Abstract
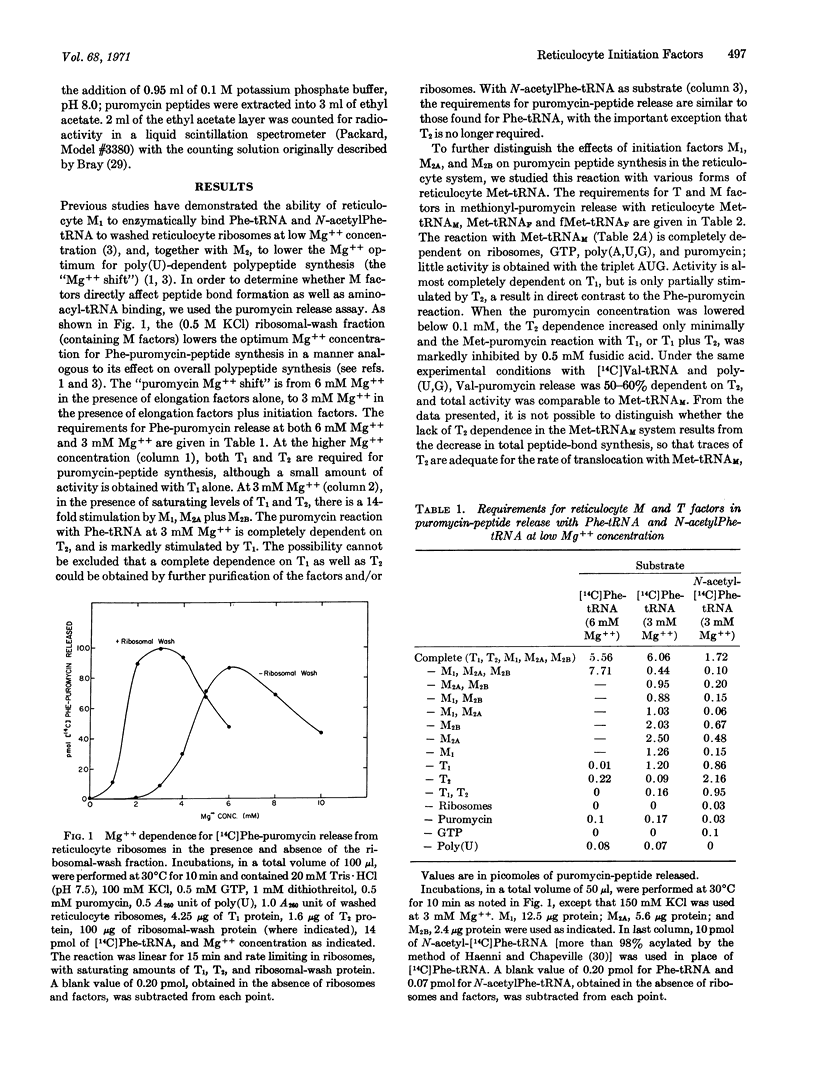
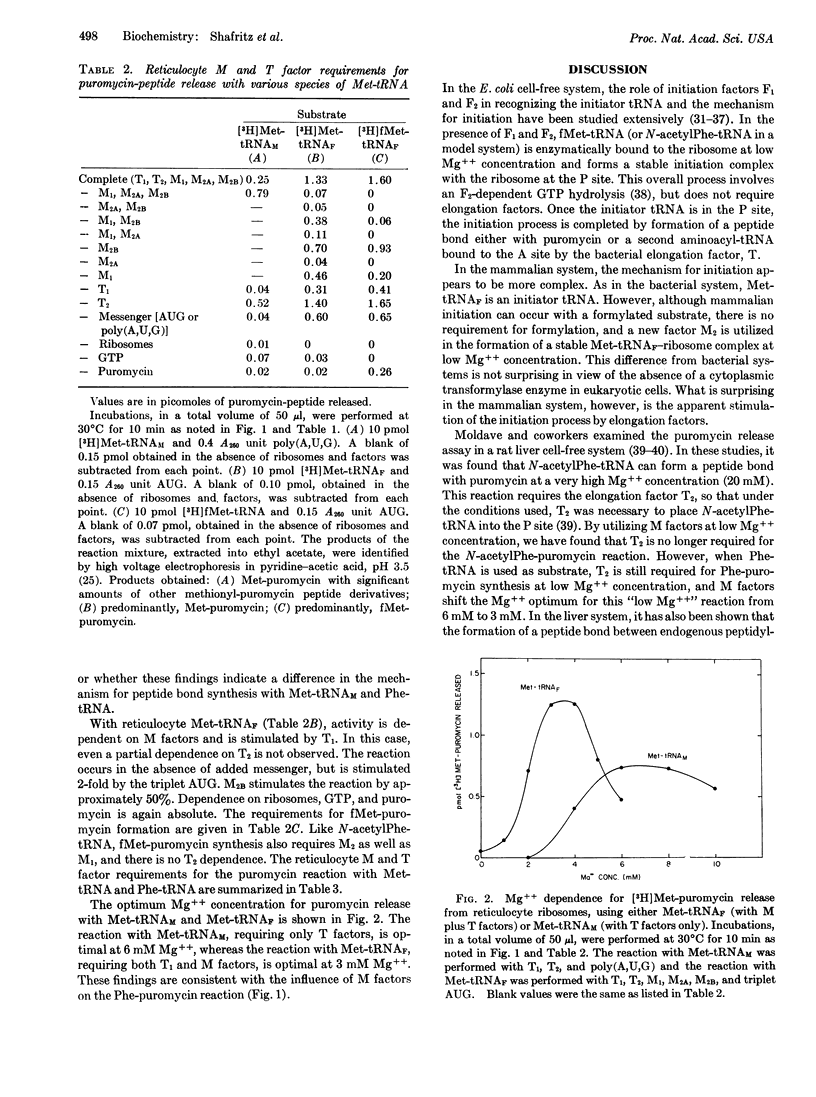
The ability to form a “peptide” bond between various forms of Met-tRNA or Phe-tRNA and puromycin has been studied in the reticulocyte cell-free system. When Met-tRNAF, fMet-tRNAF, or N-acetylPhe-tRNA are used as substrate at low Mg++ concentration (3 mM), reticulocyte initiation factors M1 and M2 (M2A + M2B) are required for puromycin-peptide synthesis. In contrast to bacterial systems, this reaction is also stimulated by the elongation factor T1. When Met-tRNAM or Phe-tRNA is used as substrate, there is no M-factor requirement for the puromycin reaction; T1 is absolutely required, and the reaction is stimulated by T2. These studies indicate that reticulocyte factors M1 and M2 may function in part by placing the initiator tRNA into the P site. The detailed mechanism for mammalian initiation, however, may be more complex than that for bacterial systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhaduri S., Chatterjee N. K., Bose K. K., Gupta N. K. Initiation of protein synthesis in rabbit reticulocytes. Biochem Biophys Res Commun. 1970 Jul 27;40(2):402–407. doi: 10.1016/0006-291x(70)91023-5. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Marcker K. A. Polypeptidyl-sigma-ribonucleic acid and amino-acyl-sigma-ribonucleic acid binding sites on ribosomes. Nature. 1966 Jul 23;211(5047):380–384. doi: 10.1038/211380a0. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Smith A. E. Initiator codons in eukaryotes. Nature. 1970 May 16;226(5246):610–612. doi: 10.1038/226610a0. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Redfield B., Weissbach H. Formylation of guinea pig liver methionyl-sRNA. Arch Biochem Biophys. 1967 Apr;120(1):119–123. doi: 10.1016/0003-9861(67)90605-4. [DOI] [PubMed] [Google Scholar]
- Chae Y. B., Mazumder R., Ochoa S. Polypeptide chain initiation in E. coli: studies on the function of initiation factor F1. Proc Natl Acad Sci U S A. 1969 Jul;63(3):828–833. doi: 10.1073/pnas.63.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp W., Morrisey J., Hardesty B. Initiator tRNA for the synthesis of globin peptides. Biochem Biophys Res Commun. 1970 Aug 24;40(4):777–785. doi: 10.1016/0006-291x(70)90970-8. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Chapeville F. The behaviour of acetylphenylalanyl soluble ribonucleic acid in polyphenylalanine synthesis. Biochim Biophys Acta. 1966 Jan 18;114(1):135–148. doi: 10.1016/0005-2787(66)90261-9. [DOI] [PubMed] [Google Scholar]
- Hille M. B., Miller M. J., Iwasaki K., Wahba A. J. Translation of the genetic message. VI. The role of ribosomal subunits in binding of formylmethionyl-tRNA and its reaction with puromycin. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1652–1654. doi: 10.1073/pnas.58.4.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Jackson R., Hunter T. Role of methionine in the initiation of haemoglobin synthesis. Nature. 1970 Aug 15;227(5259):672–676. doi: 10.1038/227672a0. [DOI] [PubMed] [Google Scholar]
- Kerwar S. S., Spears C., Weissbach H. Studies on the initiation of protein synthesis in animal tissues. Biochem Biophys Res Commun. 1970 Oct 9;41(1):78–84. doi: 10.1016/0006-291x(70)90471-7. [DOI] [PubMed] [Google Scholar]
- Leder P., Bursztyn H. Initiation of protein synthesis II. A convenient assay for the ribosome-dependent synthesis of N-formyl-C14-methionylpuromycin. Biochem Biophys Res Commun. 1966 Oct 20;25(2):233–238. doi: 10.1016/0006-291x(66)90586-9. [DOI] [PubMed] [Google Scholar]
- Leder P., Nau M. M. Initiation of protein synthesis. 3. Factor-GTP-codon-dependent binding of F-met-tRNA to ribosomes. Proc Natl Acad Sci U S A. 1967 Aug;58(2):774–781. doi: 10.1073/pnas.58.2.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Keller E. B. Protein chain initiation by methionyl-tRNA. Biochem Biophys Res Commun. 1970 Jul 27;40(2):416–421. doi: 10.1016/0006-291x(70)91025-9. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J., Lipmann F. Initiation of polyphenylalanine synthesis by N-acetylphenylalanyl-SRNA. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1050–1057. doi: 10.1073/pnas.57.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRIS A., ARLIGHAUS R., FAVELUKES S., SCHWEET R. INHIBITION OF HEMOGLOBIN SYNTHESIS BY PUROMYCIN. Biochemistry. 1963 Sep-Oct;2:1084–1090. doi: 10.1021/bi00905a030. [DOI] [PubMed] [Google Scholar]
- Mazumder R., Chae Y. B., Ochoa S. Polypeptide chain initiation in E. coli: sulfhydryl groups and the function of initiation factor F2. Proc Natl Acad Sci U S A. 1969 May;63(1):98–103. doi: 10.1073/pnas.63.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. M., Collins J. F., Maxwell E. S. Discrimination by rat liver aminoacyltransferase I against Met-tRNA-F. Biochem Biophys Res Commun. 1970 Oct 9;41(1):170–176. doi: 10.1016/0006-291x(70)90484-5. [DOI] [PubMed] [Google Scholar]
- NATHANS D. PUROMYCIN INHIBITION OF PROTEIN SYNTHESIS: INCORPORATION OF PUROMYCIN INTO PEPTIDE CHAINS. Proc Natl Acad Sci U S A. 1964 Apr;51:585–592. doi: 10.1073/pnas.51.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard P. M., Gilbert J. M., Shafritz D. A., Anderson W. F. Factors for the initiation of haemoglobin synthesis by rabbit reticulocyte ribosomes. Nature. 1970 May 9;226(5245):511–514. doi: 10.1038/226511a0. [DOI] [PubMed] [Google Scholar]
- Revel M., Lelong J. C., Brawerman G., Gros F. Function of three protein factors and ribosomal subunits in the initiation of protein synthesis in E. coli. Nature. 1968 Sep 7;219(5158):1016–1021. doi: 10.1038/2191016a0. [DOI] [PubMed] [Google Scholar]
- Richter D., Lipmann F. Formation of a ternary complex between formylatable yeast Met-tRNA, GTP and binding factor T of yeast and of E. coli. Nature. 1970 Sep 19;227(5264):1212–1214. doi: 10.1038/2271212a0. [DOI] [PubMed] [Google Scholar]
- Salas M., Hille M. B., Last J. A., Wahba A. J., Ochoa S. Translation of the genetic message, ii. Effect of initiation factors on the binding of formyl-methionyl-trna to ribosomes. Proc Natl Acad Sci U S A. 1967 Feb;57(2):387–394. doi: 10.1073/pnas.57.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Factor dependent binding of methionyl-tRNAs to reticulocyte ribosomes. Nature. 1970 Aug 29;227(5261):918–920. doi: 10.1038/227918a0. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Isolation and partial characterization of reticulocyte factors M1 and M2. J Biol Chem. 1970 Nov 10;245(21):5553–5559. [PubMed] [Google Scholar]
- Shafritz D. A., Prichard P. M., Gilbert J. M., Anderson W. F. Separation of two factors, M1 and M2, required for poly U dependent polypeptide synthesis by rabbit reticulocyte ribosomes at low magnesium ion concentration. Biochem Biophys Res Commun. 1970 Feb 20;38(4):721–727. doi: 10.1016/0006-291x(70)90641-8. [DOI] [PubMed] [Google Scholar]
- Siler J., Moldave K. Reactions of N-acetylphenylalanyl transfer RNA with rat-liver ribosomes. Biochim Biophys Acta. 1969 Nov 19;195(1):130–137. doi: 10.1016/0005-2787(69)90609-1. [DOI] [PubMed] [Google Scholar]
- Skogerson L., Moldave K. Evidence for the role of aminoacyltransferase II in peptidyl transfer ribonucleic acid translocation. J Biol Chem. 1968 Oct 25;243(20):5361–5367. [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. Cytoplasmic methionine transfer RNAs from eukaryotes. Nature. 1970 May 16;226(5246):607–610. doi: 10.1038/226607a0. [DOI] [PubMed] [Google Scholar]
- Stanley W. M., Jr, Salas M., Wahba A. J., Ochoa S. Translation of the genetic message: factors involved in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jul;56(1):290–295. doi: 10.1073/pnas.56.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUT R. R., MONRO R. E. THE PUROMYCIN REACTION AND ITS RELATION TO PROTEIN SYNTHESIS. J Mol Biol. 1964 Oct;10:63–72. doi: 10.1016/s0022-2836(64)80028-0. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Sekiya T., Ukita T. Selective utilization of nonformylatable species of methionyl-tRNA's from Escherichia coli and yeast in a reticulocyte cell-free system. Biochim Biophys Acta. 1970 Feb 18;199(2):559–561. doi: 10.1016/0005-2787(70)90108-5. [DOI] [PubMed] [Google Scholar]
- Tarragó A., Monasterio O., Allende J. E. Initiator-like properties of a methionyl-tRNA from wheat embryos. Biochem Biophys Res Commun. 1970 Nov 9;41(3):765–773. doi: 10.1016/0006-291x(70)90079-3. [DOI] [PubMed] [Google Scholar]
- Thach R. E., Hershey J. W., Kolakofsky D., Dewey K. F., Remold-O'Donnell E. Purification and properties of initiation factors F1 and F2. Cold Spring Harb Symp Quant Biol. 1969;34:277–284. doi: 10.1101/sqb.1969.034.01.033. [DOI] [PubMed] [Google Scholar]
- Wigle D. T., Dixon G. H. Transient incorporation of methionine at the N-terminus of protamine newly synthesized in trout testis cells. Nature. 1970 Aug 15;227(5259):676–680. doi: 10.1038/227676a0. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Dintzis H. M. Protein chain initiation in rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1282–1289. doi: 10.1073/pnas.66.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Watanabe S., Morris J. Initiation of rabbit hemoglobin synthesis: methionine and formylmethionine at the N-terminal. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1600–1607. doi: 10.1073/pnas.67.3.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]



