, and/or GATA3, suggesting that a complex developmental circuitry directs pyloric development. At both E14.5 and E16.5, the expression domains of GATA3 and NKX2-5 are exclusively mesenchymal, while SOX9 is expressed in both epithelium and mesenchyme, as previously noted (Figure 3)16. A line of enteric neurons that are strongly positive for SOX9 and the neuronal marker peripherin, but negative for GATA3 and NKX2-5 (Supplemental Figure 2), separates the OLM and ICM domains (white lines in Figure 3). At E14.5, cells that are exclusively NKX2-5 positive are detected in the sub-epithelial mesenchyme and in the ICM (Figure 3Aviii, yellow arrowheads), while cells that express only GATA3 are located in the outermost dorsal pylorus, where it mixes with pancreatic mesenchyme (Figure 3Aix, white arrowheads). Some of these GATA3 positive cells are squamous in morphology and may be part of the developing serosa (Supplemental Figure 3).
Figure 3. NKX2-5, GATA3, and SOX9 are co-expressed in dorsal pyloric OLM.
Immunofluorescence of WT pylorus at (A) E14.5 or (B) E16.5 : (i,vi) DAPI; (ii,vii) SOX9; (iii,viii) NKX2-5; (iv,ix) GATA3; or (v,x) merged. (A–B) NKX2-5 single positive cells are found in the sub-epithelial mesenchyme and ICM (yellow arrowheads in Aviii), while GATA3 single positive cells are present in the pancreatic mesenchyme (white arrowheads in Aix). NKX2-5, GATA3, and SOX9 are co-expressed in the dorsal OLM (asterisks in Av,x and Bv,x). Stomach is left; duodenum is right; and, dorsal is top. Green lines mark the epithelial basement membrane, and white lines separate ICM and OLM. SM = sub-epithelial mesenchyme. Scale bars = 100 μm.
Cells that are triple positive for NKX2-5, GATA3, and SOX9 are visible at E14.5 as a loose cluster of cells located primarily within the nascent OLM territory on the dorsal side (Figure 3Av,x, asterisk). Comparison with Figure 1 indicates that some of these cells are weakly αSMA positive (Figure 1Bvi, asterisk). Cross sections of the E14.5 pylorus confirm the low expression of αSMA in the OLM, which is marked by expression of both GATA3 and NKX2-5 (Supplemental Figure 4). Notably, analysis of multiple cross sections confirms that the OLM is absent on the ventral side of the pylorus between the nascent gastric ligaments, accounting for the apparent gap in Gata3 expression seen in X-gal stained whole mount tissue above (Figure 2Bii).
At E16.5, cells of the OLM strongly express αSMA (Figure 1Dvi, asterisk). Additionally, OLM cells continue to express SOX9, NKX2-5 and GATA3 (Figure 3Bv,x, asterisk). Thus, cells that co-express all three transcription factors appear to contribute primarily to the OLM.
Nkx2-5 is required for development and maintenance of the pyloric OLM
The expression pattern of Nkx2-5 suggests a potential role for this transcription factor in pyloric development. However, germline deficiency of Nkx2-5 results in embryonic lethality by E107, precluding analysis of Nkx2-5 function in late embryogenesis. Thus, we paired a conditional Nkx2-5 allele (Nkx2-5flox/flox) with a tamoxifen-inducible Cre recombinase transgene driven from a ubiquitously expressed transgenic promoter (CAGGCre-ER™)19. Two cohorts of pregnant dams were treated with once-daily tamoxifen injections, beginning at E14.5 or E16.5; each cohort was sacrificed two days later for analysis.
Deletion of Nkx2-5 beginning at E14.5 leads to nearly complete absence of the αSMA positive pyloric OLM smooth muscle at E16.5 (Figure 4Av,vi, asterisks), suggesting that Nkx2-5 is necessary for the maturation of the OLM. Interestingly, Nkx-5 deletion beginning at E16.5, when the OLM is already formed and strongly expresses αSMA (Figure 1Dv,vi), significantly reduces OLM smooth muscle by E18.5 (Figure 4Bv,vi, asterisks and Supplemental Figure 5C). Remaining cells are loosely organized and weakly rather than strongly αSMA positive, suggesting that Nkx2-5 function is continuously required for the maintenance of differentiated smooth muscle cells within this fascicle.
Figure 4. Nkx2-5 is required for the development and maintenance of the dorsal pyloric OLM.
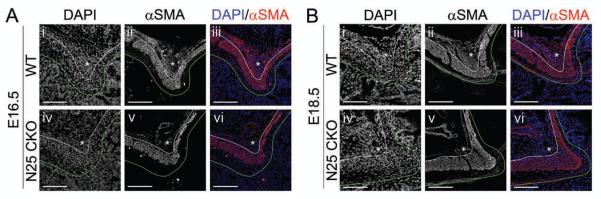
Immunofluorescence of (i–iii) Nkx2-5flox/flox (WT) or (iv–vi) CAGGCre-ER™;Nkx2-5flox/flox (N25 CKO) pylorus (after two days of intraperitoneal tamoxifen injections) harvested at (A) E16.5 or (B) E18.5: (i,iv) DAPI; (ii,v) αSMA; or (iii,vi) merged. (A) In N25 CKO mice at E16.5, cell mass and αSMA expression is reduced in the dorsal OLM (asterisks). (B) At E18.5, the dorsal OLM in WT mice has expanded further and shows increased αSMA expression (Bi–iii). Deletion of Nkx2-5 beginning at E16.5, when the dorsal OLM is already αSMA positive (Aii–iii), leads to its regression (Biv–vi). Stomach is left; duodenum is right; and, dorsal is top. Green lines mark the epithelial basement membrane, and white lines separate ICM and OLM. Asterisk = dorsal pyloric OLM. Scale bars = 100 μm.
Increased apoptosis and reduced proliferation in pyloric OLM after loss of Nkx2-5
Our data show that loss of Nkx2-5 beginning at E14.5 prevents the maturation of the pyloric OLM fascicle, and deletion of this gene after the fascicle has formed at E16.5 leads to its regression. Reduced smooth muscle cell proliferation and/or increased apoptosis are potential mechanisms underlying these OLM responses. To examine proliferative changes, timed-pregnant dams were injected with BrdU two hours prior to sacrifice. In WT animals, BrdU positive cells were found scattered throughout the ICM and OLM regions at E14.5 and E16.5 (Supplemental Figure 6A). Conditional deletion of Nkx2-5 beginning at E14.5 was associated with a 25% reduction in the proportion of proliferative cells in the OLM fascicle at E16.5 (19.6% in WT versus 14.8% after Nkx2-5 deletion, P < 0.05) (Supplemental Figure 6B,Ci). Though Nkx2-5 is also expressed in the ICM, no significant change in proliferative activity was detectable in that domain (Supplemental Figure 6Cii).
To assess the impact on apoptosis, we examined caspase 3 (CASP3) expression 24 and 48 hours after deletion of Nkx2-5. At 48 hours post-deletion, the OLM fascicle was largely absent, and the remaining cells were not CASP3 positive (data not shown). At 24 hours post-deletion, though the total number of cells (as well as the number of NKX2-5 positive cells) had already decreased, the proportion of CASP3 positive cells within the OLM had nearly doubled (2.6% in WT versus 4.8% after Nkx2-5 deletion, P < 0.05) (Supplemental Figure 7). We conclude that deletion of Nkx2-5 results in decreased proliferation, increased apoptotic activity and rapid loss of the dorsal pyloric OLM fascicle. Interestingly, though the ICM also expresses Nkx2-5, proliferation and apoptosis within this domain does not appear to be altered, indicating that a distinct molecular network directs the maturation of this muscle cell population.
Gata3 is necessary for the formation of the pyloric OLM fascicle
Since cells of the dorsal pyloric OLM express both Gata3 and Nkx2-5 (Figure 3), we next examined the consequences of germline Gata3 loss on the development of this fascicle. Though germline Gata3 deficiency results in early embryonic lethality, Gata3 null embryos can be pharmacologically rescued by catecholamine administration in utero and will survive until birth, permitting the analysis of Gata3 function in later fetal development21, 22. We found no obvious pyloric abnormalities in Gata3 null animals at E14.5 (data not shown). However, by E16.5, Gata3 deficiency results in nearly complete absence of the OLM fascicle (Figure 5Aiv–vi, asterisks and Supplemental Figure 5B), a phenotype highly similar to that seen after Nkx2-5 conditional deletion.
Figure 5. Gata3 is required for formation of the dorsal pyloric OLM; absence of Gata3 or loss of Nkx2-5 alters ICM shape and pyloric sphincter constriction.
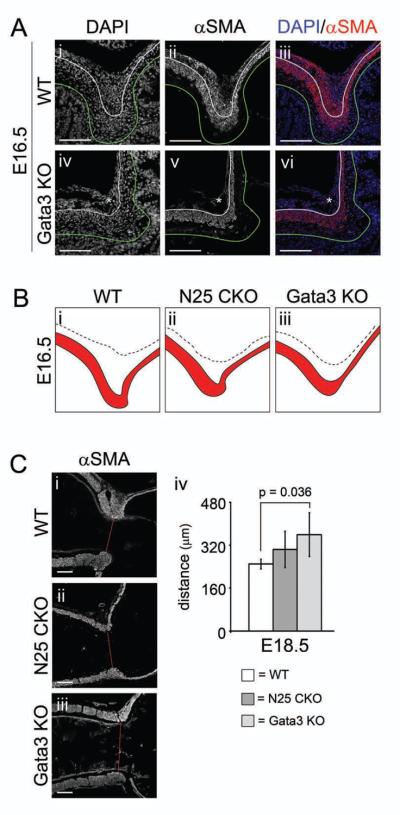
(A) Immunofluorescence of (i–iii) WT or (iv–vi) Gata3lacZ/lacZ (Gata3 KO) pylorus at E16.5: (i,iv) DAPI; (ii,v) αSMA; or (iii,vi) merged. Germline deficiency of Gata3 results in nearly complete absence of αSMA positive cells in the dorsal pyloric OLM (asterisks in Aiv–vi). (B) Tracings of the pyloric ICM (red) and OLM (white area defined by the dotted line) in WT, CAGGCre-ER™;Nkx2-5flox/flox (N25 CKO), and Gata3 KO mice at E16.5. Subtle but reproducible changes occur in the shape of the ICM in N25 CKO and Gata3 KO mice. (C) αSMA immunofluorescence of (i) WT, (ii) N25 CKO, or (iii) Gata3 KO pylorus at E18.5. Compared to WT, the pyloric sphincter constriction is somewhat reduced (wider) in N25 CKO animals and significantly attenuated in Gata3 KO (sphincter constriction measurements are shown in Civ). Stomach is left; duodenum is right; and, dorsal is top. Green lines mark the epithelial basement membrane, and white lines separate ICM and OLM. Red lines in (Ci–iii) denote width of pyloric sphincter constriction. Asterisk = dorsal pyloric OLM. Scale bars = 100 μm. Error bars represent one standard deviation from the mean.
Loss of Gata3 or Nkx2-5 alters ICM morphogenesis and pyloric sphincter constriction
As shown in Figures 4A and 5A, conditional Nkx2-5 deletion or germline Gata3 deficiency leads to loss of the dorsal OLM fascicle. In both cases, at E16.5, the shape of the dorsal ICM is clearly altered, as depicted in the tracings shown in Figure 5B. Since Gata3 is not widely expressed in the ICM (Figure 3), the shape change detected in this mutant model must be secondary to the loss of the OLM. In addition, at E18.5, the pyloric sphincter constriction is attenuated in both genetic deficiency models; compared to WT (95% CI = 231–266 μm), the constriction is 40% wider in Gata3 null animals (95% CI = 267–450 μm, P < 0.05) and 22% wider after conditional Nkx2-5 deletion (95% CI = 228–379 μm, P = 0.13) (Figure 5C).
A regulatory hierarchy of pyloric transcription factors
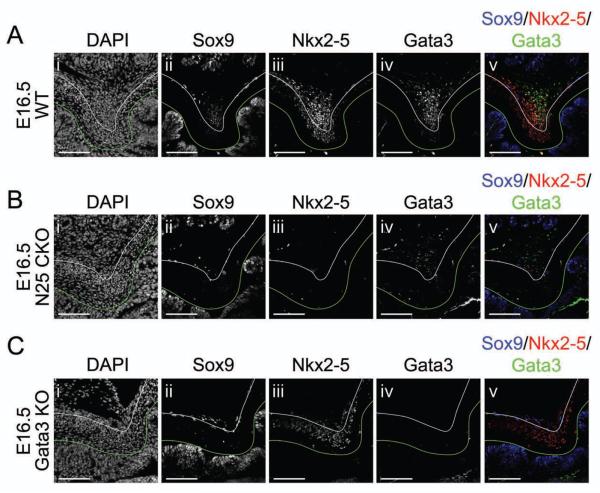
We next examined the expression of NKX2-5, GATA3 and SOX9 in E16.5 pyloric smooth muscle after conditional Nkx2-5 deletion or in Gata3 null mice. Deletion of Nkx2-5 starting at E14.5 effectively ablates NKX2-5 expression in the majority of cells at the E16.5 pylorus (Figure 6Biii). While SOX9 staining is absent in smooth muscle cells (Figure 6Bii), GATA3 expression persists in NKX2-5 negative cells of the OLM remnant (Figure 6Biv), suggesting that Nkx2-5 is required for Sox9 but not Gata3 expression in this domain.
Figure 6. Regulatory hierarchy among Nkx2-5, Gata3 and Sox9 in the dorsal pyloric OLM.
Immunofluorescence of (A) WT, (B) CAGGCre-ER™;Nkx2-5flox/flox (N25 CKO), or (C) ) Gata3lacZ/lacZ (Gata3 KO) pylorus at E16.5: (i) DAPI; (ii) SOX9; (iii) NKX2-5; (iv) GATA3; or (v) merged. (A–C) Conditional loss of Nkx2-5 effectively eliminates dorsal OLM NKX2-5 expression (Biii), with concomitant loss of SOX9 expression (Bii), but does not abrogate GATA3 expression (Biv). Germline deficiency of Gata3 is associated with the complete absence of dorsal OLM SOX9 expression (Cii), but NKX2-5 is expressed in rare remaining dorsal OLM cells (Ciii). Stomach is left; duodenum is right; and, dorsal is top. Green lines mark the epithelial basement membrane, and white lines separate ICM and OLM. Scale bars = 100 μm.
In Gata3 null mice, both GATA3 (Figure 6Civ) and SOX9 (Figure 6Cii) are absent in smooth muscle cells, but NKX2-5 expression is apparent in cells of the reduced OLM territory at E16.5 (Figure 6Ciii). Persistence of NKX2-5 expression was additionally confirmed at E14.5 (Supplemental Figure 8). Thus, Gata3 is required for Sox9 expression, but not Nkx2-5 expression, in the dorsal pyloric OLM. Taken together, these data reveal a pyloric transcriptional hierarchy in which Nkx2-5 and Gata3 are independently required to drive Sox9 expression and, consequently, smooth muscle development within the dorsal OLM fascicle.
Loss of Nkx2-5 or Gata3 does not affect the epithelial pyloric border
In the chick, perturbation of mesenchymal NKX2-5 activity changes the character of the pyloric epithelium, suggesting that signaling molecules driven by NKX2-5 are responsible for dictating epithelial phenotype10, 11. In the mouse, the distinct characters of the stomach and duodenal epithelia are established between E14.5 and E16.517, 27 , a time coincident with the development of the OLM fascicle (Figure 1). Therefore, we assessed the integrity of the epithelial stomach-intestinal (pyloric) border at E18.5 after conditional Nkx2-5 deletion and in Gata3 null animals by examining the expression of CDX2, an intestine-specific epithelial marker. In both models, the position of the epithelial pyloric border was similar to WT (Supplemental Figure 9). Thus, in the mouse, neither Gata3 nor Nkx2-5 is required for positioning of the epithelial pyloric border.
Discussion
Here, we provide a detailed analysis of normal pyloric muscle development at the cellular level, revealing an unexpectedly complex expression pattern of three critical transcription factors in these muscles. We provide evidence for a redundant regulatory mechanism that controls the development of a specific dorsal OLM fascicle at the pylorus. This fascicle is first detectable as a population of loosely organized, weakly αSMA positive smooth muscle cells at E14.5; it organizes considerably over the next two days, becoming highly αSMA positive and displacing the ICM to generate a distinct pyloric sphincter constriction by E16.5. Cells within this dorsal OLM fascicle express NKX2-5, GATA3, and SOX9. Deletion of either Nkx2-5 or Gata3 abrogates development of this OLM fascicle, results in loss of SOX9 expression, and alters pyloric muscular shape. While both Nkx2-5 and Gata3 are also expressed in other cells at the pylorus, loss of either factor appears to affect only the phenotype of triple positive cells (NKX2-5, GATA3, and SOX9) within the dorsal OLM fascicle.
This is the first study to characterize Gata3 pyloric expression at the cellular level and to establish a role for Gata3 in pyloric development. We find that GATA3 is expressed in cells on the serosal surface (Supplemental Figure 3) and in cells that intermix with pancreatic mesenchyme (Figure 3); neither of these cell types co-expresses NKX2-5 or SOX9, and their functional role remains undetermined since no obvious phenotype was detected in serosa or pancreas in Gata3 deficient mice. In contrast, the effect of Gata3 loss on the pyloric OLM fascicle is dramatic and directly mirrors the effects of Nkx2-5 loss. Despite this similarity in phenotype, we found no clear evidence of a transcriptional relationship between Gata3 and Nkx2-5. Further supporting the independent regulation of these factors, conditional overexpression of Wnt9b, or activation of a stabilized form of beta-catenin in the distal stomach and pylorus affects Nkx2-5 and Gata3 expression oppositely: the Nkx2-5 domain is expanded, while expression of Gata3 is abolished12.
Interestingly, both Nkx2-5 and Gata3 are required for pyloric sphincter morphogenesis and both directly or indirectly regulate Sox9 expression. Three other transcription factors, Barx1, Bapx1, and Six2, are also important for pyloric sphincter development and Sox9 expression13–16. All three factors are expressed in distal stomach as well as pylorus. It has been proposed that Bapx1 functions downstream of Barx1, since its expression is lost in Barx1 mutant mice14. Interestingly, loss of Bapx1 does not affect Nkx2-5 expression, but gene expression microarrays demonstrate decreased Sox9 in Bapx1 null animals14, 15. Thus, Bapx1 may regulate Sox9 expression directly or indirectly via Gata3. Loss of Six2 also results in attenuation of the pyloric sphincter constriction and is associated with transient loss of Nkx2-5 and complete loss of SOX9 expression16. Thus, all of these models converge on Sox9 and given its expression in the OLM (Figure 3), it will be important to directly determine whether Sox9 is required for development of this OLM fascicle and establishment of pyloric sphincter constriction. Testing this possibility will require a transgenic Cre driver that is active in the pyloric mesenchyme but not the epithelium, where Sox9 plays a critical role in establishing the stem cell zone28, 29.
Both Nkx2-5 and Gata3 are important for proper differentiation of the OLM fascicle, since in their absence, cells are disorganized and only weakly αSMA positive. Even very late deletion of Nkx2-5 (after the OLM has fully developed) results in an OLM fascicle that is reduced in size, organization, and differentiation. Studies in the chick suggested that a zone of Bmp4 exclusion is important for initial positioning of the Nkx2-5 and Sox9 expression domains at the pylorus8–11 , and ectopic expression of Bmp4 in this zone compromised smooth muscle differentiation9. Once positioned, if Nkx2-5 and/or Gata3 function to suppress Bmp4, ectopic BMP signaling might be responsible for the increased apoptosis and reduced proliferation and differentiation seen after deletion of these factors. However, by Smad1/5/8 staining, we were unable to detect a zone of low BMP pathway activity at the E16.5 WT pylorus and saw no change in staining after loss of Nxk2-5 or Gata3 (data not shown). In accord with this finding, loss of Gata3 (due to overexpression of Wnt9b at the pylorus) compromises pyloric sphincter formation but does not alter Bmp4 expression12. Thus, the importance of BMP signaling in later pyloric development remains to be clarified.
Co-regulation of muscle differentiation genes by NKX and GATA family members has been seen in other systems: NKX3-2 and GATA6 cooperatively activate smooth muscle genes (e.g., Itga1, SM22α, and Cald1), while NKX2-5 and GATA4 co-activate cardiac muscle genes (e.g., Anf)30. In both cases, co-regulation also involves serum response factor (SRF), an important regulator of muscle gene expression in differentiating card iac and smooth muscle. These three proteins together (SRF/GATA/NKX) synergistically activate target gene expression at levels 5–10 fold greater than SRF/GATA or SRF/NKX alone30. Thus, it is possible that loss of either Nkx2-5 or Gata3 disrupts ternary complexes of GATA3, NKX2-5, and SRF that are required for the expression of genes involved in the differentiation and/or maintenance of pyloric smooth muscle. This remains to be directly tested.
Failure to establish or maintain the proper pyloric musculature, as seen in both the conditional Nkx2-5 deletion and Gata3 null models examined here, could potentially underlie some cases of primary duodenogastric reflux, a rare and poorly understood condition involving excessive reflux of bile acids from the duodenum to the stomach31. IHPS, in contrast, presents as pyloric obstruction. Given that recent GWAS data link nucleotide polymorphisms near the NKX2-5 gene locus to IHPS5, our results would predict that some forms of IHPS may result from overexpression of NKX2-5 at the pylorus. For example, a change in an intergenic regulatory element could readily account for increased pyloric NKX2-5 expression. Indeed, an upstream enhancer that drives pyloric Nkx2-5 expression in mice has already been described24, and it will be interesting to examine the homologous region in patients with IHPS. Additionally, our data suggest that the search for linked polymorphisms in IHPS should be expanded to include GATA3 and, potentially, SOX9.
Supplementary Material
Acknowledgements
We thank for Brianna Sabol, Tomonori Hosoya, Kate Walton, and William Zacharias for technical and intellectual contributions, as well as Richard Harvey for providing Nkx2-5lacZ/+ mice. We also appreciate excellent technical support from the Microscopy and Image Analysis Laboratory of the Department of Cell and Developmental Biology (University of Michigan).
Grant Support:
F30 DK082144 (A.M.U.)
F30 DK089712 (A.P.)
R01 DK065850 (D.L.G.)
P01 DK062041 (D.L.G.)
R01 GM28896 (J.D.E)
R01 AI94642 (J.D.E.)
Abbreviations
- αSMA
alpha smooth muscle actin
- BrdU
bromodeoxyuridine
- BMP
bone morphogenetic protein
- CASP3
caspase 3
- DGR
duodenogastric reflux
- E
embryonic day
- FRT
FLP recognition target
- GWAS
genome-wide association study
- H&E
hematoxylin and eosin
- HMG
high mobility group
- ICM
inner circular muscle
- IHPS
infantile hypertrophic pyloric stenosis
- OLM
outer longitudinal muscle
- SRF
serum response factor
- SRY
sex-determining region Y
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
None (A.M.U., A.P., D.A.S., T.M., P.Y.J., K.-C.L., J.D.E., D.L.G)
Currently employed by Novartis Institutes for BioMedical Research, Inc., a division of Novartis International AG (M.S.)
Author Contributions:
A.M.U. = study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; study supervision
A.P. = study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding
D.S. = acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
M.S. = provision of critical reagents
T.M. = provision of critical reagents, acquisition of data; analysis and interpretation of data
P.Y.J. = provision of critical reagents; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
K.-C.L. = provision of critical reagents; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
J. D.E. = provision of critical reagents; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
D.L.G. = study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; study supervision
References
- 1.Daniel EE. Sphincters : normal function--changes in diseases. CRC Press; Boca Raton, Fla.: 1992. [Google Scholar]
- 2.Panteli C. New insights into the pathogenesis of infantile pyloric stenosis. Pediatr Surg Int. 2009 doi: 10.1007/s00383-009-2484-x. [DOI] [PubMed] [Google Scholar]
- 3.Ranells JD, Carver JD, Kirby RS. Infantile hypertrophic pyloric stenosis: epidemiology, genetics, and clinical update. Adv Pediatr. 2011;58:195–206. doi: 10.1016/j.yapd.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Peeters B, Benninga MA, Hennekam RC. Infantile hypertrophic pyloric stenosis-genetics and syndromes. Nat Rev Gastroenterol Hepatol. 2012;9:646–60. doi: 10.1038/nrgastro.2012.133. [DOI] [PubMed] [Google Scholar]
- 5.Feenstra B, Geller F, Krogh C, et al. Common variants near MBNL1 and NKX2-5 are associated with infantile hypertrophic pyloric stenosis. Nat Genet. 2012;44:334–7. doi: 10.1038/ng.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith DM, Grasty RC, Theodosiou NA, et al. Evolutionary relationships between the amphibian, avian, and mammalian stomachs. Evol Dev. 2000;2:348–59. doi: 10.1046/j.1525-142x.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- 7.Lyons I, Parsons LM, Hartley L, et al. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9:1654–66. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 8.Smith DM, Tabin CJ. BMP signalling specifies the pyloric sphincter. Nature. 1999;402:748–9. doi: 10.1038/45439. [DOI] [PubMed] [Google Scholar]
- 9.Smith DM, Nielsen C, Tabin CJ, et al. Roles of BMP signaling and Nkx2.5 in patterning at the chick midgut-foregut boundary. Development. 2000;127:3671–81. doi: 10.1242/dev.127.17.3671. [DOI] [PubMed] [Google Scholar]
- 10.Theodosiou NA, Tabin CJ. Sox9 and Nkx2.5 determine the pyloric sphincter epithelium under the control of BMP signaling. Dev Biol. 2005;279:481–90. doi: 10.1016/j.ydbio.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Moniot B, Biau S, Faure S, et al. SOX9 specifies the pyloric sphincter epithelium through mesenchymal-epithelial signals. Development. 2004;131:3795–804. doi: 10.1242/dev.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiefer SM, Robbins L, Rauchman M. Conditional expression of Wnt9b in Six2-positive cells disrupts stomach and kidney function. PLoS One. 2012;7:e43098. doi: 10.1371/journal.pone.0043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akazawa H, Komuro I, Sugitani Y, et al. Targeted disruption of the homeobox transcription factor Bapx1 results in lethal skeletal dysplasia with asplenia and gastroduodenal malformation. Genes Cells. 2000;5:499–513. doi: 10.1046/j.1365-2443.2000.00339.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim BM, Miletich I, Mao J, et al. Independent functions and mechanisms for homeobox gene Barx1 in patterning mouse stomach and spleen. Development. 2007;134:3603–13. doi: 10.1242/dev.009308. [DOI] [PubMed] [Google Scholar]
- 15.Verzi MP, Stanfel MN, Moses KA, et al. Role of the homeodomain transcription factor Bapx1 in mouse distal stomach development. Gastroenterology. 2009;136:1701–10. doi: 10.1053/j.gastro.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Self M, Geng X, Oliver G. Six2 activity is required for the formation of the mammalian pyloric sphincter. Dev Biol. 2009;334:409–17. doi: 10.1016/j.ydbio.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Udager AM, Hu C, et al. Dynamic patterning at the pylorus: formation of an epithelial intestine-stomach boundary in late fetal life. Dev Dyn. 2009;238:3205–17. doi: 10.1002/dvdy.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Doorninck JH, van Der Wees J, Karis A, et al. GATA-3 is involved in the development of serotonergic neurons in the caudal raphe nuclei. J Neurosci. 1999;19:RC12. doi: 10.1523/JNEUROSCI.19-12-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–18. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 20.Elliott DA, Solloway MJ, Wise N, et al. A tyrosine-rich domain within homeodomain transcription factor Nkx2-5 is an essential element in the early cardiac transcriptional regulatory machinery. Development. 2006;133:1311–22. doi: 10.1242/dev.02305. [DOI] [PubMed] [Google Scholar]
- 21.Lim KC, Lakshmanan G, Crawford SE, et al. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet. 2000;25:209–12. doi: 10.1038/76080. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman CK, Zhou P, Pasolli HA, et al. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–22. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka M, Chen Z, Bartunkova S, et al. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126:1269–80. doi: 10.1242/dev.126.6.1269. [DOI] [PubMed] [Google Scholar]
- 24.Jay PY, Harris BS, Maguire CT, et al. Nkx2-5 mutation causes anatomic hypoplasia of the cardiac conduction system. J Clin Invest. 2004;113:1130–7. doi: 10.1172/JCI19846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burn SF, Boot MJ, de Angelis C, et al. The dynamics of spleen morphogenesis. Dev Biol. 2008;318:303–11. doi: 10.1016/j.ydbio.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 26.Torgersen J. The muscular build and movements of the stomach and duodenal bulb. Acta radiol. 1942;(Suppl 45):1–191. [Google Scholar]
- 27.Braunstein EM, Qiao XT, Madison B, et al. Villin: A marker for development of the epithelial pyloric border. Dev Dyn. 2002;224:90–102. doi: 10.1002/dvdy.10091. [DOI] [PubMed] [Google Scholar]
- 28.Bastide P, Darido C, Pannequin J, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–48. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Formeister EJ, Sionas AL, Lorance DK, et al. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1108–18. doi: 10.1152/ajpgi.00004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishida W, Nakamura M, Mori S, et al. A triad of serum response factor and the GATA and NK families governs the transcription of smooth and cardiac muscle genes. J Biol Chem. 2002;277:7308–17. doi: 10.1074/jbc.M111824200. [DOI] [PubMed] [Google Scholar]
- 31.Hermans D, Sokal EM, Collard JM, et al. Primary duodenogastric reflux in children and adolescents. Eur J Pediatr. 2003;162:598–602. doi: 10.1007/s00431-003-1259-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.