Abstract
Detection of microbial constituents by membrane associated and cytoplasmic pattern recognition receptors is the essence of innate immunity, leading to activation of protective host responses. However, it is still unclear how immune cells specifically respond to pathogenic bacteria. Using virulent and non-virulent strains of Bacillus anthracis, we have shown that secretion of ATP by infected macrophages and the sequential activation of the P2X7 purinergic receptor and nucleotide binding oligomerization domain (NOD)- like receptors are critical for IL-1-dependent host protection from virulent B. anthracis. Importantly, lethal toxin produced by virulent B. anthracis blocked activation of protein kinases, p38 MAPK and AKT, resulting in opening of a connexin ATP release channel and induction of macrophage death. Prevention of cell death or ATP release through constitutive p38 or AKT activation interfered with inflammasome activation and IL-1β production, thereby compromising anti-microbial immunity.
INTRODUCTION
Two major types of pattern recognition receptors (PRRs)- Toll like receptors (TLRs) and NOD like receptors (NLRs) recognize microbial components and activate mammalian innate immunity (Meylan et al., 2006). TLRs are directly activated by microbial components collectively known as pathogen associated molecular patterns (PAMPs), but the mechanism of NLR activation is more obscure and complex (Martinon et al., 2009). Despite their name, most PAMPs, including lipopolysaccharides, peptidoglycans, lipopeptides, and flagellin are expressed by virulent and non-virulent bacteria alike. Thus, it is not entirely clear how immune cells discriminate between non-virulent commensals and virulent pathogens or non-virulent and virulent variants of the same bacterial species. One signaling system that seems capable of such discrimination is the NLR-activated inflammasome system (Martinon et al., 2009). Inflammasomes are NLR-containing multiprotein complexes that control activation of caspase-1 and the processing and secretion of pro-inflammatory cytokines interleukin (IL)-1β and IL-18 (Martinon et al., 2009). Inflammasome activators include certain PAMPs and danger associated molecular patterns (DAMPs), such as proteins released by dying cells, extracellular ATP, reactive oxygen species (ROS), urea crystals and bacterial pore-forming toxins such as listeriolysin O of Listeria monocytogenes and alpha-hemolysin of Staphylococcus aureus (Franchi et al., 2009; Hruz et al., 2009; Mariathasan et al., 2004). The pathways connecting these elicitors of inflammasome activation remain largely unknown.
We have investigated the mechanisms that allow a toxin-producing strain of the Gram-positive bacterial pathogen Bacillus anthracis, the causative agent of anthrax (Tournier et al., 2009), to activate the inflammasome, whereas non-toxin producing derivatives of the same strain do not. Anthrax pathogenesis depends on production of lethal toxin (LT) and edema toxin (ET) (Moayeri and Leppla, 2009). LT is composed of two subunits, protective antigen (PA), which also serves as a subunit of ET, and lethal factor (LF), a zinc metalloprotease that specifically cleaves the N-termini of mitogen-activated protein kinase (MAPK)-kinases (MEK or MKK) within the host cell (Moayeri and Leppla, 2009). Importantly, LF-dependent inhibition of p38 MAPK results in macrophage apoptosis (Park et al., 2002). LT can also induce assembly of the NALP1 inflammasome (Boyden and Dietrich, 2006; Bruey et al., 2007; Faustin et al., 2007; Hsu et al., 2008). However, it is not clear whether LT is directly recognized as a PAMP by NALP1 or whether its catalytic activity promotes inflammasome activation indirectly. Another enigma is the host-based variation in NALP1 inflammasome activation by LT, caused by genetic polymorphisms in the Nalp1b locus (Boyden and Dietrich, 2006). Notably, live B. anthracis can induce IL-1β production also in mouse strains, such as C57BL/6 (Hsu et al., 2008), whose macrophages are refractory to LT-induced NALP1 activation (Boyden and Dietrich, 2006). Given these differences and complications, we pursued studies with isogenic live bacterial strains that differ in LT production rather than use purified LT in the absence of other bacterial components.
Using isogenic B. anthracis strains that differ only in LF expression, we found that LT affects the host response in a manner dependent on activation of NALP1 and caspase-1, and that a LF-deficient strain cannot induce inflammasome activation. Specifically, LT-dependent inhibition of p38 and AKT signaling resulted in release of ATP from infected macrophages through connexin channels leading to subsequent inflammasome activation mediated by the ATP-responsive P2X7 purinergic receptor. This pathway plays a critical role in protecting the host from LT-producing B. anthracis.
RESULTS
The NLR associated molecules, RIP2 and IPAF, are required for B. anthracis-mediated NALP1 inflammasome activation
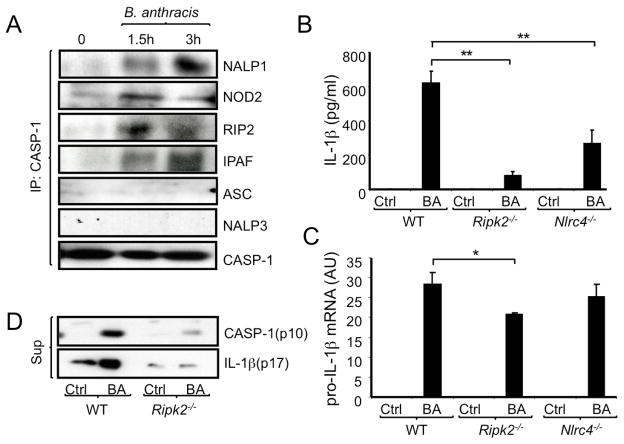
B. anthracis infection activates a NALP1 inflammasome complex leading to IL-1β processing and secretion (Hsu et al., 2008). To elucidate the composition of the B. anthracis-activated NALP1 inflammasome complex, murine peritoneal macrophages were infected with B. anthracis (Sterne strain), at a multiplicity of infection (MOI) of 2 bacteria/cell. Cell lysates were immunoprecipitated with a caspase-1 antibody followed by immunoblotting for candidate NLRs and adaptor proteins. B. anthracis infection induced association of NALP1, NOD2, RIP2 and IPAF (NLRC4) with caspase-1 (Figure 1A). By contrast, NALP3 and ASC were not present in caspase-1 immunoprecipitates following B. anthracis infection.
Figure 1. Molecular constituents of B. anthracis-activated inflammasomes.
(A) Peritoneal macrophages from C57BL/6 mice were infected with B. anthracis at a multiplicity of infection (MOI) = 2. When indicated, macrophage lysates were prepared and immunoprecipitated (IP) with a caspase-1 antibody. Composition of the immunoprecipitates was examined by immunoblotting of three different blots of the same experiment, which was repeated twice with similar results.
(B) Macrophages from the indicated mouse strains were infected with B. anthracis as above. After 8 hr, IL-1β in the culture medium was measured by ELISA. This and all similar experiments were repeated at least 3 times and the results of one representative experiment done in triplicates are shown. Values represent means ± SD. **p < 0.01 denote significant differences between the groups.
(C) Macrophages were infected as above and 3 hr later amounts of pro-IL-1β mRNA were measured by quantitative reverse transcriptase polymerase chain reaction (Q-RT-PCR). Results were normalized to the amount of GAPDH mRNA. Values represent means ± SD. *p < 0.05 denotes significant differences between the groups. This experiment was repeated 3 times and the results of one representative experiment done in triplicates are shown.
(D) WT and Ripk2−/− macrophages were infected with B. anthracis as above and release of active caspase-1 (p10) and IL-1β (p17) to the culture medium was examined after 12 hr by immunoblotting. This experiment was repeated twice with similar results.
To determine if IPAF and RIP2 are required for B. anthracis-induced IL-1β secretion, wild type (WT), Nlrc4−/− or Ripk2−/− macrophages were infected as above and IL-1β secretion was analyzed by ELISA. Ripk2−/− and Nlrc4−/− macrophages released significantly less IL-1β upon B. anthracis infection than WT macrophages (Figure 1B). By contrast, RIP2 or IPAF made little contribution to induction of pro-IL-1β mRNA in B. anthracis infected macrophages (Figure 1C). Ripk2 ablation also did not decrease the expression of caspase-1, Nod2, Nalp1 and Nalp3 mRNAs (Figure S1). Notably, RIP2 was important for B. anthracis-induced caspase-1 activation and processing as detected by release of the caspase-1 p10 fragment along with mature (p17) IL-1β (Figure 1D).
B. anthracis activates the NALP1 inflammasome in a p38-dependent manner
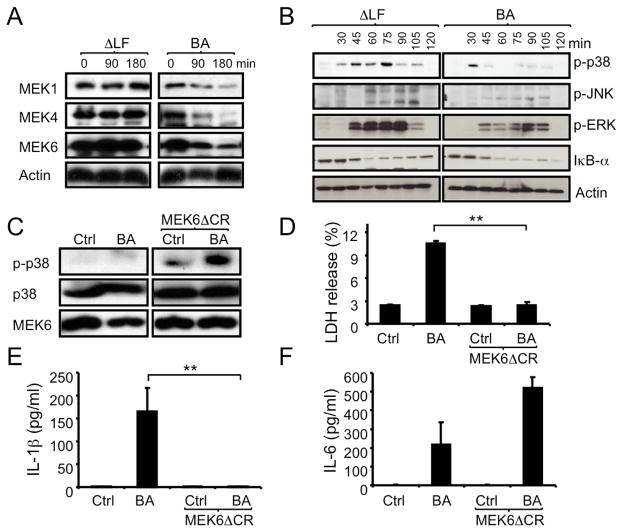
LF, the active component of LT, is a protease that cleaves the N-termini of all MEKs (or MKKs) with the exception of MEK5 (Vitale et al., 2000). As expected, infection of macrophages with WT B. anthracis Sterne strain led to disappearance of full length MEK1, MEK4 and MEK6 (Figures 2A and S2A). All 3 proteins remained intact in macrophages infected with an isogenic LF-deficient mutant of the B. anthracis Sterne strain (ΔLF). Correspondingly, infection of macrophages with the ΔLF mutant triggered much stronger activation of ERK, JNK and p38 MAPKs than did infection with the parental B. anthracis strain (WT; Figure 2B). Whereas WT B. anthracis induced IL-1β release, infection with the ΔLF strain did not (Figure S2B). Accordingly, WT but not ΔLF B. anthracis induced association of caspase-1 with NOD2 (Figure S2C). To determine whether LF-mediated inhibition of MAPK activation contributes to inflammasome activation and IL-1β secretion, we treated ΔLF-infected macrophages with different MAPK and MEK inhibitors [PD98059, an inhibitor of MEK1 and MEK2 which activate ERK, SP600125 to inhibit JNK and SB202190 for p38α and β isoforms]. However, inhibition of p38, JNK or ERK signaling in ΔLF-infected macrophages failed to restore IL-1β secretion (Figure S2B). However, as previously reported (Park et al., 2002), the p38 inhibitor reduced IL-1β mRNA in ΔLF-infected macrophages to amounts similar to those found in WT B. anthracis-infected cells (Figure S2B). Activation of p38 regulates CREB (c-AMP response element binding transcription factor) activity that is required for transcriptional regulation of several molecules including plasminogen activator inhibitor-2 (PAI-2), cyclooxygenase-2 (COX-2) and IL-1β (Park et al., 2005). Notably, the amounts of IL-1β mRNA induced by the ΔLF mutant were considerably higher than the amounts of IL-1β mRNA induced by WT B. anthracis.
Figure 2. B. anthracis-induced inflammasome activation depends on LF and inhibition of p38.
(A,B) C57BL/6 macrophages were infected with WT (BA) or ΔLF B. anthracis. At the indicated times, cell lysates were analyzed by immunoblotting of 2 separate gels of the same experiment for MEK proteolysis (A) and MAPK phosphorylation (B).
(C,D) RAW264.7 macrophages were transfected with MEK6ΔCR or an empty vector. Transfected cells were left uninfected or infected with B. anthracis and 1 hr later cell lysates were prepared and analyzed for p38 phosphorylation by immunoblotting (C). Lactate dehydrogenase (LDH) release to the culture supernatant was measured after 4 hr of infection (D). Results are means ± SD, **p < 0.01 denote significant differences between the groups.
(E,F) RAW264.7 cells were transfected and infected as above. After 8 hr, IL-1β (E) and IL-6 (F) in culture supernatants were measured by ELISA. Results are means ± SD, **p < 0.01 denote significant differences between the groups. All experiments were repeated at least 3 times and the results of one representative experiment are shown.
Due to the interference of p38 inhibition with optimal IL-1β mRNA induction, we undertook a different approach to understand whether MEK6 cleavage and inhibition of p38 activation contribute to inflammasome activation. We thus transfected RAW264.7 macrophages with a construct encoding MEK6ΔCR, a variant form of MEK6 that is resistant to the inhibitory effect of LF (Park et al., 2002). RT-PCR with mutant gene-specific primers confirmed MEK6ΔCR mRNA expression in transfected RAW 264.7 macrophages (Figure S2D); amounts were comparable to those of WT MEK6 mRNA in these cells (data not shown). Over-expression of MEK6ΔCR in B.anthracis-infected macrophages restored p38 MAPK activation (Figure 2C). As found previously using purified LT (Park et al., 2002), over-expression of MEK6ΔCR prevented B.anthracis-induced cell death (Figure 2D). MEK6ΔCR expression also completely blocked IL-1β release in response to WT B. anthracis infection (Figure 2E). In contrast, IL-6 release was increased by MEK6ΔCR expression (Figure 2F) and induction of IL-1β mRNA was also slightly enhanced (Figure S2E). In conclusion, MEK6 cleavage by LF is required for IL-1β release by B. anthracis infected macrophages.
LF-mediated inhibition of AKT contributes to IL-1β release
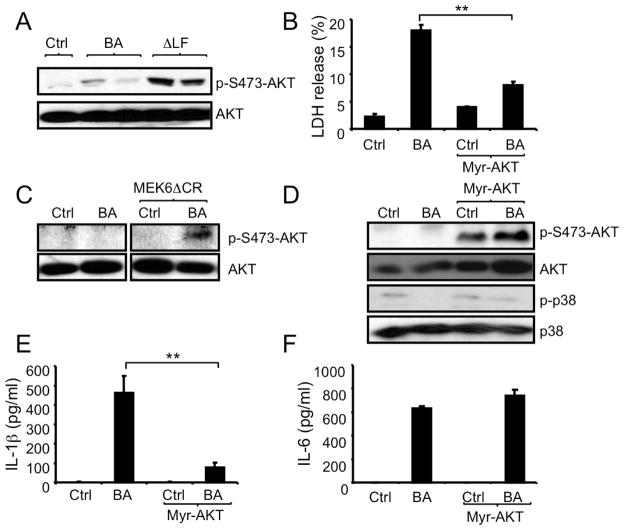
AKT is a serine-threonine protein kinase with important roles in multiple cellular processes. LF inhibits T-cell receptor-induced AKT activation (Comer et al., 2005). We observed LF-dependent inhibition of AKT activation in macrophages, as evidenced by elevated AKT S473 phosphorylation in ΔLF-infected cells relative to cells infected with WT B. anthracis (Figure 3A). We postulated that reduced AKT activity could also contribute to B. anthracis-induced cell death and IL-1β release along with inhibition of p38 activation. To test this hypothesis, macrophages were transduced with retroviral vectors containing a constitutively active variant of AKT (myristoylated-AKT or myr-AKT) or no insert and were then infected with B. anthracis. Myr-AKT expressing macrophages were significantly protected from B. anthracis-induced cell death (Figure 3B), suggesting an important contribution of AKT to host cell survival during infection. Given the protective functions of both p38 and AKT, we explored the possibility of cross-talk between the two kinases. Using MEK6ΔCR-expressing RAW264.7 cells, we found that expression of cleavage-resistant MEK6ΔCR enhanced AKT activation in B. anthracis infected cells (Figure 3C). However, expression of myr-AKT did not prevent inhibition of p38 activation in B. anthracis infected macrophages (Figure 3D). Thus, B. anthracis-mediated inhibition of p38 activation prevents AKT phosphorylation in infected macrophages, but AKT activation does not affect p38 activity. Nonetheless, expression of myr-AKT blocked IL-1β release without affecting IL-6 release by infected macrophages (Figure 3E and 3F). Thus, inhibition of both p38 and AKT is required for inflammasome activation and IL-1β release in B. anthracis infected macrophages.
Figure 3. B. anthracis-induced cell death and inflammasome activation depend on AKT inhibition.
(A) RAW264.7 cells were infected with WT (BA) or ΔLF B. anthracis and 2 hr later, cell lysates were prepared and analyzed by immunoblotting for AKT Ser473 phosphorylation.
(B) RAW264.7 cells were retrovirally transduced with myr-AKT or an empty vector. Transduced cells were left uninfected or infected with B. anthracis. LDH in culture supernatants was measured 4 hr later. Results are means ± SD, **p < 0.01 denote significant differences between the groups.
(C) RAW264.7 cells were transfected with MEKΔ6CR or an empty vector. Transfected cells were left uninfected or infected with B. anthracis and 2 hr later, cell lysates were prepared and analyzed by immunoblotting for AKT phosphorylation.
(D) Myr-AKT-transduced or control RAW264.7 cells were infected with B. anthracis as above. After 1 hr, cell lysates were prepared and analyzed by immunoblotting of 2 separate gels for AKT and p38 phosphorylation.
(E,F) RAW264.7 cells transfected with myr-AKT or an empty vector were infected as above. IL-1β (E) and IL-6 (F) in culture supernatants were measured 8 hr after infection by ELISA. Results are means ± SD, **p < 0.01. All experiments were repeated at least 3 times and the results of one representative experiment are shown.
ATP secretion is required for inflammasome activation upon B. anthracis infection
ATP is released from mononuclear phagocytes stimulated with various PAMPs (Piccini et al., 2008) and extracellular ATP is one of the most pleiotropic inflammasome activators (Ogura et al., 2006). To examine a potential role for ATP in B. anthracis- induced inflammasome activation, we measured extracellular ATP released from macrophages infected with either WT or the ΔLF mutant B. anthracis. Infection with WT B. anthracis resulted in time-dependent ATP release, but this was not seen in macrophages infected with the ΔLF mutant (Figure 4A). Cell death was minimal (around 3-5%) at 3 hr post-infection suggesting that macrophage death may not be the direct cause of early ATP release. To determine if the secreted ATP contributed to inflammasome activation, we blocked ATP signaling via the P2X7 purinergic receptor with the antagonist KN-62 (Di Virgilio, 2007b; Piccini et al., 2008). P2X7 blockade abolished B. anthracis-induced IL-1β secretion (Figure 4B), without affecting pro-IL-1β mRNA synthesis (data not shown). Secretion of IL-6 by B. anthracis-infected macrophages was unaffected by P2X7 blockade (Figure 4C), suggesting an inflammasome-specific phenomenon rather than a general requirement for P2X7 in cell activation or cytokine secretion. If lack of ATP accounts for inability of ΔLF mutant-infected macrophages to activate caspase-1, we hypothesized that co-treatment with exogenous ATP should compensate for this difference. Indeed, IL-1β release was detected in the culture medium of ΔLF mutant-infected macrophages upon concomitant ATP treatment (Figure S3A). In fact, in the presence of exogenous ATP, the ΔLF mutant induced more IL-1β secretion than WT B. anthracis.
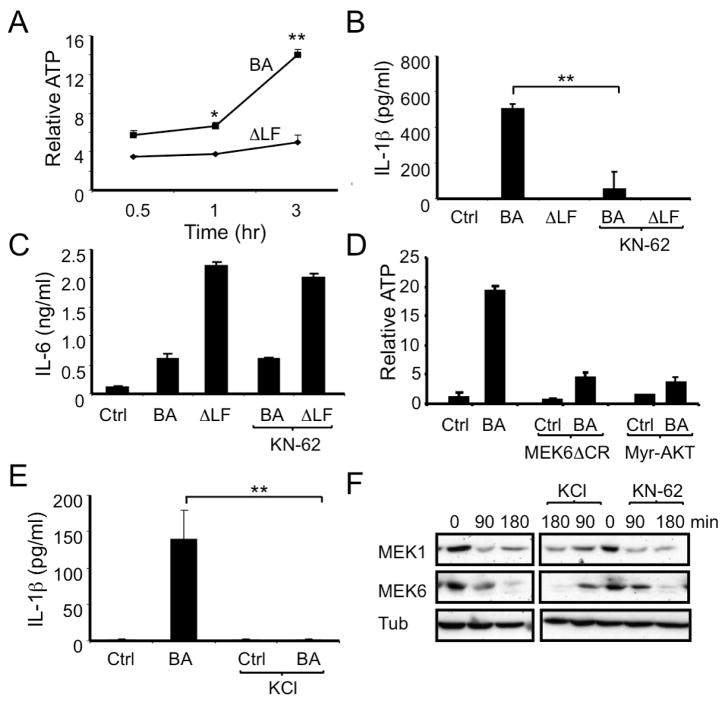
Figure 4. B. anthracis induces ATP release from infected macrophages in LF-, p38- and AKT-dependent manner.
(A) C57BL/6 macrophages were infected with WT (BA) or ΔLF B. anthracis. At the indicated times points, ATP in the culture supernatants was measured. This experiment was repeated at least 4 times and the results of one representative experiment done in triplicates are shown. Results are means ± SD, *p < 0.05, **p < 0.01 denote significant differences between the groups.
(B,C) Mouse macrophages were infected with WT (BA) or ΔLF B. anthracis in the absence or presence of KN-62 (10 μM). After 8 hr, IL-1β (B) and IL-6 (C) in culture supernatants were measured by ELISA. This experiment was repeated 3 times and the results of one representative experiment done in triplicates are shown. Results are means ± SD, **p < 0.01 denote significant differences between the groups.
(D) RAW264.7 cells transfected with MEK6ΔCR, myr-AKT or empty vector were left uninfected or infected with B. anthracis and 2 hr later ATP release to culture supernatants was measured. This experiment was repeated 3 times and the results of one representative experiment done in triplicates are shown.
(E) C57BL/6 macrophages were infected with WT B. anthracis in the absence or presence of 130 mM KCl and 8 hr later the amount of IL-1β in culture supernatants was determined by ELISA. This experiment was repeated 3 times and the results of one representative experiment done in duplicates are shown. Results are means ± SD, **p < 0.01 denote significant differences between the groups.
(F) Mouse macrophages were infected with WT B. anthracis in the absence or presence of KN-62 (10 μM) or KCl (130 mM) and at the indicated times cell lysates were prepared and analyzed by immunoblotting of 2 separate gels from the same experiment for MEK proteolysis. This experiment was repeated 3 times and the results of one representative experiment are shown.
Furthermore, release of ATP from B. anthracis-infected macrophages was blocked by expression of constitutively active myr-AKT or MEK6ΔCR (Figure 4D), suggesting a role for LF-mediated inhibition of p38 and AKT signaling in triggering ATP release. Overexpression of either p38 or AKT by itself had no inhibitory effect on inflammaosme activation, as induction of IL-1β release by co-treatment of ΔLF + ATP was unaffected, by MEK6ΔCR or myr-AKT expression in RAW264.7 cells (Figure S3B and data not shown). In contrast to p38 and AKT- signaling, caspase-1 was dispensable for ATP secretion as similar amounts of ATP were released from B. anthracis-infected WT and Casp1−/− macrophages (Figure S3C).
Activation of P2X7 by ATP causes rapid K+ efflux from the cytosol and release of intracellular Ca++ (Di Virgilio, 2007b). To study the role of these events in B. anthracis- induced IL-1β release, we blocked K+ efflux by increasing extracellular KCl to 130 mM. Blockade of K+ efflux inhibited B. anthracis-induced IL-1β secretion without affecting pro-IL-1β mRNA expression (Figure 4E and data not shown). Neither extracellular KCl nor KN-62 -mediated blockade of P2X7 affected MEK proteolysis in B. anthracis-infected macrophages (Figures 4F and S3D), indicating that the observed inhibition of IL-1β release is not due to alterations in the infection process or LF catalytic activity.
AKT prevents ATP release by controlling a connexin-43 channel
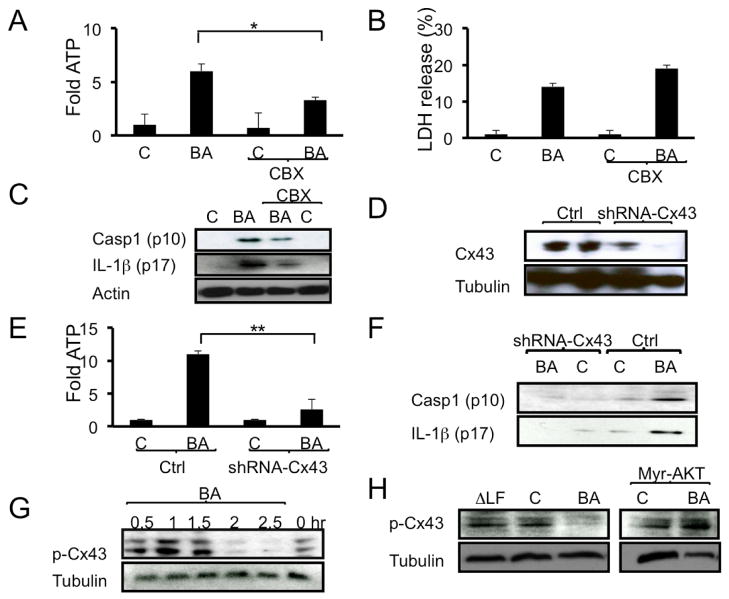
The above experiments suggested that ATP released by B. anthracis-infected macrophages is a key signal for activation of inflammasome-dependent host immunity. Live cells release ATP through a number of channels, including volume-sensitive channels, pannexin and connexin channels (Chekeni et al., 2010; Faigle et al., 2008; Pangrsic et al., 2007; Sabirov et al., 2001), as well as through vesicular exocytosis (Pangrsic et al., 2007). To examine the potential involvement of the connexin-43 channel (Eltzschig et al., 2006; Faigle et al., 2008) in B. anthracis induced ATP release, mouse macrophages were infected with B. anthracis in the presence or absence of the connexin-43 inhibitor, carbenoxolone (CBX) (Kang et al., 2008). Notably, release of ATP by B. anthracis-infected macrophages was attenuated following CBX exposure (Figure 5A). In contrast, CBX treatment did not inhibit lactate dehydrogenase (LDH) release; on the contrary, CBX treated macrophages exhibited enhanced LDH release (Figure 5B). To analyze the role of connexin-43 in inflammasome activation, mouse macrophages were infected with B. anthracis in the presence or absence of CBX, and IL-1β and caspase-1 secretion were examined. Incubation with CBX inhibited secretion of activated caspase-1 and IL-1β by B. anthracis-infected macrophages (Figures 5C and S4A). Since, CBX may affect other pathways (Chekeni et al., 2010; Faigle et al., 2008; Pangrsic et al., 2007; Sabirov et al., 2001), we validated our results using shRNA-mediated silencing of connexin-43. shRNA-mediated silencing of connexin-43 (Figure 5D), reduced B. anthracis-induced ATP (Figure 5E), caspase-1 and IL-1β release (Figure 5F) compared to control treated cells. Neither B. anthracis-induced IL-1β mRNA expression nor cell death were affected by connexin-43 silencing (data not shown). Collectively, the connexin-43 silencing studies and pharmacological inhibition by CBX support a role for connexin-43 in ATP release and inflammasome activation during B. anthracis infection.
Figure 5. B. anthracis-induced ATP release and inflammasome activation depend on connexin-43.
(A-C) C57BL/6 macrophages were infected with B. anthracis as above and 45 min later the connexin-43 inhibitor carbenoxolone (CBX, 2.5 μM) was added to the culture medium. ATP release was measured 1 hr later (A) and LDH release was measured after 2 hr (B). Secretion of active caspase-1 (p10) and IL-1β (p17) to the culture medium was examined 12 hr after CBX addition. Cellular actin was used as a control. This experiment was repeated 3 times and the results of one representative experiment are shown.
(D) RAW264.7 cells were transduced with lentiviruses expressing connexin-43 shRNA or control shRNA (Ctrl.). Connexin-43 expression in the transduced cells was analyzed by immunoblotting. This experiment was repeated 2 times and the results of one representative experiment are shown.
(E,F) RAW264.7 cells were transduced with lentiviruses expressing connexin-43 shRNA or control shRNA. Transduced cells were left uninfected or infected with B. anthracis. ATP release was measured 2 hr later (E). Caspase-1 (p10) and IL-1β (p17) secretion was examined after 12 hr (F). This experiment was repeated twice and the results of one representative experiment are shown. Results are means ± SD, **p < 0.01 denote significant differences between the groups.
(G) C57BL/6 macrophages were treated as above and at the indicated times cell lysates were prepared and analyzed by immunoblotting for phosphorylation of connexin-43 at Ser279 and Ser282. This experiment was repeated 3 times and the results of one representative experiment are shown.
(H) Myr-AKT-expressing or parental RAW264.7 cells were infected as above. After 2 hr, cell lysates were prepared and analyzed by immunoblotting for connexin-43 phosphorylation and tubulin content. This experiment was repeated 3 times and the results of one representative experiment are shown.
Connexin-43 has multiple serine residues that can be phosphorylated by PKC, MAPK, or AKT (Kang et al., 2008) and the phosphorylation of connexins negatively regulates their opening (Faigle et al., 2008). Since B. anthracis inhibits activation of MAPKs and AKT, we asked whether inhibition of these protein kinases impacts the phosphorylation of connexin-43. In non-infected macrophages, connexin-43 was phosphorylated at Ser279 and Ser282 based on reactivity with a phospho-specific connexin-43 antibody (Leykauf et al., 2003). Initially, B. anthracis infection enhanced connexin-43 phosphorylation, but after 2 hr connexin-43 phosphorylation was almost completely abolished (Figure 5G). In contrast, infection of macrophages with ΔLF B. anthracis had no inhibitory effect on connexin-43 phosphorylation (Figure 5H). The kinetics of connexin-43 dephosphorylation correlated with the kinetics of ATP release (Figure S4B). To gain further insight into the regulation of connexin-43 phosphorylation, we examined it in RAW264.7 cells expressing myr-AKT. Notably, expression of constitutively active myr-AKT, which inhibits ATP release and inflammasome activation in infected macrophages (Figures 4D and 3E), prevented the B. anthracis-induced decrease in connexin-43 phosphorylation (Figure 5H). Together, our results suggest that LT-dependent inhibition of p38 and AKT signaling results in opening of connexin-43 channel and ATP release for inflammasome activation (Table S1).
IL-1β production protects mice from B. anthracis
IL-1β signaling plays a prominent role in immune and inflammatory responses (Meylan et al., 2006; Miller et al., 2006). To determine the immunological role of B. anthracis-induced inflammasome activation and IL-1β production, we infected WT C57BL/6 mice and different mutants in the same genetic background with WT or ΔLF B. anthracis. Although C57BL/6 mice are resistant to pharmacological challenge with purified LF (Boyden et al., 2006), they died when infected with WT B. anthracis but not upon infection with the ΔLF mutant (Figure S5A). Interestingly, the C57BL/6 strain exhibited enhanced B. anthracis-induced mortality relative to the Balb/c strain (Figure S5B), in contrast to the response elicited by purified LT in the two strains (Moayeri et al., 2003). Although purified edema toxin causes lethality of Balb/c mice (Moayeri and Leppla, 2009), ΔLF that expresses intact ET was unable to induce mortality in C57BL/6 (Figure S5A) as well as in Swiss mice (Pezard et al., 1991). Infected mice cleared ΔLF mutant bacteria from their spleen more efficiently than WT B. anthracis (Figures S5C). Furthermore, WT B. anthracis, but not the ΔLF mutant, induced cell death in spleens of infected mice (data not shown). These results demonstrate that lethal B. anthracis infection of C57BL/6 mice is indeed LF-dependent.
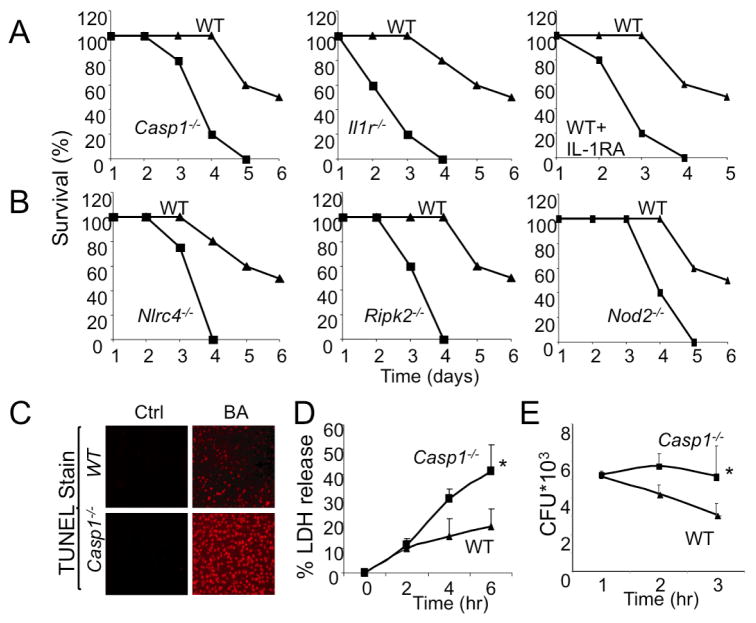
Both caspase-1 (Casp1−/−) and IL-1 receptor (Il1r−/−) deficient mice exhibited enhanced mortality upon infection with WT B. anthracis relative to WT mice (Figure 6A). Furthermore, B. anthracis-infected mice displayed enhanced mortality when treated with Anakinra (Kineret), an FDA-approved IL-1 receptor (IL-1R) antagonist (Figure 6A). No mortality of either WT or Casp1−/− mice was seen upon infection with the ΔLF mutant (data not shown). Furthermore, infection with a low inoculum (0.5 × 106 cfu/mouse) that had no effect on survival of WT mice was sufficient to induce mortality of Casp1−/− mice (Figure S5D). Together, the results suggest that inflammasome activation and IL-1β signaling are critical for innate immunity to LT-producing B. anthracis. Furthermore, counter to previous expectations (Boyden and Dietrich, 2006), assembly of inflammasome components and caspase-1 activation was not the cause of B. anthracis-induced mortality. Consistent with these observations, mice deficient in critical LF-responsive inflammasome components, including Nlrc4−/−, Ripk2−/− and Nod2−/− mice, also exhibited enhanced mortality upon B. anthracis infection (Figure 6B).
Figure 6. Inflammasome activation protects mice from B. anthracis infection.
(A,B) Mice of the indicated genotypes were infected with WT B. anthracis (2×106 cfu/mouse given i.p., n=5–8 mice per group). Where indicated, Anakinra (Kineret, IL-1RA, 100 mg/kg) was s.c. injected every 6 hr over a period of 24 hr post-infection. Mouse survival was analyzed over the course of 6 days post-infection. This experiment was repeated 3 times and the results of one representative experiment are shown.
(C) WT and Casp1−/− mice were infected as above and 48 hr later their spleens were collected and examined for cell death by a TUNEL assay. The experiment was repeated twice with similar results.
(D) Macrophages from WT and Casp1−/− mice were infected with WT B. anthracis and at the indicated times, LDH release was measured. This experiment was repeated 3 times and the results of one representative experiment done in triplicates are shown. Results are means ± SD, *p < 0.05 denote significant differences between the groups.
(E) Macrophages from WT and Casp1−/− mice were infected with WT B. anthracis and at the indicated times intracellular bacterial counts were determined. This experiment was repeated 3 times and the results of one representative experiment done in triplicates are shown. Results are means ± SD, *p < 0.05 denote significant differences between the groups.
To determine the effect of reduced inflammasome responses in Casp1−/− mice on host leukocytes, we harvested spleens from B. anthracis-infected WT and Casp1−/− mice 3 days after infection and evaluated cell death by TUNEL staining. In contrast to previous reports that LT-induced cell death in vitro is caspase-1-dependent (Boyden and Dietrich, 2006), we observed a dramatic increase in splenocyte death in Casp1−/− mice relative to WT mice (Figure 6C). This effect correlated with decreased bacterial clearance from spleens of Casp1−/− mice (data not shown).
Although neutrophils dominate IL-1β-dependent responses to bacterial challenge (Miller et al., 2007), downstream effects of IL-1β signaling during bacterial infection have not been well studied. To determine how inflammasome activation benefits the infected host, peritoneal macrophages from WT and Casp1−/− mice were challenged with B. anthracis and cell death was assessed by LDH release. As found in vivo, Casp1−/− macrophages exhibited enhanced death (Figure 6D) and impaired bacterial clearance (Figure 6E). Corroborating that caspase-1 exerts its effect through IL-1β, Anakinra treatment also increased the extent of macrophage death in response to infection with WT, but not ΔLF, B. anthracis (Figure S5E). Since survival of pathogen-activated macrophages depends on p38 and NF-κB activation (Park et al., 2002), we examined whether caspase-1 was required for induction of NF-κB target genes in B. anthracis-infected macrophages. Indeed, expression of several known NF-κB target genes in mouse macrophages, including Ptgs2 and Nos2 was higher in WT macrophages relative to Casp1−/− macrophages 4 hr after infection with WT B. anthracis (Figure S5F and data not shown). Nonetheless and despite existence of this amplification loop, at very early stages of macrophage infection, both WT and ΔLF B. anthracis led to similar NF-κB activation responses as indicated by the kinetics and extend of IκBα degradation (Figure 2B). Collectively, these findings indicate a critical role of IL-1β in mounting immunity against virulent B. anthracis.
DISCUSSION
We have used two B. anthracis strains that differ in expression of LF, an essential component of LT, a critical virulence factor, to interrogate how the host innate immune system discriminates between virulent and non-virulent bacteria. Our results indicate that the major and most critical difference in the host responses elicited by the two bacteria lies in their ability to activate the inflammasome system and induce IL-1β secretion. Yet, both B. anthracis strains were equally effective in their ability to induce IκB degradation, a response that is most likely dependent on TLR activation. In fact, at early time points after infection, the non-virulent bacterium led to more effective induction of IL-1β mRNA and IκBα re-synthesis, responses that depend on activation of both NF-κB and MAPKs (Park et al., 2005). IL-1β secretion was essential for host protection against WT B. anthracis, attenuation of macrophage death and bacterial clearance. Dissection of the molecular events involved in B. anthracis-mediated inflammasome activation revealed that the key difference between the two strains lies in their ability to induce ATP release from infected macrophages. Our results strongly suggest that the major source of ATP release from B. anthracis infected macrophages is the connexin-43 ATP-release channel. Whereas WT B. anthracis induces dephosphorylation and presumably opening of connexin-43, the non-virulent ΔLF mutant fails to do so. Most likely, connexin-43 is dephosphorylated in WT B. anthracis infected cells due to inhibition of AKT, one of several protein kinases known to keep connexin-43 in a closed state (Park et al., 2007; Solan and Lampe, 2007; Spinella et al., 2003; Warn-Cramer et al., 1996) In addition to elucidating the mechanism of B. anthracis-induced inflammasome activation, our results demonstrate that inflammasomes, containing NALP1, NOD2, RIP2 and IPAF, were responsible for discrimination between virulent and non-virulent B. anthracis. Interestingly, however, inflammasomes are not directly responsible for recognition of LF, the one molecule that differs between the two bacterial strains used in this study. Rather, the inflammasomes that are activated upon B. anthracis infection are responsive to K+ ions, whose efflux is stimulated by the ATP that is released from infected cells. Thus, it is the inflammasome, and not the TLR system, that is responsible for recognition of “danger” (Meylan et al., 2006), which in this case is caused by infection with virulent, LT-producing, B. anthracis. The initial induction of IL-1β mRNA, which depends on activation of NF-κB and other transcription factors, is most likely due to recognition of PAMPs, which are present on both virulent and non-virulent bacteria, by different TLRs expressed by the infected macrophage.
Our results suggest that upon virulent B. anthracis infection, caspase-1 interacts with NALP1, NOD2, RIP2 and IPAF, but it is not clear whether all of these molecules form a single inflammasome complex. In vitro studies using purified LT in the absence of other B. anthracis components has identified NALP1 as a key component of the LT-activated inflammasome (Boyden and Dietrich, 2006). In these studies, however, LT activated the NALP1 inflammasome only in Balb/c, but not in C57BL/6, macrophages and the differential response was attributed to polymorphisms at the Nalp1 locus (Boyden and Dietrich, 2006). The molecular mechanism by which these polymorphisms affect NALP1 activation was never identified, neither was it shown that LT or LF is directly recognized by NALP1. Our results indicate that although less responsive to purified LT, C57BL/6 macrophages are perfectly capable of responding to live B. anthracis, which is the only physiologically relevant route of LT delivery into these cells. Furthermore, C57BL/6 mice succumbed to infection with WT B. anthracis and the mortality was fully dependent on LT production. As NALP1 is unlikely to be directly involved in LT (or LF) recognition, it is plausible that when encountering recombinant LT, which may not be as active as the bacterially-produced toxin within the host macrophage, Balb/c macrophages may be more sensitive to ATP or ATP-induced K+ efflux than C57BL/6 macrophages, a difference that is due to expression of different NALP1 variants.. It should also be noted that in vitro, macrophage killing by LT is caspase-1-dependent, whereas in vivo, at least in C57BL/6 mice, caspase-1 activation is important for host protection. Furthermore, the C57BL/6 strain is more susceptible to B. anthracis-induced mortality than the Balb/c strain, in contrast to the in vitro response elicited by purified LT in macrophages of the two strains (Boyden and Dietrich, 2006), but in agreement with another in vivo infection study using the Sterne strain of B. anthracis (Welkos et al., 1986). Furthermore, purified LT induced enhanced mortality in Balb/c mice relative to C57BL/6 mice (Moayeri et al., 2003). Clearly, the response to the intact bacterium is entirely different from the response to the purified toxin.
Previously, we found that B. anthracis infection of macrophages induces NALP1- and NOD2-dependent caspase-1 activation and IL-1β release (Hsu et al., 2008). These NLRs could be involved in sensing of peptidoglycans or other PAMPs produced by this Gram positive bacterium (Martinon, 2007; Schroder and Tschopp, 2010). However, both toxigenic and non-toxigenic B. anthracis are probably identical in the PAMPs they express and the key to their differential ability to activate caspase-1 lies in their differential ability to induce ATP release from infected macrophages. Addition of exogenous ATP to macrophages infected with the non-virulent ΔLF mutant restored inflammasome activation and IL-1β secretion. Consistent with previous publications (Di Virgilio, 2007a, b; Piccini et al., 2008), we find that ATP is likely to cause inflammasome activation through engagement of the P2X7 purinergic receptor and induction of K+ efflux.
Multiple mechanisms can mediate release of intracellular ATP in response to mechanical stimulation, stress, osmotic swelling or shrinking of cells, physical perturbation, or host-pathogen interactions (Corriden and Insel, 2010; Piccini et al., 2008). Various membrane channels can release ATP, including connexin and pannexin hemichannels, maxi-anion channels, and volume-regulated anion channels (Corriden and Insel, 2010). In addition to channel-mediated release, ATP can be released by exocytotic mechanisms in neurons, or by membrane permeabilization in dying cells (Chekeni et al., 2010; Faigle et al., 2008; Pangrsic et al., 2007; Sabirov et al., 2001). ATP release was previously shown to be associated with induction of macrophage death through a process that has been named pyroptosis (Iyer et al., 2009), which was implicated in IL-1β release nearly 20 years ago (Hogquist et al., 1991). Indeed, LT by virtue of its ability to inhibit p38 activation can promote macrophage death (Park et al., 2002). Activation of p38 is required for synthesis of several anti-apoptotic molecules including Bfl1 and PAI-2 (Park et al., 2005) and as we show here, it is also needed for maintenance of AKT activity. Furthermore, the release of ATP from B. anthracis-infected macrophages is strongly inhibited by activation of either p38 or AKT but is independent of caspase-1 activation. Rather than be a direct and non-specific consequence of cell death, a major part of the early ATP release by B. anthracis infected macrophages depends on the opening of connexin-43 channels as indicated by its inhibition by carbenoxolone, a connexin-43 gap junction inhibitor (De Vuyst et al., 2007) and by connexin-43 silencing.
Previous work has implicated connexin-43 channels in ATP release from various cells including astrocytes, neuronal precursors, neutrophils and myocytes, in response to different stimuli such as hypoxia, ischemia as well as PAMPs (Clarke et al., 2009; Dobrowolski et al., 2008; Eltzschig et al., 2006; Faigle et al., 2008; Goldberg et al., 2002; Kang et al., 2008; Lampe and Lau, 2004; Liu et al., 2010; Solan and Lampe, 2007). Connexin-43 is maintained in a closed state through phosphorylation by several kinases including PKC, MAPK and AKT (Faigle et al., 2008; Lampe and Lau, 2004) and is opened upon its dephosphorylation (Faigle et al., 2008). We found that macrophage infection with B. anthracis resulted in dephosphorylation of connexin-43, which could be prevented by constitutive AKT activation. Although AKT is not a direct target for LF-mediated proteolysis, it is phosphorylated by the p38-responsive protein kinase MK2 at Ser473 (Seimon et al., 2009; Taniyama et al., 2004). In fact, MK2 in a complex with p38, hsp27, and AKT, is activated by p38 to subsequently phosphorylate AKT at Ser473 (Seimon et al., 2009; Taniyama et al., 2004). Thus, cleavage of MEK6 by LF is directly responsible for loss of p38 MAPK activity in B. anthracis-infected macrophages and this leads to diminished expression of survival genes and loss of AKT activity. The latter is likely to be responsible for connexin-43 opening and initial ATP release by infected macrophages. The amount of ATP released prior to the onset of massive macrophage death is sufficient for inflammasome activation and IL-1β secretion (Figure S13). Interestingly, IL-1β maintains macrophage survival and this may limit further ATP release. However, if the initial release of IL-1β is not sufficient for prevention of macrophage death, more ATP is likely to be released at later stages when membrane integrity is lost, leading to inflammasome activation in neighboring macrophages that are yet to die. Thus, inflammasome-activation may be controlled by a competition between B. anthracis-induced macrophage death and IL-1β-induced macrophage survival. Importantly, by directly responding to a “danger” signal, the inflammasome is only activated upon infection with a virulent bacterium that cannot evade this critical component of innate immunity whose activation depends on an abundant molecule that is important to all life forms-ATP.
Supplementary Material
Acknowledgments
This work was supported by NIH grants to M.K. and V.N and L.E. We thank Drs. R.A. Flavell, and G. Cheng for various genetically targeted mouse strains. We also thank D. G. Guiney for B. anthracis strains. S.R.A. and A.M.T. were supported by postdoctoral fellowships from the Philip Morris and the A. P. Giannini Foundations, respectively. M.K. is an American Cancer Society Research Professor. We dedicate this paper to the memory of Jürg Tschopp, the discoverer of the inflammasome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, Kress CL, Bailly-Maitre B, Li X, Osterman A, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, et al. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TC, Williams OJ, Martin PE, Evans WH. ATP release by cardiac myocytes in a simulated ischaemia model: inhibition by a connexin mimetic and enhancement by an antiarrhythmic peptide. Eur J Pharmacol. 2009;605:9–14. doi: 10.1016/j.ejphar.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Comer JE, Chopra AK, Peterson JW, Konig R. Direct inhibition of T-lymphocyte activation by anthrax toxins in vivo. Infect Immun. 2005;73:8275–8281. doi: 10.1128/IAI.73.12.8275-8281.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal. 2010;3:re1. doi: 10.1126/scisignal.3104re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst E, Decrock E, De Bock M, Yamasaki H, Naus CC, Evans WH, Leybaert L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell. 2007;18:34–46. doi: 10.1091/mbc.E06-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007a;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F. Purinergic signalling in the immune system. A brief update. Purinergic Signal. 2007b;3:1–3. doi: 10.1007/s11302-006-9048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, Troatz C, Ghanem A, Tiemann K, Degen J, et al. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum Mol Genet. 2008;17:539–554. doi: 10.1093/hmg/ddm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS One. 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Lampe PD. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem. 2002;277:36725–36730. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Nett MA, Unanue ER, Chaplin DD. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci U S A. 1991;88:8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz P, Zinkernagel AS, Jenikova G, Botwin GJ, Hugot JP, Karin M, Nizet V, Eckmann L. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc Natl Acad Sci U S A. 2009;106:12873–12878. doi: 10.1073/pnas.0904958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, Karin M. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leykauf K, Durst M, Alonso A. Phosphorylation and subcellular distribution of connexin43 in normal and stressed cells. Cell Tissue Res. 2003;311:23–30. doi: 10.1007/s00441-002-0645-5. [DOI] [PubMed] [Google Scholar]
- Liu X, Hashimoto-Torii K, Torii M, Ding C, Rakic P. Gap junctions/hemichannels modulate interkinetic nuclear migration in the forebrain precursors. J Neurosci. 2010;30:4197–4209. doi: 10.1523/JNEUROSCI.4187-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- Martinon F. Orchestration of pathogen recognition by inflammasome diversity: Variations on a common theme. Eur J Immunol. 2007;37:3003–3006. doi: 10.1002/eji.200737871. [DOI] [PubMed] [Google Scholar]
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, O'Connell RM, Iwakura Y, Cheung AL, Cheng G, Modlin RL. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007;179:6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayeri M, Leppla SH. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med. 2009;30:439–455. doi: 10.1016/j.mam.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- Park DJ, Wallick CJ, Martyn KD, Lau AF, Jin C, Warn-Cramer BJ. Akt phosphorylates Connexin43 on Ser373, a "mode-1" binding site for 14-3-3. Cell Commun Adhes. 2007;14:211–226. doi: 10.1080/15419060701755958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science. 2002;297:2048–2051. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- Park JM, Greten FR, Wong A, Westrick RJ, Arthur JS, Otsu K, Hoffmann A, Montminy M, Karin M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis--CREB and NF-kappaB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pezard C, Berche P, Mock M. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect Immun. 1991;59:3472–3477. doi: 10.1128/iai.59.10.3472-3477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov RZ, Dutta AK, Okada Y. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J Gen Physiol. 2001;118:251–266. doi: 10.1085/jgp.118.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Seimon TA, Wang Y, Han S, Senokuchi T, Schrijvers DM, Kuriakose G, Tall AR, Tabas IA. Macrophage deficiency of p38alpha MAPK promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. J Clin Invest. 2009;119:886–898. doi: 10.1172/JCI37262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J Membr Biol. 2007;217:35–41. doi: 10.1007/s00232-007-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinella F, Rosano L, Di Castro V, Nicotra MR, Natali PG, Bagnato A. Endothelin-1 decreases gap junctional intercellular communication by inducing phosphorylation of connexin 43 in human ovarian carcinoma cells. J Biol Chem. 2003;278:41294–41301. doi: 10.1074/jbc.M304785200. [DOI] [PubMed] [Google Scholar]
- Taniyama Y, Ushio-Fukai M, Hitomi H, Rocic P, Kingsley MJ, Pfahnl C, Weber DS, Alexander RW, Griendling KK. Role of p38 MAPK and MAPKAPK-2 in angiotensin II-induced Akt activation in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2004;287:C494–499. doi: 10.1152/ajpcell.00439.2003. [DOI] [PubMed] [Google Scholar]
- Tournier JN, Rossi Paccani S, Quesnel-Hellmann A, Baldari CT. Anthrax toxins: a weapon to systematically dismantle the host immune defenses. Mol Aspects Med. 2009;30:456–466. doi: 10.1016/j.mam.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Vitale G, Bernardi L, Napolitani G, Mock M, Montecucco C. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem J. 2000;352(Pt 3):739–745. [PMC free article] [PubMed] [Google Scholar]
- Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LW, Eckhart W, Lau AF. Characterization of the mitogen-activated protein kinase phosphorylation sites on the connexin-43 gap junction protein. J Biol Chem. 1996;271:3779–3786. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- Welkos SL, Keener TJ, Gibbs PH. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect Immun. 1986;51:795–800. doi: 10.1128/iai.51.3.795-800.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.