Abstract
Environmental challenges are part of daily life for any individual. In fact, stress appears to be increasingly present in our modern, and demanding, industrialized society. Virtually every aspect of our body and brain can be influenced by stress and although its effects are partly mediated by powerful corticosteroid hormones that target the nervous system, relatively little is known about when, and how, the effects of stress shift from being beneficial and protective to becoming deleterious. Decades of stress research have provided valuable insights into whether stress can directly induce dysfunction and/or pathological alterations, which elements of stress exposure are responsible, and which structural substrates are involved. Using a broad definition of pathology, we here review the “neuropathology of stress” and focus on structural consequences of stress exposure for different regions of the rodent, primate and human brain. We discuss cytoarchitectural, neuropathological and structural plasticity measures as well as more recent neuroimaging techniques that allow direct monitoring of the spatiotemporal effects of stress and the role of different CNS structures in the regulation of the hypothalamic–pituitary–adrenal axis in human brain. We focus on the hypothalamus, hippocampus, amygdala, nucleus accumbens, prefrontal and orbitofrontal cortex, key brain regions that not only modulate emotions and cognition but also the response to stress itself, and discuss disorders like depression, post-traumatic stress disorder, Cushing syndrome and dementia.
Keywords: HPA axis, Hippocampus, Prefrontal cortex, Amygdala, Mood disorder, PTSD
Stress and the brain
The concept of stress
Whenever an endogenous or exogenous challenge is perceived as unpleasant, aversive or threatening, a series of systems and processes is activated that generates a coordinated response to that particular challenge, or stressor. This so-called stress response, an integral part of any adaptive biological system, is conserved throughout evolution. It is particularly active when an individual’s homeostasis, well-being, overall health or survival is threatened. An unpleasant surprise, relational or financial problems, the loss of a loved one, bereavement, unpredictability, an acute threat, e.g., when faced with an animal predator, or with psychosocial demand in humans, can all initiate a stress response. The same is true for perturbations of a more biological nature, such as an energy crisis, physical injury, hemorrhage or inflammation.
According to the popular press, stress is ever present in our modern, performance oriented and demanding society [109]. Stress contributes to several disabilities worldwide and as such represents a severe economical burden. The WHO expects that mental disease, including stress-related disorders, will be the second leading cause of disabilities by 2020. In the US, job stress alone, e.g., has been estimated to cost several hundred billions of USD every year in absenteeism, turnover, diminished productivity and medical, legal and insurance costs.
Stress, however, is no single entity and several different types of stressors can be distinguished: stressful challenges can be acute (being confronted with a predator or giving an important oral presentation) or of a chronic nature (living in poverty or in a broken family). It may occur only once, or may rather take place in a repetitive manner, that can be anticipated. Conversely, stress can be unpredictable and uncontrollable, mild or severe, and occurring in or out of context, e.g., of a learning experience [93, 102]. In addition, how stress exposure is actually perceived by an individual varies greatly, as does the persistence of its consequences.
Importantly, physiological ‘stress’ responses also occur following rewarding, “positive” and appetitive stimuli (e.g., winning a competition, sexual activity). Although they are often not considered to be stressors in classic, generally “negative”, terms, the physiological responses elicited by them can be as large as those seen after more aversive stimuli [104]. For the purpose of definition, a stressor in the context of this review will refer to any environmental demand that exceeds the physiological regulatory capacity of an organism, in particular during situations of unpredictability and uncontrollability. They are characterized by the absence of an anticipatory response (unpredictable), or a reduced recovery (uncontrollable) of the neuroendocrine reactions to stress. Taken together, depending on the type of stressor, various signals converge to orchestrate an integrated stress response that ‘resets’ many peripheral and central processes and allow an individual to adapt to the changes in its environment and thus to restore homeostasis.
The physiological stress response can be divided into two different time domains with a very quick response and a delayed response. The first phase of the stress response is considered the “alarm reaction” or the “fight-fright-or-flight” response, which involves the rapid activation of the autonomic nervous system (ANS) that causes the release of epinephrine and norepinephrine from the adrenal medulla. These hormones quickly elevate basal metabolic rate, blood pressure and respiration, and increase blood flow to the more vital organs that are essential for the “fight-or-flight” response, such as the heart and skeletal muscles. At a later stage, the hypothalamic–pituitary–adrenal (HPA) axis is activated as well. In this classic neuroendocrine circuit, limbic and hypothalamic brain structures coordinate emotional, cognitive, neuroendocrine and autonomic inputs, which together determine the magnitude and specificity of an individual’s behavioral, neural and hormonal responses to stress.
This second response is mediated by glucocorticoid (GC) hormones (corticosterone in rodents and cortisol in humans) which generally act in a slow, genomic manner as transcriptional regulators of glucocorticoid responsive genes. Fast (non-genomic) GC actions have also been described and their actions are mediated by putative membrane-bound receptors. It should be emphasized that other signalling pathways act in concert with the HPA axis like the gonadal axis, the adipose axis, and the immune system. All these help to direct energy resources such that attention can be focused on the most urgent and important elements of the challenge while other, less urgent functions, e.g., food intake, digestion or reproduction, are temporarily suppressed [93]. The profound change in activational patterns that is induced by perceiving a situation as stressful can nowadays be visualized by functional magnetic resonance imaging (fMRI) studies, and as can be seen in Fig. 1, encompasses a variety of limbic system and frontal lobe structures, including hippocampus, amygdala and the anterior cingulate cortex. Throughout the text, we focus on the HPA axis, because this system is most heavily investigated, but it should be emphasized that several other systems contribute to the stress response as well (see “Inflammatory changes and depression”, “Gender differences in depression: relationship with HPA axis activity”, “The glutamatergic and GABAergic systems in depression”, “Glial plasticity”, “Water content, volume changes and altered vasculature”).
Fig. 1.
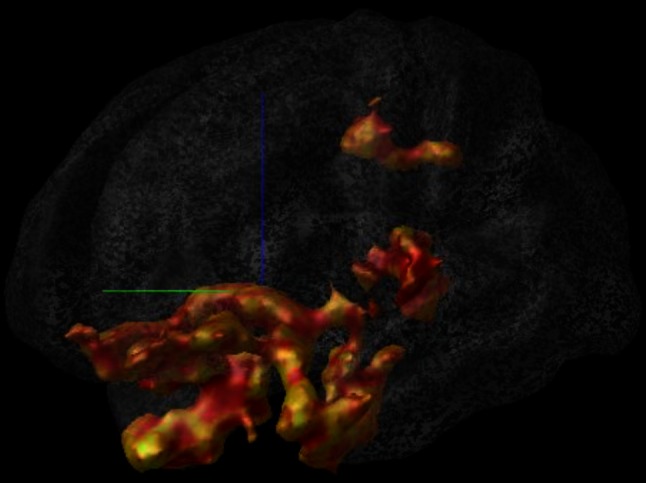
Identification of the brain regions that show activational changes during acute stress. Data are based on averages of 21 healthy subjects who were exposed to the Montreal Imaging Stress Task and showed subsequent cortisol increases, for details see [159]. Activation is prominent in a variety of limbic system and frontal lobe structures, including hippocampus, amygdala and the anterior cingulate cortex
HPA axis, stress hormones and their receptors
HPA axis activation is triggered by corticotropin-releasing hormone (CRH) in the paraventricular nucleus (PVN) that induces adrenocorticotropic hormone (ACTH) release from the pituitary, which in turn releases GCs from the adrenal. Regulation occurs through negative feedback after GC binding to high-affinity mineralocorticoid (MR) and lower affinity glucocorticoid receptors (GR) [47]. HPA axis activity is not only affected by stress, (see “Inflammatory changes and depression”) and a bi-directional communication exists, e.g., between the immune and neuroendocrine stress systems.
The GR helps to maintain GC levels within physiological limits [57, 104]. Aberrant GR expression has been implicated in stress resistance, anxiety and depression [47, 172, 234]. GC plasma levels are under circadian and ultradian control [115, 163]. Together, MR and GR determine sensitivity of the brain to stress [77, 135, 160, 203] and thereby modulate attention, vigilance, behavior and memory formation and eventually adaptation and coping with stress.
Glucocorticoid hormone actions and hippocampal pathology; the ‘glucocorticoid cascade’ hypothesis
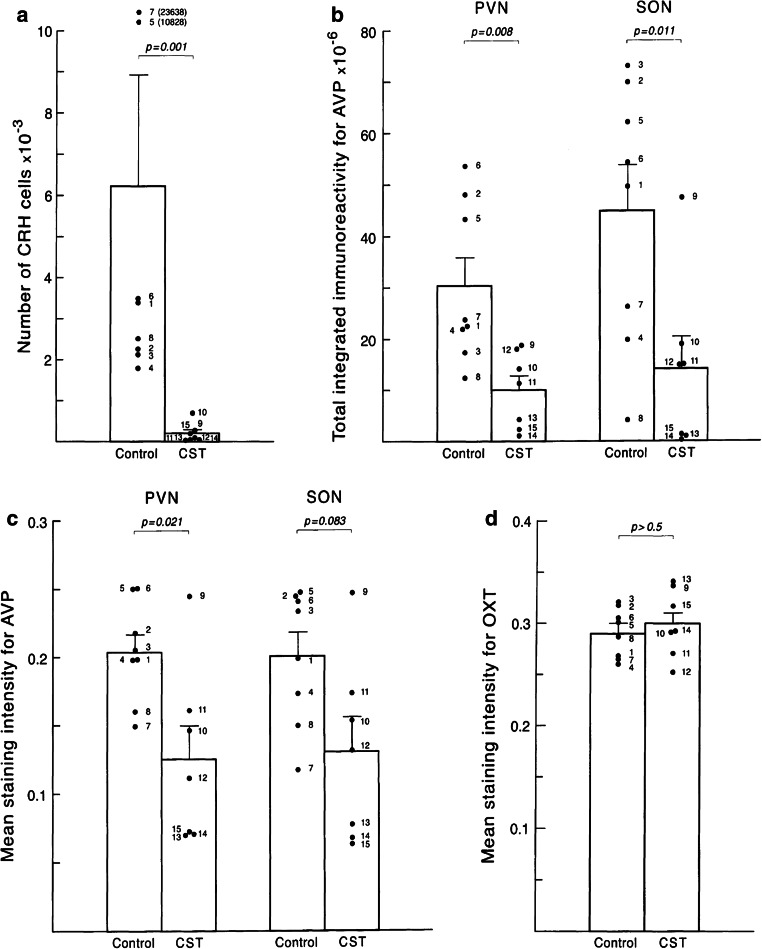
Upon their release in the periphery, GCs enable an individual to engage in an adaptive response to a stressor by affecting energy and lipid metabolism, among others. The large numbers of GRs in the brain, and particularly the hippocampus, make it vulnerable to elevated GC levels [47, 120, 209]. The GR occurs in at least two isoforms (GRalpha and GRbeta), with the GRalpha isoform being the most predominant one in brain. In addition to rodent studies, [171] several human brain regions express GR and MR [77, 162, 189, 233]. In humans, considerable diversity of GR and MR transcripts exists [49, 100], which includes 13 exon 1 mRNA variants and 8 N-terminal variants, that arise from the GRalpha isoform. In the human hypothalamus, GRalpha protein is selectively expressed in CRH-containing parvocellular, but not magnocellular, neurons of the PVN [231]. Application of exogenous, synthetic GCs strongly suppresses CRH and vasopressin production in neurons of the human PVN (Fig. 2) [59], whereas oxytocin (OXT) neurons are not affected.
Fig. 2.
Exogenous or synthetic glucocorticoid treatment affects the human brain and reduces the numbers of CRH-immunoreactive cells in the hypothalamic PVN (a) but has no effect on oxytocin immunoreactivity in the PVN of corticosteroid-exposed subjects (d; CST). Reproduced, with permission, from [59]
In contrast to the relative paucity of GR in the rhesus monkey [175], the human hippocampus shows abundant GR protein expression in CA1 and DG neurons, although the CA3 subregion has lower levels. GR is additionally expressed in astrocytes and overall GR levels remain stable with age. Increases have been found in the hippocampus and amygdala of patients with major depression (MDD) [229, 231, 232]. Thus, even though less is known about the MR [162], the prominent expression of GR in human brain makes these regions important targets for stress exposure [2, 93, 100]. Whereas acute and short-term stress is generally considered beneficial and adaptive, chronic stress may cause an MR/GR imbalance or down-regulation [47, 162] which can alter HPA feedback and result in overexposure of the brain and peripheral tissues to these powerful steroids.
When considering neuropathological aspects of stress, earlier animal experiments had reported brain damage after prolonged periods of stress, especially in aged animals and mainly in the hippocampus. For example, Landfield et al. [107] showed that cumulative GC exposure influenced hippocampal viability and compromised cognition. Subsequently, Sapolsky et al. [178, 182] reported that chronic stress causes a loss of pyramidal neurons in the hippocampus, accompanied by cognitive deficits in rats. Another study described that training rats for 6 months in a two-way shuttle escape task, using low intensity foot shock stress (4 h/days) resulted in endogenous hypercortisolism and CA1 pyramidal neuronal loss in senescent rats [98], while others reported reactive gliosis, reduced dendritic branching and reductions in volume and in CA1/3 cell numbers [181, 183].
The hippocampus was previously thought to inhibit CRH activity directly, and given its high MR and GR density [171, 189, 231], damage to the hippocampus was expected to cause a disinhibition of CRH activity, thus increasing the drive on the HPA axis, which in turn would further stimulate GC levels and aggravate hippocampal damage. This feed-forward “glucocorticoid cascade hypothesis” was proposed to be a pathogenic mechanism underlying stress effects on the brain, and considered relevant for human disorders associated with peripheral HPA axis changes that were paralleled by structural changes in the hippocampus, like Alzheimer’s disease (AD), post-traumatic stress disorder (PTSD), Cushing’s disease and depression [182].
These interpretations were supported by studies on subordinate, wild-born vervet monkeys that had experienced prolonged, severe social stress in captivity and that displayed post-mortem adrenal hypertrophy. Although the coincident hippocampal degeneration in these animals was consistent with the glucocorticoid cascade hypothesis, the morphological alterations and neuron loss in these, and also in other non-human primates implanted with cortisol pellets in their hippocampi [183], were most pronounced in CA neurons [217], brain regions particularly sensitive to pressure artifacts; after death, the soft tissue of the brain can be compressed by its own weight or by manipulation during brain collection, which refers to “post-mortem compression”.
While the initial studies had used rather extreme, physical stressors or extremely high pharmacological GC concentrations [101, 181], later studies in non-human primates and rodents that used more relevant psychosocial stressors [112, 219, 223], added conflicting data insofar that they did not observe massive neuronal loss or obvious neuropathology following chronic stress when this was assessed using unbiased stereological tools [157, 201, 223]. For example, when adult male rats were chronically treated with the selective GR agonist dexamethasone, with dexamethasone plus the selective MR agonist aldosterone, or with supraphysiological doses of corticosterone, only dexamethasone treatment reduced DG granule and CA3 pyramidal cell numbers [202]. However, this synthetic steroid differs from the endogenous corticosterone in terms of brain penetrance and retention and although it can induce cognitive deficits in aged mice [238], it did not influence the CA1 or hilar subfields. In contrast to dexamethasone, animals injected with corticosterone failed to reveal any change in cell number in any of the hippocampal subfields, although volume reductions in hilus and CA3 were observed [201]. Also in tree shrews exposed to chronic subordination stress, the CA1 and CA3 pyramidal neuron numbers were not different from controls, despite elevated cortisol levels [223]. Chronic studies in pigs, non-human primates and chickadees yielded similar results while changes in apoptosis were also not demonstrated [112, 157, 219].
It is now known that the tonic inhibitory control on HPA axis activity [82] is exerted through several, often indirect, neural pathways including the bed nucleus of the stria terminalis (BST), the amygdala and the endocannabinoid system [212, 221]. The GC-mediated negative feedback of the HPA axis thus takes place at several levels, including the hypothalamus and pituitary, and not only at the hippocampus [82, 84]. Also, although GCs target hippocampal cells, they can increase vulnerability to, but not directly cause, subsequent insults [33]. Moreover, an absence of massive structural changes after GC exposure does not necessarily imply that no functional, molecular or changes in responsivity [43] are induced. Furthermore, variables like genetic risk factors, early life programming and structural plasticity were not considered at the time. As a consequence, the GC cascade hypothesis has been refined and rephrased over time [31, 119, 134, 154, 160]. Together with the corticosteroid receptor hypothesis of depression [87] and the realization that GCs are on the other hand, essential for, e.g., dentate viability [197], it has sparked the development of drugs selectively targeting specific stress system components [88, 188, 243].
Molecular, cellular and functional changes in stress-related psychiatric disorders
It is difficult to describe the complete and exact neuropathological consequences of stress exposure in the human brain as stress can occur in so many different forms and depends on an individuals coping strategy and stress sensitivity. Also, there is no single unique disease or direct neuropathological hallmark that is always, only and directly, caused by stress exposure per se. Notably, many of the stress-induced changes are plastic and reversible in nature, for this phenomena we use the term “neuroplasticity”, which refers to the general capacity of the brain to adapt functionally or structurally to a change in demands, and we further specify when particular forms of plasticity are discussed.
However, severe or prolonged stress is well known to increase the risk to develop psychopathologies such as PTSD, depression, schizophrenia or anxiety disorders in susceptible individuals and may even trigger psychotic episodes. Clear brain changes have been described in these conditions and our aim is to summarize those. We focus on disorders where the relationship between stress and the disease occurrence is well documented and where obvious structural alterations have been reported, like in depression, PTSD, Alzheimer’s and Cushing’s disease.
Stress-related changes in major depressive disorder (MDD)
Stress is the most common risk factor for the development of mood disorders like MDD that are thought to result from interactions between genetic predispositions and the environment [173]. Especially stressful life events experienced during early childhood or adolescence can significantly increase the risk to develop depression [78]. Indeed, in a large proportion of depressed patients the HPA axis activation and GC feedback resistance is common. This is reflected by the high percentage of dexamethasone non-suppressors in this population as well as hypertrophy of the adrenals and pituitary and increased plasma levels of cortisol, particularly during the through of the circadian rhythm, and increases in CRH and AVP expression in the PVN [209]. Notably, depressed subjects show remarkable heterogeneity in neuroendocrine function and the proportion of depressed individuals demonstrating overt HPA axis abnormalities may range from 35 to 65 %.
Hypothalamic neuropeptide changes in depression
CRH in depression
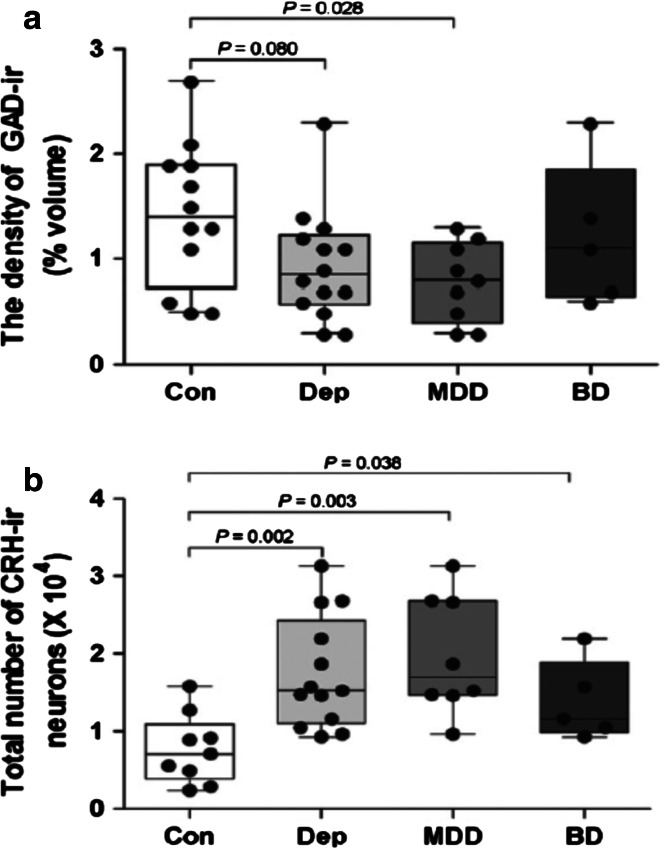
CRH-expressing neurons in the hypothalamic PVN are the central driving force of the HPA axis. The number of CRH expressing neurons, the number of CRH neurons co-expressing AVP, and the amount of CRH-mRNA in the PVN are significantly increased in depressed subjects, independent of whether they died during a depressive state or not (Fig. 3) [166]. Since intracerebroventricular injection of CRH induces symptoms of depression in rodent [87], centrally released CRH may be implicated in depression etiology. During post-natal development of the stress system, CRH controls HPA axis activity and mediates effects of early disturbances like maternal deprivation, through the CRH receptor CRH-R1. Both basic and clinical studies further suggest that disrupting CRH signaling through CRH-R1 ameliorates stress-related clinical conditions. Although CRH in CSF is also derived from other brain areas like the thalamus, CRH concentrations of healthy controls and depressed patients decrease after treatment with antidepressant drugs [83]. Lastly, in depressed patients the significantly increased CRH-mRNA levels in the PVN are accompanied by an increased expression of genes involved in CRH activation, such as CRH-R1, MR, estrogen receptor-alpha (ER-α) and AVPR1a, and with a significantly decreased expression of genes involved in the inhibition of CRH neurons, such as the androgen receptor (AR) mRNA [8, 228]. These findings [228] raise the possibility that a disturbed receptor balance in the PVN contributes to a CRH-mediated HPA axis activation in depression.
Fig. 3.
Quantification of glutamic acid decarboxylase (GAD)65/67-immunoreactivity (GAD-ir) (a), the total number of corticotropin-releasing hormone (CRH-ir) neurons in the hypothalamic paraventricular nucleus (PVN) (b) of depressed patients and controls. Con controls, Dep depression, MDD major depressive disorder, BD bipolar disorder. Significant increases are found in total numbers of CRH-ir in the depressed, major depressed and bipolar groups (b, see also [166]), whereas GAD65/67-ir is significantly reduced in the major depressed group (a). From [65], with permission
Arginine vasopressin (AVP), stress and depression
There are at least four different vasopressinergic systems intimately involved in the signs and symptoms of depression [209]. First, AVP is produced by the magnocellular neurons of the hypothalamic SON and PVN, whose axons run to the neurohypophysis where they release AVP and OXT into the general circulation. Circulating AVP targets the anterior pituitary while high levels also affect mood. Secondly, the parvocellular neurons of the PVN secrete CRH and AVP also as neurohormones from their axons in the median eminence, into the portal capillaries that transport them to the anterior lobe of the pituitary. AVP strongly potentiates ACTH-releasing activity.
Third, vasopressinergic fibers project from the hypothalamus to subregions of the hippocampus, septum, amygdala and brainstem, where AVP serves as a neurotransmitter/neuromodulator via AVPR1a and AVPR1b receptors. Moreover, magnocellular neurons release AVP from their dendrites and somata to act as local neuromodulators on receptors close to their site of release. Fourth, AVP is released into the brain with a circadian rhythm by neurons of the biological clock or SCN, which shows significant changes in depression. Once overexpressed, AVP may contribute to hyper-anxiety and depression-like behaviors, whereas AVP deficits may, in addition to diabetes insipidus, cause signs of hypo-anxiety and disturbed rhythmicity [108].
A misbalance of multiple genes involved in HPA axis regulation may occur in the PVN in depression with a possible role for AVPR1a [228]. Since also early types of stress can epigenetically program the AVP gene in a long-lasting manner [139, 140], the AVP-driven HPA axis hyperactivity in depression is receiving more attention [136].
In the PVN of depressed patients, the number of AVP and OXT protein expressing neurons is increased while for AVP mRNA, a 60 % increase in expression was found in the SON in melancholic but not in non-melancholic depression [136]. Enhanced AVP mRNA production leads to increased plasma levels of AVP [220] that have been related to an enhanced suicide risk in depression, as well as to an anxious-retarded type of depression, psychomotor retardation and memory disturbances in depression.
Hippocampal changes in depressed patients
Maladaptive responses to stress and the associated GC hypersecretion can induce hyperemotional states, mood dysfunction and cognitive impairments in depressed patients. This is often paralleled by volume changes in various brain regions including the hippocampus. In early studies, considerable variation was found in hippocampal volume in depression [124, 160]. Several explanations for this variation have been put forward including the higher spatial resolution in the more recent studies or differences in disease duration [195], anatomical delineation [179], lateralization, early life conditions and genotype [22, 158, 160, 215], the presence of abuse [215] and/or pharmacological treatments [116]. Hippocampal volume reductions in depression are by now one of the best-replicated findings in biological psychiatry [97], but whether it is cause or consequence of the disorder remains unclear. Predictors of lower hippocampal volumes in patients were: a more extensive depressive episode duration and recurrence, the size of their integrated cortisol responses and a history of early life stress [46, 62, 160, 226], while a smaller hippocampal volume could also predispose for the development of psychopathology [180].
Although classic MRI studies generally demonstrated a lower volume of the entire hippocampus in depression, spatial resolution generally precluded in vivo measurement of distinct hippocampal subfields, even though preclinical and some post-mortem studies indicated that chronic stress and depression affect hippocampal subfields, and different structural substrates, to a different extent. In addition to subfields across its transversal axis, the hippocampus also shows topographical segregation along its longitudinal axis [61]. Improved spatial resolution of high field strength MRI has now enabled to identify connectivity changes [245] and detailed measurements of subfield areas [90, 92, 235]. They revealed that the mean volumes of the DG and CA1-3 subregion were smaller in non-medicated or recently unmedicated depressed patients than in healthy controls. Along the longitudinal axis, a smaller volume was mainly found posteriorly, i.e., in the hippocampal body and tail, rather than anteriorly in the hippocampal head [90].
Of interest, both the subfield and posterior hippocampal volume reductions were seen only in unmedicated depression but were absent in patients treated with antidepressants. The posterior hippocampus may thus be particularly susceptible to volume changes [130, 143, 184], the extent of which may even predict a worse treatment outcome [128]. Furthermore, successful long-term antidepressant treatment also seems to increase posterior hippocampal volume [184]. In agreement with preclinical studies of chronic stress, decreases in CA3 volumes were shown [90] that, based on rodent studies, it could be related to different structural substrates, as will be discussed below.
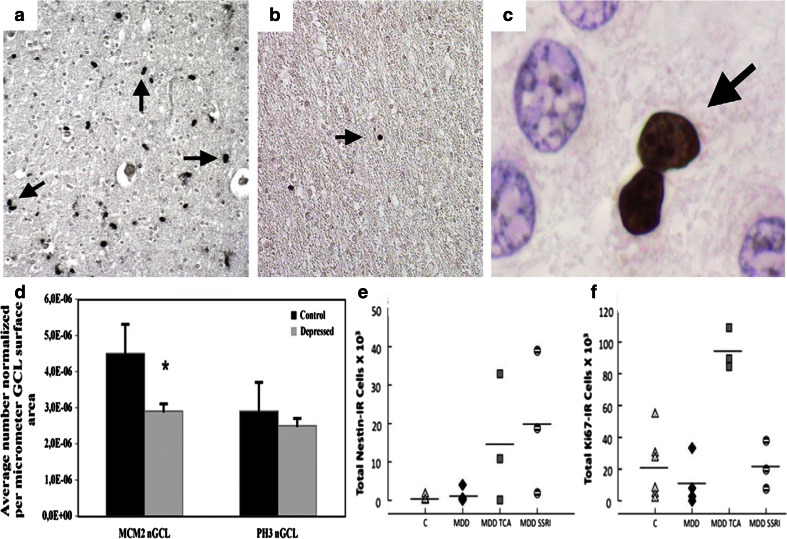
In a recent post-mortem study, hippocampal tissue of 17 pairs of MDD and control subjects, all around 50 years of age, were analyzed stereologically. While hippocampal volume in all MDD subjects was not significantly smaller compared to control subjects, total volume in MDD was decreased with duration of depressive illness. Also, there was no significant difference between MDD and controls in total number or density of the pyramidal neurons and granule cells or glial cells in the CA1, CA2/3, hilus, or DG subregion [29]. However, CA1 pyramidal neuron density increased with duration of illness in MDD and both granule cell and glial cell numbers increased with age in MDD patients on medication which may reflect proliferative effects of antidepressants (see Fig. 4, “Adult hippocampal neurogenesis”). This suggests that reductions in volume parallel to increased cellular densities are best explained by assuming cell shrinkage and hence changes in neuropil rather than cell loss [29, 206]. Also changes in water content may be implicated (see “Water content, volume changes and altered vasculature”).
Fig. 4.
Changes in proliferation in the human brain of depressed and anti-depressant-treated patients. a Cells immunopositive for the cell cycle marker minichromosome maintenance protein 2 (MCM2) that is involved in the control of DNA replication. In the hippocampus, many MCM2-immunopositive cells and doublets (arrows) are observed in cortical tissue of a 2-year-old subject that served as positive control. b MCM2-ir cell numbers are strongly reduced to very low numbers (arrow) in a 69-year-old control subject. c MCM2-ir doublet of 2 still closely attached cells that appear to be about to separate in the hippocampus of a depressed patient (arrow), cresyl violet counterstain. Cells are viewed under a 10× magnification (a, b) or at 63× (c). d Graphs depicting numbers of MCM2 and phosphorylated histone H3 (PH3) immunopositive cells (the latter marker reflecting late G2 and mitotic phase of cell division). PH3 immunoreactive cells in the subgranular zone and granular cell layer of the dentate gyrus, normalized to the surface area of the GCL and expressed per square micrometer. A significant reduction is found for MCM2, but not PH3, in a cohort of 10 elderly (average age of 68 years) depressed patients compared to 10 controls. e Neural progenitor and f dividing cells (Nestin and Ki-67 as respective immunocytochemical markers) are increased in the dentate gyrus of a younger cohort of (average ages of 40 and 54 years) of patients with major depressive disorder (MDD) who were treated with antidepressants compared to untreated MDDs and control subjects. Progenitor numbers (e, Nestin-ir) were higher in MDD patients treated with tryciclics (TCA) or with selective serotonin reuptake inhibitors (SSRI), compared to untreated MDD and Control cases whereas the numbers of dividing cells (f, Ki-67-ir) were higher only in the TCA but not SSRI-treated group (n = 5–7 cases per subgroup). Reproduced, with permission, from [15, 121]. [121] was used for (a–d) and [15] was used for (e, f)
Imaging studies in patients with stress-related affective and emotional disorders have shown that also the volume of structures other than the hippocampus like the PFC, cingulate cortex, hypothalamus and amygdala, as well as their interactions and coherence, is altered by stress [45, 147, 160, 200]. Finally, it should be noted that hippocampal shrinkage is not stress specific and has also been reported in several other brain disorders including schizophrenia, dementia, Parkinson’s and Huntington’s diseases, epilepsy and alcoholism [36].
Stress effects on the amygdala
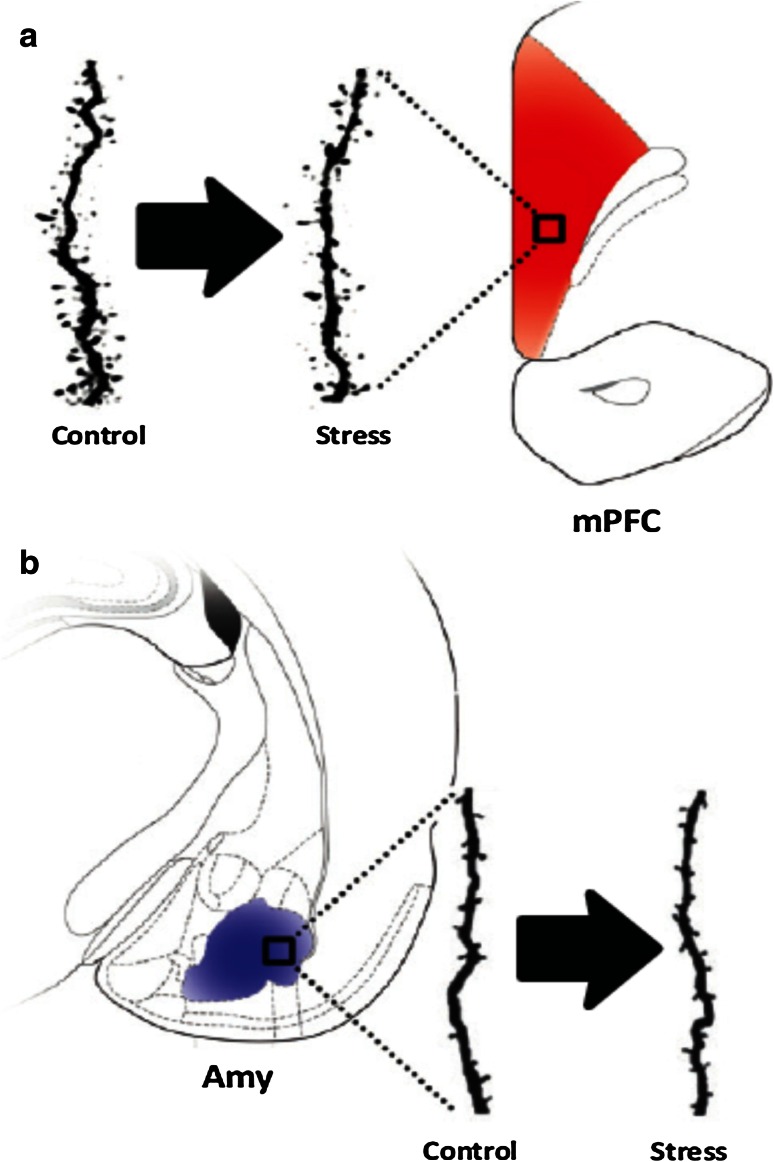
While the hippocampus mediates spatiotemporal aspects of behavioral impairment, the amygdala contributes to the affective and emotional aspects of cognition [110]. Clinical studies have shown increases, decreases or no change in volume of the amygdala in MDD [23] and a meta-analysis even found no changes in amygdala volume in depressed patients although increases in cerebral blood flow to the amygdala, a correlate of neuronal activity were reported [54]. Recent experimental studies have identified cellular and molecular correlates of stress-induced amygdaloid plasticity that may underlie anxiety and depressive-like behavior. Animals exposed to chronic stress exhibited enhanced anxiety in the elevated plus-maze while at the cellular level, a persistent increase in dendritic arborization and higher spine density was found across the primary and secondary branches of the basolateral amygdala (BLA) spiny neurons and even spine formation was induced [137].
This dendritic hypertrophy in the BLA after stress is distinct from the retractions seen in the hippocampal CA3, which is reversible after a stress-free period [225]. In contrast, the BLA does not re-adjust morphologically or functionally after such a recovery period [137, 225]. This adds to accumulating evidence that structural encoding of aversive experiences, through enhanced availability of post-synaptic dendritic surface and synaptic inputs on principal neurons of the BLA, may facilitate symptoms of chronic anxiety and disorders like MDD and PTSD by enhancing synaptic connectivity in the BLA [154].
Such changes resemble findings in humans where larger amygdala volumes have been found, partly related to early stress exposure [125, 160]. The apparently conflicting clinical and basic findings could be due to heterogeneous clinical populations, where depression is comorbid with other psychiatric illness, or medication effects, as some patients were undergoing treatment during the investigations. At the same time, the stress paradigms used in basic research are incomplete models not capable of fully recapitulating the human disease state. Furthermore, most of the basic science findings were gathered from adult rodent stress and did not take into account early stress, which has been implicated in depression vulnerability [122].
The prefrontal cortex (PFC)
The PFC participates in cognitive, socio-emotional and executive functions that are sensitive to stress [4, 26, 40, 50]. Furthermore, the PFC modulates autonomic and neuroendocrine responses to stress [82, 151]. As the human PFC is one of the last regions to complete maturation, it contains neurons with more complex dendritic trees than earlier maturing cortical structures. This prolonged development makes the PFC susceptible to disruption and it indeed is affected in developmental neuropsychiatric disorders like autism and schizophrenia [214]. GRs and MRs are abundantly expressed by neurons and glia in the PFC of rodents and primates [175] and regulated by stress [153]. A recent human study investigated developmental changes in GR expression in the dorsolateral PFC from infancy to adulthood and found dynamic patterns of GR isoform expression across the lifespan, suggesting that the neonatal and late adolescent periods represent vulnerability windows to stress during human cortical development [191]. Furthermore, abnormal GR isoform expression levels have been found in the PFC of patients with psychiatric disorders like schizophrenia, bipolar and major depressive disorders [162, 190, 192]. Transcript level of MR was significantly decreased, while the ratio of GRα to MR mRNA was increased in the anterior cingulate cortex (ACC) and the dorsolateral PFC (DL–PFC) of depressed patients. Thus, a selective disturbance of MR and of GRα/MR ratio may exist in the ACC/DL–PFC in depression that is inversely correlated to the corresponding levels in the PVN and may thereby contribute to HPA axis hyperactivity and depression etiology [162].
A large body of evidence further demonstrates that repeated stressful experiences have a profound impact on neuronal plasticity in the PFC. The most thoroughly investigated neuromorphological change is the regression of the geometrical length of apical dendrites of pyramidal neurons in layers II–III of the mPFC [26, 40, 164]. The main results from these studies are a significant reduction in total dendritic length of 20–35 % with a significant decrease in branching and spine density of the distal apical dendrites. These chronic stress-induced effects are likely to be mediated partially by the activation of GRs and NMDA receptors [156] because artificially elevated levels of GCs result in morphological changes similar to those seen following chronic stress exposure [26, 40, 164], while blocking NMDA receptors could prevent these stress-induced effects [132]. It also appears that these changes are plastic and not degenerative in nature, because they reverse spontaneously after a recovery period [71, 165], at least when animals are young, as middle-aged and aged rats failed to show this reversible dendritic remodeling [13].
Notably, most rodent studies [26, 40, 164] report a significant impact of stress on the apical but not basal dendrites. However, the only comparable human study that examined dendritic branching of pyramidal neurons in the ACC of depressed suicides found significantly reduced numbers of third-order branches in the basilar dendritic arbor [81]. These data are the first evidence of altered cortical dendritic branching in a psychiatric disorder. As the proximal dendritic branches grow during perinatal development, and are generally less plastic at maturity than the more distal segments, this led the authors to speculate that differences in dendritic branching may reflect a biological predisposition to depression and suicide [81], or could result from perinatal stress exposure.
Notably, not only the glutamatergic pyramidal cells are affected by chronic stress, but also GABAergic interneurons undergo dendritic reorganization in the mPFC and dendritic hypertrophy was found in a subpopulation of interneurons identified as Martinotti cells [68]. Chronic adverse experiences further decrease GAD67 expression levels and the number of GAD67 expressing neurons [68, 185]. GAD67 is the 67 kDa isoform of glutamate decarboxylase enzyme which synthetizes GABA and is a marker for GABAergic neurons. Chronic stress also decreased the number of parvalbumin-positive interneurons in the PFC [185].
Not only PFC neurons are affected by chronic stress, but glial cells as well. Stress, e.g., inhibits gliogenesis in the PFC [39], results in impaired activity of microglia [86] and in a profound shortening of astrocytic branching and process length, as well as reduced GFAP expression [216]. These findings are in line with the astrocytic pathology in the PFC of depressed patients, that includes reduced astroglial cell numbers and related markers [167]. Finally, many of the cellular changes after stress can be hemisphere specific, and stress often abolishes or even reverses asymmetries at the cellular level [39, 40].
Studies on the mPFC have been critical in showing that the impact of stress on one brain region can spread to other areas that are synaptically linked [200] involving, e.g., the corticostriatal network and indeed stress shifts decision-making to more habitual processing [50]. Thus, chronic stress-induced morphological and functional changes take place in the PFC and can result in various executive, cognitive and affective dysfunctions (Figs. 5, 6) (e.g., [18, 76, 114, 198]).
Fig. 5.
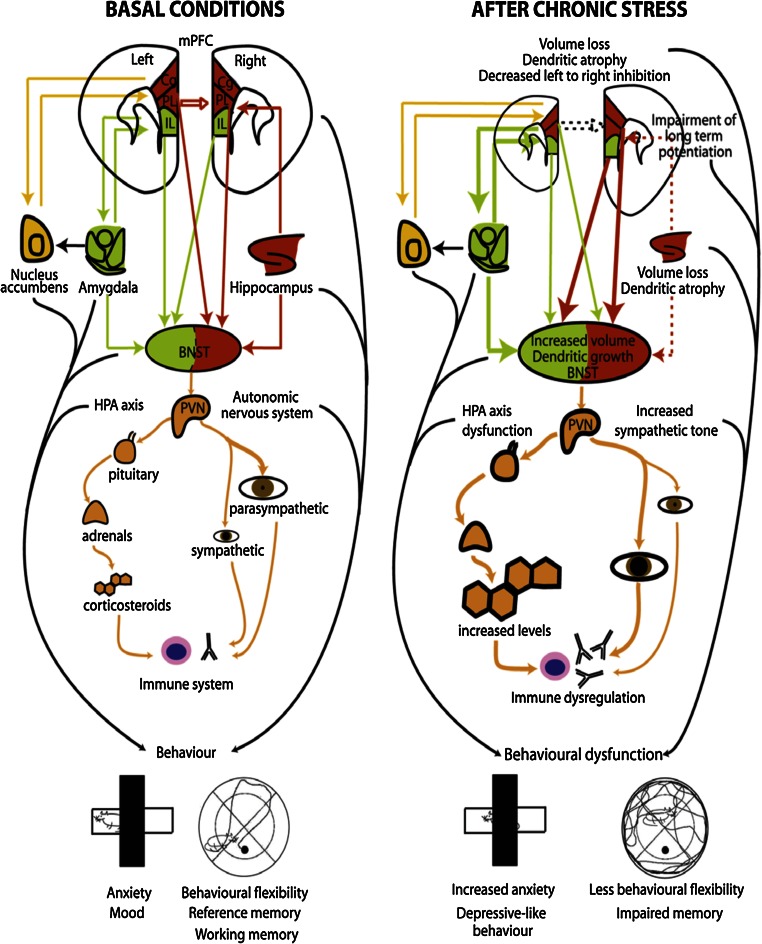
Schematic representation of the connections between the prefrontal cortex, the stress response and the immune system and the changed interactions during conditions of chronic stress. At basal conditions (left panel), the right medial prefrontal cortex (mPFC) is mainly under tonic inhibition from its left counterpart. Modulatory inputs from the mPFC, amygdala and hippocampus to the PVN relay on the bed nucleus of the stria terminalis (BNST). Furthermore, whereas activation of the infralimbic cortex (IL) and amygdala increases PVN activity, activation of the cingulate (Cg) and prelimbic (PL) parts of the PFC and from the hippocampus decreases it. In basal conditions the parasympathetic tone of the autonomic nervous system predominates. After chronic stress (right panel), which elevates glucocorticoid levels, changes are induced in the brain that include a decreased volume and dendritic retraction in the mPFC and hippocampus, but opposite changes in the BNST and amygdala (see Fig. 4). Damage to the hippocampus may decrease the influence of this brain structure on the mPFC and BNST (dotted lines); as a result, a reduced activity of the mPFC (especially in the left hemisphere) may occur, but an overactivation of the amygdala and over the neuroendocrine and autonomic control centers (BNST/hypothalamus). This may trigger HPA axis dysfunction, increase corticosteroid levels and activate the sympathetic nervous system, which, together, may induce immune dysregulation and contribute to behavioral dysfunction. Reproduced from [26], with permission
Fig. 6.
Scheme representing the contrasting effects of chronic stress on dendritic spine numbers in the prefrontal cortex (PFC) and amygdala. a A decrease in the number of spines occurs in pyramidal neurons of the infralimbic cortex of rats after repeated restraint stress (21 days). b By contrast, chronic immobilization stress (10 days) triggers an increase in the number of spines in basolateral amygdala spiny neurons in rats. Amy amygdala, mPFC medial prefrontal cortex. Reproduced, with permission, from [26]
Effects of chronic stress on structure and function of the PFC are supported by human imaging studies. For example, significant reductions in the gray matter of DL–PFC and ACC were found in subjects with long-term occupational stress by MRI-based voxel-based morphometry [12]. Subjects reporting more uncontrollable and overwhelming stressors displayed blunted neural responses in mPFC following feedback processing as established by fMRI. In another fMRI study, patients with MDD and a history of childhood maltreatment showed reduced functional connectivity strength within the prefrontal-limbic-thalamic-cerebellar circuitry and these reductions were significantly correlated with measures of childhood neglect [230]. Maltreated children had volume reductions in the medial orbitofrontal cortex and middle temporal gyrus [44]. Similarly, early life stress in depressed patients decreased local connectivity of the ventrolateral PFC and correlated negatively with global connectivity for the DL–PFC [28].
In summary, there is a clear effect of early life stress or stress in adulthood on reductions in volume and alteration in functional connectivity of the PFC and related cortical limbic regions. Interestingly, these effects are present not only in depression but also in healthy controls who were exposed either to occupational or early life stress.
Inflammatory changes and depression
Although inflammatory changes alone may not typically initiate pathology, emerging evidence indicates that sustained inflammation can affect disease progression of various brain disorders, and is implicated in depression etiology [42, 218]. When activated, cells of the immune system produce pro-inflammatory cytokines that can induce behavioral withdrawal and activate the HPA axis [11]. Conversely, stress and/or elevated GC levels themselves are generally immunosuppressive and prevent the immune system from overshooting. Psychological stress is known to stimulate pro-inflammatory cytokine production in patients experiencing stress and anxiety, and elevations in interleukin-1β (IL-1β) and 6 (IL-6) and increases in macrophage activity have been reported in depression [42, 222]. Stress can provoke depression-like behaviors, mediated through the activation of inflammatory and anti-neurogenic mechanisms and pathways, some of which are even normalized by antidepressants [105]. Inflammation can further affect hippocampal function and cognition [138, 240, 246] and reducing it by anti-inflammatory drugs restored functional and structural plasticity in animal models [56, 138].
Stress-related changes in PTSD
Post-traumatic stress disorder (PTSD) is a multisystem disorder with multiple comorbidities. PTSD patients have experienced severe, often life-threatening, episodes of stress that cause flashbacks, nightmares and sleep problems, emotional numbness or emotional outbursts, anhedonia, inappropriate startle reflexes and problems with memory and concentration [21]. Notably, the HPA changes in PTSD indicate low cortisol levels consistent with increased glucocorticoid sensitivity. In one particularly intriguing experimental protocol, 40 pairs of identical twins were studied, one of whom participated in the Vietnam war while the other stayed at home. Of those who experienced combat, 43 % developed PTSD. They turned out to have smaller hippocampi, but their stay-at-home twin brother did too. In contrast, those who did not develop PTSD had larger hippocampi, and so did their stay-at-home twin as well. A small hippocampus thus seems to be present already prior to the stressful experience and in fact to infer an increased vulnerability to PTSD [69, 180]. This goes back to the idea of either genetic factors, or life history and shaping events during critical development periods, or an interaction between the two, determining hippocampal volume early in life, and putting the individual on a trajectory for (psycho)pathology [30, 124, 161].
Thus, it remains elusive whether hypercortisolism is indeed responsible for hippocampal shrinkage, since combat-related PTSD is associated with decreased HPA axis activity and steroid feedback super-sensitivity that often lasts for decades after the initial trauma. It has been presumed that early on in the process the HPA axis may have been strongly activated. This is based on the observation that soldiers who had undergone random bombardments in the Korean war displayed markedly increased levels of cortisol, with the highest levels of cortisol in soldiers who had been in the greatest danger. It has therefore been hypothesized that high levels of cortisol at the time of the stressor would result in damage to the hippocampal neurons that may persist for many years after the original trauma and that could lead to reductions in hippocampal volume and subsequent differences in feedback or stress responsivity. However, those victims of rape or motor vehicle accidents who later developed PTSD appeared to have—already a few hours after the traumatic event—lower cortisol levels than victims who had no subsequent psychiatric disorder, or those who developed major depression. Yet, pituitary and adrenal hyperactivity to exogenous CRH and ACTH has been demonstrated in these patients. An increased sensitivity or up-regulation of GRs in PTSD and a pre-existing smaller hippocampal volume thus seems, at present, the best explanation [180, 239].
Stress-related effects: relation to sex, aging, Alzheimer’s disease and Cushing’s disease
Gender differences in depression: relationship with HPA axis activity
Gender differences in stress regulation have important implications for understanding the physiological differences in the male and female brain and their impact on vulnerability to stress disorders. Women are generally better at expressing emotions, tend to score higher on emotional ratings on, e.g., neuroticism [73] and have an increased risk of suffering from mood disorders. Morphometrical studies have shown sexual dimorphisms in several brain structures implicated in emotional processing, including the cingulate and ventrolateral prefrontal cortices (larger in women) and the medial temporal structures, including the amygdala and BST (larger in men) that are implicated in emotional processing [70]. These structural differences are thought to be programmed by sex steroids during early development [70].
The possible importance of fluctuating levels of sex hormones as a risk factor for depression is underlined by the higher prevalence of premenstrual depression, antepartum or post-partum depression, and depression during the transition to menopause. Studies in rodents have further shown intrinsic differences in the way female and male brains respond to stress [113]. Human post-mortem brain, animal and cell-line studies confirm a key stimulating role of estrogens on CRH production, while androgens diminish CRH production [152]. These opposite effects on CRH neurons may underlie sex difference in the prevalence of depression.
Stress-related changes in Alzheimer’s disease (AD)
Stressful life experiences are implicated in sporadic forms of AD. A considerable portion of AD patients hypersecrete glucocorticoids or are non-suppressors of plasma cortisol following dexamethasone administration [145, 169, 208, 209]. Their GC levels generally correlate with their rates of cognitive impairment and the extent of neuronal remodeling [48, 89, 123]. The cognitive deficits in AD correlate primarily with hyperphosphorylated forms of the cytoskeletal protein tau, which, together with amyloid β (Aβ), has a pathogenic role in AD. Increased GC levels may not only induce hippocampal damage but can also potentiate Aβ toxicity [25, 91]. Conversely, dehydro-epiandrosterone (DHEA) and its sulphate (DHEAS) or GR blockade may exert a neuroprotective action [5, 141].
Some aging animals and mouse models of AD show changes in stress regulation too but also, in wild-type, middle-aged rats, chronic stress and GCs induce abnormal hyperphosphorylation of Tau in the hippocampus and PFC, with parallel impairments of hippocampus- and PFC-dependent behaviors. Exogenous GC application further potentiates the ability of centrally infused Aβ to induce hyperphosphorylation of Tau epitopes. Moreover, previous exposure to stress further aggravated the biochemical and behavioral effects of GCs in Aβ-infused animals [25, 193]. Thus, stress and GC exposure may have a cumulative impact on the onset and progression of AD pathology. Tau hyperphosphorylation may be instrumental in the negative effects of stress and GC on cognition, initially by increasing the production of pathogenic products of amyloid precursor protein (APP), followed by up-regulation of Tau kinases such as GSK3β and cdk5 and aberrantly hyperphosphorylated Tau [199].
Stress-related changes in Cushing’s syndrome
Cushing’s syndrome can occur after prolonged exposure to (tumor-derived) cortisol or synthetic steroids and usually induces some brain atrophy and cognitive dysfunction. Positive correlations exist between hippocampal volume and memory tests and negative ones with plasma cortisol levels. As at least partial recovery of brain shrinkage occurs after reversal of high GC exposure [19, 201] making massive, irreversible, cell loss unlikely [53, 75, 85, 242].
Effects of stress on structural and molecular plasticity
Traditionally, affective and stress-related brain disorders were explained by neurochemical (mainly monoaminergic) imbalances. More recent studies indicate that impairments in structural plasticity and volumetric changes of specific limbic areas also contribute to their pathophysiology. Various candidate cellular substrates, like dendritic retraction, neuronal loss or glial changes have been proposed that are indeed all stress-sensitive [36, 127, 167]. Reciprocal relationships may exist between stress-related behaviors and changes in structural plasticity. Overall, it still remains unclear whether the above substrates should be classified as truly pathological or whether they may represent plastic and/or dynamic adaptations to a stressor that can to some extent be reversible.
Dendritic remodeling
One of the best known forms of structural plasticity is dendritic retraction that was first observed in CA3 and CA1 hippocampal neurons following chronic stress exposure. At the structural level, prolonged exposure to high doses of corticosterone reduces apical (but not basal) dendritic complexity of CA3 pyramidal neurons [237] and a prolonged exposure to various types of chronic stress results in similar changes in different species [64, 129]. These changes in hippocampal dendritic morphology generally need several weeks to develop. In addition, chronic stress also leads to a loss of mossy fiber synapses, increased surface area of the post-synaptic density, and rearrangements of synaptic mitochondria and vesicles at the presynaptic terminals [176, 213].
Spine density
The effects of stress on spine density are less clear. While increase in the number of spines were reported on CA3 pyramidal dendrites and in the size of the post-synaptic densities on CA1 synapses, others could not find changes in spine density [129] or reported a decrease in spines, that was notably rapidly reversible already after a recovery period or subsequent training [1, 176, 205]. Oscillating GC plasma concentrations along the circadian rhythm appear to be important for the elimination of spines present before motor learning, and also for the maintenance of new spines along with the retention of motor memory [115]. Spine elimination, but not their formation, was shown to require MR activation [115]. Together, these studies suggest that prolonged exposure to chronic stress and glucocorticoids markedly alters the number and morphology of both pre- and post-synaptic structural elements and thus strength of excitatory synapses in the hippocampus.
Dendritic spines are of particular relevance as they are critically involved in the storage of information and their density can be increased by stress under specific conditions [212]. Pyramidal cells of the hippocampus and PFC respond with reduced dendritic complexity and spine loss to chronic stress [71, 164], both of which are reversible following a recovery period [71, 165], but other regions appear resistant [194]. Consistent with circuit-specific effects of stress (Fig. 5), also increases were found in the dendritic arborization of orbital frontal cortex neurons, an effect opposite to what is observed in other cortical neuron populations. Interestingly, in the basolateral amygdala and nucleus accumbens (NAc), stress generally results in hypertrophy of dendritic arborization and increases in spine density (Fig. 6). In the amygdala, these changes do not normalize following a recovery period after stress [137, 225]. In addition to changes in spine density, chronic unpredictable stress reduces density of synapses in the rat PFC [94]. Similar reductions in synaptic density and a lower expression of synaptic function-related genes occur in the DL–PFC of MDD subjects [94].
Apoptosis, neuronal death and neuropil loss
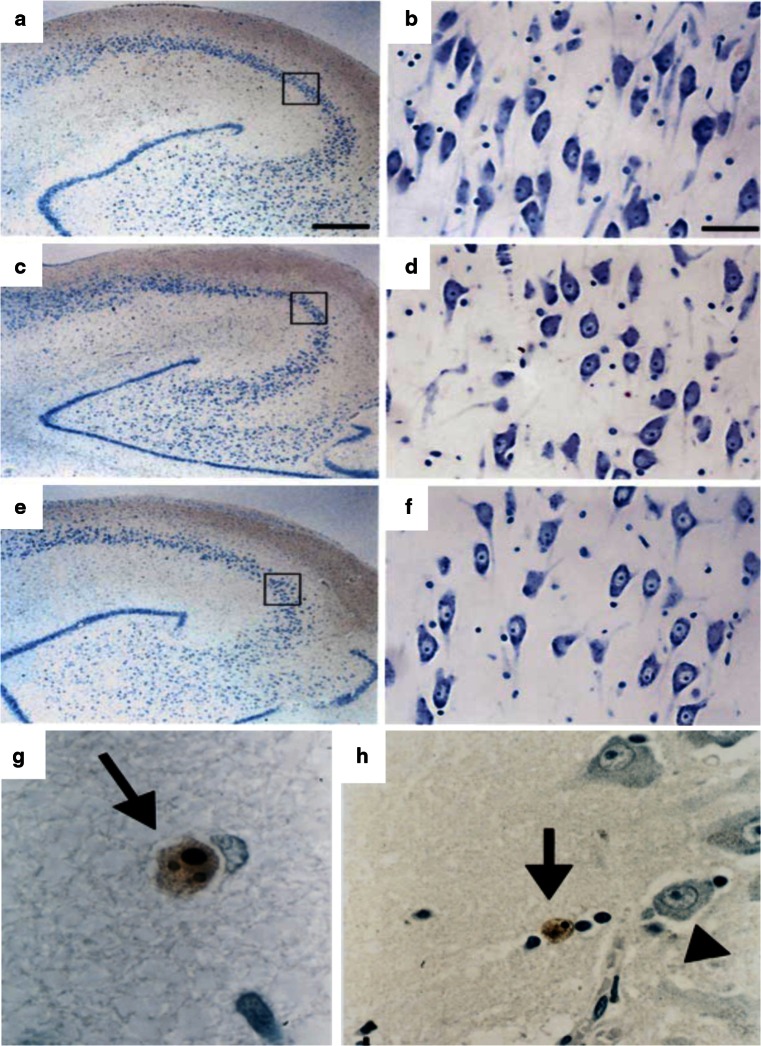
According to the glucocorticoid cascade hypothesis, neuronal apoptosis may underlie hippocampal volume shrinkage observed after stress [182]. Neuronal death has also been implicated in the cerebral shrinkage that occurs following prednisone administration and/or in the inflammatory changes in the cortex in depression [119, 134, 196, 209]. However, initial histological and neuropathological examination of the hippocampus of depression models or from patients that had been depressed or were exposed to synthetic corticosteroids, could not support this notion. In hippocampi from established depressed patients, no indications for obvious neuronal loss or for significant neuropathology could be found using a variety of relevant architectural, synaptic and glia markers [29, 117, 119, 142] (see Figs. 7, 8). In a very recent study, Boldrini et al. [17] reported fewer granule cells in the dentate gyrus of unmedicated depressed patients (without fewer neuronal progenitor cells) suggesting that cell maturation or turnover defects in this plastic hippocampal subregion might be related to the duration of the illness. Notably, the hippocampal volume reductions present in Cushing’s disease were reversed after cessation of steroid exposure [204]. Similar findings exist on ventricular enlargements that are reversible in certain conditions (e.g., following recovery from alcoholism or prolonged steroid use), thereby challenging the hypothesis that ventricular enlargement predicts neuronal loss [210]. This agrees with the general clinical experience with depressive or Cushing’s patients, in which treatment or operation can relieve their depressive symptoms, several of the HPA alterations, and even the hippocampal shrinkage, findings that would be hard to interpret had irreversible damage or massive cell loss been induced.
Fig. 7.
Photomicrographs of Nissl stained sections from the hippocampus of a depressed patient (a, b), a steroid-treated patient (c, d) and a control subject (e, f). b, d, f Shows the CA3 area of the same patients at higher magnification. Although some rare apoptotic cells (brown TUNEL-positive cells indicated by arrows in g and h, compared to intact, non stained neuronal nuclei nearby (arrowhead)) were seen outside of subregions predicted to be at risk for glucocorticoid overexposure like the dentate gyrus or entorhinal cortex, no morphological evidence for neuronal damage or massive cell loss was observed in any of the groups. Bar indicates 710 μm in (a) and 45 μm in (b). Reproduced, with permission, from [117, 142]
Fig. 8.
Immunohistochemical staining for synaptophysin (a, c, e) and for the neuronal growth-related phosphoprotein B-50 (b, d, f) in the hippocampus of a, b a depressed patient, c, d a steroid-treated patient and e, f a control subject. No marked qualitative difference is observed between the overall immunohistochemical staining patterns of the groups in the hippocampal subarea CA3 and the molecular layer of the dentate gyrus. Bar in e represents 50 μm, bar in f 115 μm. Reproduced, with permission, from [117, 142]
The incidence of apoptosis or cell loss in rodent stress models is rare and also seen after acute stress [79, 119, 241]. Chronic stress changed apoptosis in tree shrews only in specific hippocampal subareas and was normalized by tianeptine treatment [118]. These effects also occurred in associated cortical areas and were consistent with a general anti-apoptotic mode of antidepressant action [118, 134]. This bears considerable relevance for the interpretation of structural and/or neuropathological studies on the hippocampus in depression, where almost all patients generally receive antidepressant treatment [90, 117] and hence, effects of the disorder per se might be masked by the concomitant drug treatment. Yet, in drug-free, depressed patients [17], little differences were reported when compared to patients on treatment [17], and hippocampal volume reductions were then present as well.
Adult hippocampal neurogenesis
Another, relatively novel, form of neuroplasticity is neurogenesis that has been implicated in stress-induced hippocampal volume changes. Adult neurogenesis refers to the production of new neurons, an event that continues to occur in the adult brain of several mammalian species, including humans (Figs. 4, 9) [58, 120]. Neuron formation in the adult hippocampus received considerable attention during recent years and was proposed to contribute to depression etiology [96]. Although later studies suggested that neurogenesis was rather implicated in antidepressant drug actions [177], we still lack a coherent functional theory explaining how exactly newborn neurons in the hippocampus can contribute to mood, or to specific symptoms of depression, besides their cognitive deficits, which are related too, but not specific to mood disorders. Although a reduced rate of neurogenesis may reflect impaired hippocampal plasticity, reductions in adult neurogenesis alone are unlikely to produce depression. Lasting reductions in the turnover rate of DG granule cells, however, will alter the average age and overall composition of the DG cell population and thereby influence properties and vulnerability of the hippocampal circuit.
Fig. 9.
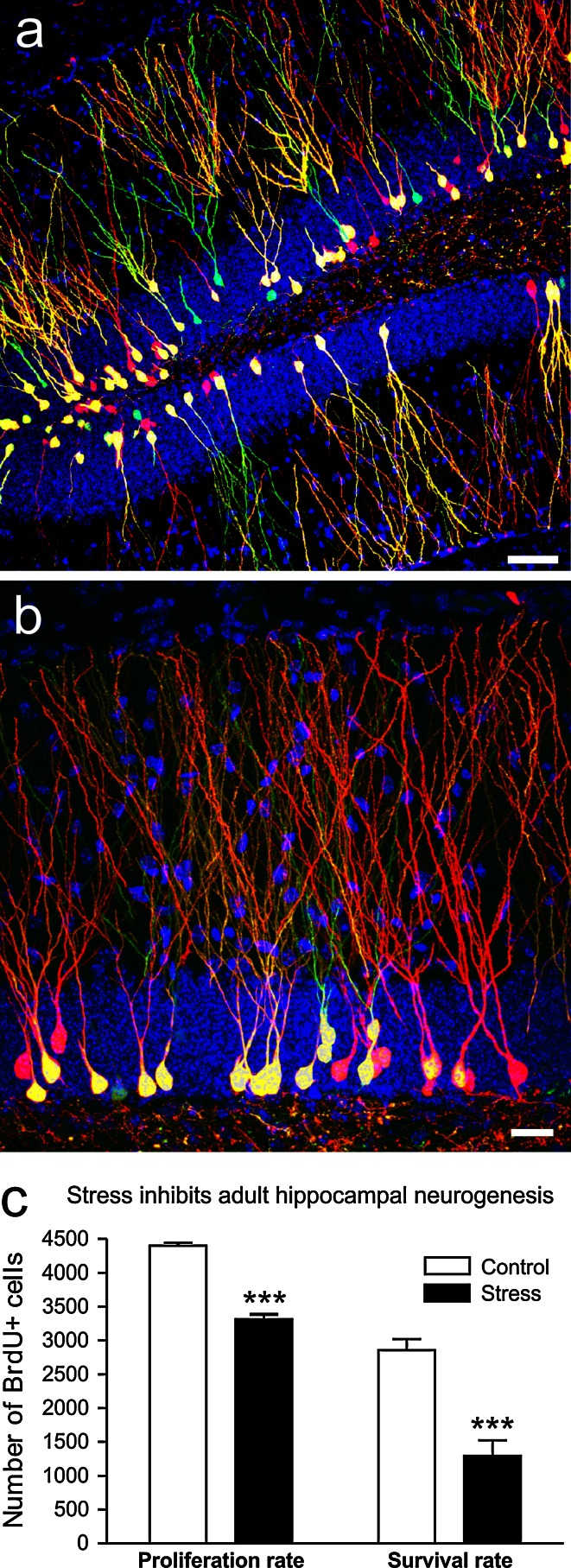
Chronic stress inhibits neurogenesis in the adult hippocampal dentate gyrus. Representative confocal images of newborn neurons in the hippocampus of adult mice with low (a) and high magnification (b). A mixture of retroviruses expressing green and red fluorescent protein (CAG-IRES-GFP and CAG-IRES-RFP) was injected into the dentate gyrus of adult mice to label the newly born cells. Double-transduced cells are in yellow and DAPI is in blue. B. Czéh, D. Refojo, and D.C. Lie unpublished observations. Scale bars a 50 μm, b 20 μm. c Chronic stress inhibits both the proliferation rate and the survival rate of the newly generated cells in the hippocampal dentate gyrus of adult rats as it was shown with BrdU-labeling in a chronic social defeat stress model (modified fom [39]). Data are mean ± SEM, group sizes n = 6 rats/group, ***p < 0.001. Similar changes are seen in depressed individuals [16, 121] and treatment with some, but not all, antidepressants can normalize these changes [16, 37, 120]
Furthermore, neurogenesis is regulated by exercise, inflammation and antidepressants (Fig. 4) [37, 120, 146, 177, 247] while stress potently inhibits neurogenesis in several species (Fig. 9) [120, 187]. Both psychosocial [74] and physical stressors [224] inhibit at least one or more phases of the neurogenesis process [74, 79]. Stress and GCs further interfere with most stages of neuronal renewal, proliferation, maturation and survival (Fig. 9) [79, 148, 187, 236].
The neurogenic hypothesis of depression proposes that prolonged reductions in neurogenesis, e.g., induced by stress, may affect hippocampal structure and volume in depression, and that successful antidepressant treatment would require increases in neurogenesis [96, 120]. In preclinical studies, the stress-induced suppression of DG neurogenesis can be prevented by antidepressant treatments, which can also have direct neurogenic effects in naive animals. They can block effects on depressive-like behavior and may also restore plasticity outside the hippocampus [24, 133, 146].
Inflammation also affects neurogenesis. Microglia are considered instrumental given their homeostatic role in inflammatory signalling that may become maladaptive in the chronically stressed brain [72, 86, 155]. Under physiological conditions, microglia exhibit a resting, ramified phenotype associated with the production of anti-inflammatory and neurotrophic factors, but when primed, e.g., by early life stress [51] or challenged by pathogens or damage during adult life, microglia can switch to an activated, amoeboid phenotype, that can initiate tissue repair, or rather produce cytokines that are detrimental for neuronal function and viability [52, 56]. Specific subsets of cytokines can be proneurogenic [10] while others decrease neurogenesis through IL-1 [247]. Pro-inflammatory mediators can further restrict neurogenesis [56, 247].
The exact underlying cellular mechanisms mediating the inhibitory effect of stress or inflammation on neurogenesis are largely unknown. Adrenal glucocorticoids have been suggested as key players and MR, GR and NMDA receptors have been identified on progenitor cells [74]. At the same time, several examples exist of a longlasting inhibition of neurogenesis after an initial stressor, despite later normalized GC levels [187]. This suggests that while glucocorticoids may be involved in the initial suppression of cell proliferation, particularly in early life, when neurogenesis is abundant, they are not always necessary for the maintenance of this effect [74].
Stress also affects levels of various neurotransmitters implicated in the regulation of neurogenesis: GABA [67], serotonin, noradrenalin, dopamine [6], cannabinoids, opioids and nitric oxide (see [6]). Stress further reduces the expression of several growth factors, such as brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1), nerve growth factor (NGF), epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF), that all can influence neurogenesis (see, e.g., [186] while gonadal steroids should not be neglected either [63]. The proximity of the precursors to blood vessels further suggests a strong interaction with the vasculature and it is this population that is particularly sensitive to stress [80].
Although there is extensive preclinical evidence that antidepressants affect hippocampal neuroplasticity, information regarding the possible impact of medication on hippocampal volume in MRI studies of MDD patients is limited. A recent study by Huang et al. [90], found smaller DG volumes in unmedicated depressed patients and a post-mortem analysis reported the same [17] which would be consistent with the neurogenic hypothesis of depression. Interestingly, both subfield and posterior hippocampal volume reductions were reported that were only seen in unmedicated depression but were absent in patients treated with antidepressants. Although it is so far not possible to reliably detect ongoing neurogenesis in vivo [35, 131], these data are consistent with preclinical studies demonstrating subregional specific effects of stress and its modulators on neurogenesis, and/or on hippocampal functionality [99].
While neurogenesis in the mature human hippocampus is a rare event [14, 58, 101], changes have been reported following antidepressant treatment in middle aged, but not older depressed patients [15, 16, 121]. In a recent post-mortem study of major depressed patients, the volume of the histologically defined dentate gyrus was in fact 68 % larger in SSRI-treated depressed subjects [16]. SSRI treatment also substantially increased neural progenitor cells (NPCs) in the dentate gyrus [15], although this was not replicated by others [170] and may depend on the age of the patients [121].
The glutamatergic and GABAergic systems in depression
The glutamatergic pathway shows alterations in patients with mood disorder [9, 174]. Particularly, since glutamate stimulates the HPA axis antiglutamatergic agents, like ketamine [95, 244] are considered promising targeta for new antidepressants. In patients with MDD, glutamine synthetase (GS) transcripts were down-regulated in the anterior cingulate cortex and dorsolateral PFC [27]. The expression of glutamate transporters, the excitatory amino acid transporters (EAAT), EAAT1, EAAT2 are reduced frontal brain regions in MDD [27]. Accumulations of extracellular glutamate may not only perturb the ratio of excitatory–inhibitory neurotransmitters [34], but could also damage neurons and glia.
Regarding the GABAergic system, the majority of studies revealed lower GABA levels in the brain of depressed patients, suggesting a hypoactive GABAergic neurotransmission, including the PVN [9, 126]. GABA also inhibits the HPA axis in the hypothalamus [103] and GABAergic drugs can help maintain a positive response during antidepressant treatment [126].
Glial plasticity
During recent years, also glial abnormalities have been implicated in the pathophysiology of mood disorders. Glial cells slightly outnumber neurons in the human hippocampus and constitute a substantial volume fraction. During pathophysiological conditions, specific glial subtypes may become activated, or may die, or, similarly to the dendritic debranching documented in neurons, may retract their elaborate branching processes, and thereby reduce hippocampal volume. A considerable proportion of the astrocytes further express GRs in the rodent [66] and human hippocampus [231] and similar to neurons, these cells are stress responsive too. Typically, astrocytes are identified by their GFAP (glial fibrillary acidic protein) expression and stress modulates GFAP expression in rodents: while acute physical stress increase GFAP immunoreactivity in several brain regions [106], chronic stress reduced GFAP mRNA and protein expression levels in the prefrontal cortex and hippocampus [3]. As corticosterone lowers GFAP levels in rat brain [144], elevated glucocorticoids are likely instrumental in these effects.
Further studies demonstrated that in laboratory animals, exposure to long-term stress, either during early life or adulthood, decreased number and somal volume of GFAP-positive astrocytes in the hippocampus and in several other stress-related brain areas [38, 111]. This implied that chronic stress may cause astrocytic loss, while more recent experiments did not substantiate that [216]. For example, in vitro experiments showed that dexamethasone treatment of astrocytes, derived from hippocampal primary cell cultures, results in growth inhibition and moderate activation of caspase 3, which is not followed by apoptosis [241], suggesting that hippocampal astrocytes are resistant to glucocorticoid-induced apoptosis.
A recent in vivo study suggests that the reduced number of GFAP-positive cells after stress may not reflect cell death. By comparing the results of different labeling methods, it was found that chronic stress was associated with a decrease in GFAP-+ cell numbers, but there was no indication for astrocytic cell loss based on Nissl staining or S100ß-immunoreactivity [216]. This latter study also showed that astrocytes respond to chronic stress by reorganizing their cellular morphology and reducing the length, complexity and volume of their processes [216].
Chronic stress can also reduce the proliferation rate of glial cells. This was shown in the medial prefrontal cortex of rats subjected to 5 weeks of social defeat, or to chronic unpredictable stress or after chronic corticosterone administration [7, 39]. Similarly, prolonged and elevated glucocorticoid treatment inhibits NG2-positive cell proliferation in the adult rat hippocampus [144] reflecting changes in oligodendrocyte precursors or in a distinct mature glial type. Chronic stress also promotes significant structural remodeling of microglia, and can enhance the release of pro-inflammatory cytokines from microglia [227].
Finally, glial cells, especially astrocytes, are key components of the “neurogenic niche” that provides the necessary local microenvironment for generation of neurons in specific brain areas. They support maturation and integration of newborn neurons, both physically and by releasing a cocktail of growth factors and cytokines. Together, this implies that GCs not only are influenced by stress, but also stimulate interactions between astrocytes and neural progenitors.
In humans, post-mortem analysis of tissue from depressed patients has revealed reductions in glial numbers in the amygdala and prefrontal, orbitofrontal and cingulate cortices [20, 32, 149]. Accumulating data demonstrates that not only astrocytic cell numbers are reduced in depressed patients, but several typical structural and functional astrocytic markers as well, like GFAP, gap junction proteins, the water channel aquaporin-4 (AQP4), a calcium-binding protein S100B and glutamatergic markers including the excitatory amino acid transporters 1 and 2 (EAAT1, EAAT2) and glutamine synthetase [167].
In contrast, studies on post-mortem hippocampal samples so far failed to find significant reductions in neuron or glial cell numbers, while confirming the volume reduction in depressed patients [29]. One should add that besides astrocytes and oligondendrocytes, also microglia have also been implicated in pathological mood regulation [55, 60]. In vivo imaging studies in depressed patients demonstrate white matter abnormalities suggesting a contribution of oligodendrocytes to MDD etiology. MRI and also diffusion tensor imaging studies revealed white matter hyperintensities particularly in elderly subjects with late-life depression (reviewed in [55]) which was substantiated by post-mortem data reporting lower density of oligodendrocytes in the MDD brain and a reduced expression of oligodendrocyte-specific gene transcripts [55]. Animal models based on chronic stress paradigms also showed cellular and molecular changes in limbic structures that indicate an involvement of oligodendrocytes [39, 43, 207].
Water content, volume changes and altered vasculature
The controversial findings on hippocampal volume decrease without significant cell loss might also be explained by shifts in water content and the volumetric reduction of limbic structures is indeed often accompanied by enlarged cerebral ventricles [97]. In support, significantly shortened T1 relaxation times were found for the hippocampus, especially in elderly depressed patients, indicative of differences in hippocampal water content. A recent study used multimodal in vivo imaging, incorporating structural magnetic resonance imaging (MRI), and MR spectroscopy (1H-MRS) in rats exposed to ethanol [242]. While MRI revealed expansion of ventricles, volume changes in dorsal or ventral hippocampi, caudate or thalamus were not detected. Also, all MR parameters returned to baseline with 7 days of recovery [242]. Thus, the rapid recovery of ventricular volume and the absence of detectable volume reductions in brain regions adjacent to the ventricles argue against neuronal or tissue atrophy as a mechanism to explain ventricular expansion but rather suggest lower tissue water content. Thus, a rapid fluid redistribution may be followed by compensatory ventricular volume changes in stress-related psychopathologies.
Water homeostasis is largely regulated by aquaporin water channels. In the CNS, their major representative is AQP4 which is expressed in the end-feet of astrocytic processes, but not in neurons [211]. In the human orbitofrontal cortex (Brodmann’s area 47; gray matter), the density of AQP4 immunoreactive astrocytic end-feet was reduced by 50 % in patients with major depression [168] while the coverage of vessels by GFAP-immunoreactive processes did not differ from controls. These data indicate possible disturbances in water homeostasis, at least in this brain region.
Finally, a recent study revealed that stress reduced numbers of microvessels and capillarization in the hippocampi of stressed rats [41, 80] which coincides with clinical studies and metareviews indicating that increased psychological distress and depression are associated with increased stroke risk and mortality [150].
Conclusions
Exposure to chronic or severe stress has profound effects on the structural and functional integrity of limbic brain areas that not only coordinate the stress response, but are also exposed to the altered expression levels of different hormones, neurotransmitters and trophic factors. The central role of the HPA axis in these events has been most thoroughly investigated. Current experimental, post-mortem and in vivo imaging techniques have revealed various subtle morphological changes detectable both at the cellular level (affecting spines, dendrites, endothelial cells of the vasculature and glial and neuronal cell numbers), and in the gross morphology of specific brain areas (MRI findings). These changes thus appear to affect almost all cellular components of the CNS and many efforts are now undertaken to uncover the exact molecular mechanisms. While earlier studies highlighted disturbances in the monoaminergic systems, the glutamatergic and GABAergic systems receive attention too.
Most of the cellular responses to stress are initially plastic in nature and can normalize following appropriate recovery periods. These changes, particularly during the early phase, are often adaptive and essential for successful coping with stress. It is still very difficult to pinpoint when adaptive changes turn into maladaptive and/or pathological, and when systems start to deteriorate. Notably, many stress-induced morphological changes are specific to selected brain areas, and even to specific cell types, where they often correlate well with the functional disturbances of that given brain structure.
Finally, in humans, severe or repeated stress often contributes to the development, or can worsen the outcome, of psychopathology. Here, it is difficult to separate initial structural and/or neuropathological changes specific to the disease from the ones that result from the (additional) stress exposure per se.
Acknowledgments
PJL is supported by the Dutch Brain Foundation, Alzheimer Nederland, Int Parkinson Foundation and ISAO. B. Czéh is supported by the TÁMOP 4.2.4.A/2-11-1-2012-0001, National Excellency Program (János Szentágothai Scholarship: A2-SZJ-TOK-13-0060).
Abbreviations
- Aβ
Amyloid beta (β) protein
- ACC
Anterior cingulate cortex
- ACTH
Adrenocorticotropic hormone
- AD
Alzheimer’s disease
- ANS
Autonomic nervous system
- APP
Amyloid precursor protein
- AQP4
Water channel aquaporin-4
- AR
Androgen receptor
- AVP
Arginine vasopressin
- BD
Bipolar depression
- BDNF
Brain-derived neurotrophic factor
- BLA
Basolateral amygdala
- BST
Bed nucleus of the stria terminalis
- CBG
Glucocorticoid binding globulins
- CDK5
Cyclin-dependent kinase 5 (cell division protein kinase 5)
- CRH
Corticotropin-releasing hormone
- CRH-R
Corticotropin-releasing hormone receptor
- DHEA
Dehydro-epi-androsterone
- DHEAS
Dehydro-epi-androsterone sulphate
- DL–PFC
Dorsolateral prefrontal cortex
- EAAT
Excitatory amino acid transporters
- EGF
Epidermal growth factor
- ER-α
Estrogen receptor-alpha
- GAD
Glutamic acid decarboxylase
- GC
Glucocorticoid hormone
- GFAP
Glial fibrillary acidic protein
- GR
Glucocorticoid receptor
- GS
Glutamine synthetase
- fMRI
Functional magnetic resonance imaging
- H-MRS
Proton magnetic resonance spectroscopy
- HPA
Hypothalamic–pituitary–adrenal
- IL-1β
Interleukin 1-beta
- IGF-1
Insulin-like growth factor-1
- LTD
Long-term depression
- LTP
Long-term potentiation
- MDD
Major depressive disorder
- mPFC
Medial prefrontal cortex
- MR
Mineralocorticoid receptor
- MRI
Magnetic resonance imaging
- NAc
Nucleus accumbens
- NGF
Nerve growth factor
- NPCs
Neural progenitor cells
- OXT
Oxytocin
- pgACC
Pregenual anterior cingulate cortex
- PFC
Prefrontal cortex
- PSD-95
Post-synaptic density-95
- PTSD
Post-traumatic stress disorder
- PVN
Paraventricular nucleus of the hypothalamus
- SNPs
Single-nucleotide polymorphisms
- SSRI
Selective serotonin reuptake inhibitor
- SVZ
Subventricular zone
- UD
Unipolar depression
- VEGF
Vascular endothelial growth factor
Contributor Information
Paul J. Lucassen, Email: p.j.lucassen@uva.nl
Boldizsár Czéh, Email: czeh.boldizsar@pte.hu.
References
- 1.Alfarez DN, De Simoni A, Velzing EH, Bracey E, Joëls M, Edwards FA, Krugers HJ. Corticosterone reduces dendritic complexity in developing hippocampal CA1 neurons. Hippocampus. 2009;19:828–836. doi: 10.1002/hipo.20566. [DOI] [PubMed] [Google Scholar]
- 2.Alt SR, Turner JD, Klok MD, Meijer OC, Lakke EA, Derijk RH, Muller CP. Differential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed. Psychoneuroendocrinology. 2010;35:544–556. doi: 10.1016/j.psyneuen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Araya-Callis C, Hiemke C, Abumaria N, Flugge G. Chronic psychosocial stress and citalopram modulate the expression of the glial proteins GFAP and NDRG2 in the hippocampus. Psychopharmacology. 2012;224:209–222. [Google Scholar]
- 4.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baglietto-Vargas D, Medeiros R, Martinez-Coria H, LaFerla FM, Green KN. Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and also reduces tau pathology. Biol Psychiatry. 2013;74:357–366. doi: 10.1016/j.biopsych.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33:232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Bao AM, Fischer DF, Wu YH, Hol EM, Balesar R, Unmehopa UA, Zhou JN, Swaab DF. A direct androgenic involvement in the expression of human corticotropin-releasing hormone. Mol Psychiatry. 2006;11:567–576. doi: 10.1038/sj.mp.4001800. [DOI] [PubMed] [Google Scholar]
- 9.Bao AM, Ruhé HG, Gao SF, Swaab DF. Neurotransmitters and neuropeptides in depression. Handb Clin Neurol. 2012;106:107–136. doi: 10.1016/B978-0-444-52002-9.00008-5. [DOI] [PubMed] [Google Scholar]
- 10.Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- 11.Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 12.Blix E, Perski A, Berglund H, Savic I. Long-term occupational stress is associated with regional reductions in brain tissue volumes. PLoS ONE. 2013;8:e64065. doi: 10.1371/journal.pone.0064065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloss EB, Janssen WG, McEwen BS, Morrison JH. Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J Neurosci. 2010;30:6726–6731. doi: 10.1523/JNEUROSCI.0759-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006;24:1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, Arango V. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry. 2012;72:562–571. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38:1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 19.Bourdeau I, Bard C, Noël B, Leclerc I, Cordeau MP, Bélair M, Lesage J, Lafontaine L, Lacroix A. Loss of brain volume in endogenous Cushing’s syndrome and its reversibility after correction of hypercortisolism. J Clin Endocrinol Metab. 2002;87:1949–1954. doi: 10.1210/jcem.87.5.8493. [DOI] [PubMed] [Google Scholar]
- 20.Bowley MP, Drevets WC, Ongür D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 21.Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci USA. 2012;109:E1312–E1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caetano SC, Hatch JP, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res. 2004;132(2):141–147. doi: 10.1016/j.pscychresns.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Castrén E, Hen R. Neuronal plasticity and antidepressant actions. Trends Neurosci. 2013;36:259–267. doi: 10.1016/j.tins.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, Almeida OF. The amyloidogenic potential and behavioral correlates of stress. Mol Psychiatry. 2009;14:95–105. doi: 10.1038/sj.mp.4002101. [DOI] [PubMed] [Google Scholar]
- 26.Cerqueira JJ, Almeida OF, Sousa N. The stressed prefrontal cortex. Left? Right! Brain Behav Immun. 2008;22:630–638. doi: 10.1016/j.bbi.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE, Jr, Akil H, Watson SJ, Jones EG. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci USA. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cisler JM, James GA, Tripathi S, Mletzko T, Heim C, Hu XP, Mayberg HS, Nemeroff CB, Kilts CD. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol Med. 2013;43:507–518. doi: 10.1017/S0033291712001390. [DOI] [PubMed] [Google Scholar]
- 29.Cobb JA, Simpson J, Mahajan GJ, Overholser JC, Jurjus GJ, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA. Hippocampal volume and total cell numbers in major depressive disorder. J Psychiatr Res. 2013;47:299–306. doi: 10.1016/j.jpsychires.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coe CL, Kramer M, Czéh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- 31.Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 33.Crochemore C, Lu J, Wu Y, Liposits Z, Sousa N, Holsboer F, Almeida OF. Direct targeting of hippocampal neurons for apoptosis by glucocorticoids is reversible by mineralocorticoid receptor activation. Mol Psychiatry. 2005;10:790–798. doi: 10.1038/sj.mp.4001679. [DOI] [PubMed] [Google Scholar]
- 34.Cryan JF, Kaupmann K. Don’t worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26:36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Curtis MA, Kam M, Faull RL. Neurogenesis in humans. Eur J Neurosci. 2011;33:1170–1174. doi: 10.1111/j.1460-9568.2011.07616.x. [DOI] [PubMed] [Google Scholar]
- 36.Czéh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- 37.Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Czéh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- 39.Czéh B, Müller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- 40.Czéh B, Perez-Cruz C, Fuchs E, Flügge G. Chronic stress-induced cellular changes in the medial prefrontal cortex and their potential clinical implications: does hemisphere location matter? Behav Brain Res. 2008;190:1–13. doi: 10.1016/j.bbr.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 41.Czéh B, Abumaria N, Rygula R, Fuchs E. Quantitative changes in hippocampal microvasculature of chronically stressed rats: no effect of fluoxetine treatment. Hippocampus. 2010;20:174–185. doi: 10.1002/hipo.20599. [DOI] [PubMed] [Google Scholar]
- 42.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Datson NA, Speksnijder N, Mayer JL, Steenbergen PJ, Korobko O, Goeman J, de Kloet ER, Joëls M, Lucassen PJ. The transcriptional response to chronic stress and glucocorticoid receptor blockade in the hippocampal dentate gyrus. Hippocampus. 2012;22:359–371. doi: 10.1002/hipo.20905. [DOI] [PubMed] [Google Scholar]
- 44.De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, McCrory EJ. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J Child Psychol Psychiatry. 2013;54:105–112. doi: 10.1111/j.1469-7610.2012.02597.x. [DOI] [PubMed] [Google Scholar]
- 45.Dedovic K, Rexroth M, Wolff E, Duchesne A, Scherling C, Beaudry T, Lue SD, Lord C, Engert V, Pruessner JC. Neural correlates of processing stressful information: an event-related fMRI study. Brain Res. 2009;1293:49–60. doi: 10.1016/j.brainres.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 46.Dedovic K, Engert V, Duchesne A, Lue SD, Andrews J, Efanov SI, Beaudry T, Pruessner JC. Cortisol awakening response and hippocampal volume: vulnerability for major depressive disorder? Biol Psychiatry. 2010;68:847–853. doi: 10.1016/j.biopsych.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 47.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 48.de Leon MJ, McRae T, Tsai JR, George AE, Marcus DL, Freedman M, Wolf AP, McEwen B. Abnormal cortisol response in Alzheimer’s disease linked to hippocampal atrophy. Lancet. 1988;2:391–392. doi: 10.1016/s0140-6736(88)92855-3. [DOI] [PubMed] [Google Scholar]
- 49.DeRijk RH, van Leeuwen N, Klok MD, Zitman FG. Corticosteroid receptor-gene variants: modulators of the stress-response and implications for mental health. Eur J Pharmacol. 2008;585:492–501. doi: 10.1016/j.ejphar.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 51.Diz-Chaves Y, Pernía O, Carrero P, Garcia-Segura LM. Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. J Neuroinflammation. 2012;9:71. doi: 10.1186/1742-2094-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doorn KJ, Lucassen PJ, Boddeke HW, Prins M, Berendse HW, Drukarch B, van Dam AM. Emerging roles of microglial activation and non-motor symptoms in Parkinson’s disease. Prog Neurobiol. 2012;98:222–238. doi: 10.1016/j.pneurobio.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75:27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edgar N, Sibille E. A putative functional role for oligodendrocytes in mood regulation. Transl Psychiatry. 2012;2:e109. doi: 10.1038/tp.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 57.Erdmann G, Berger S, Schütz G. Genetic dissection of glucocorticoid receptor function in the mouse brain. J Neuroendocrinol. 2008;20:655–659. doi: 10.1111/j.1365-2826.2008.01717.x. [DOI] [PubMed] [Google Scholar]
- 58.Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 59.Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab. 1998;83:2066–2073. doi: 10.1210/jcem.83.6.4881. [DOI] [PubMed] [Google Scholar]
- 60.Eyre H, Baune BT. Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology. 2012;37:1397–1416. doi: 10.1016/j.psyneuen.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 61.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res. 2010;44:799–807. doi: 10.1016/j.jpsychires.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 63.Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 64.Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- 65.Gao SF, Klomp A, Wu JL, Swaab DF, Bao AM. Reduced GAD(65/67) immunoreactivity in the hypothalamic paraventricular nucleus in depression: a postmortem study. J Affect Disord. 2013;149:422–425. doi: 10.1016/j.jad.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Segura LM, Chowen JA, Naftolin F. Endocrine glia: roles of glial cells in the brain actions of steroid and thyroid hormones and in the regulation of hormone secretion. Front Neuroendocrinol. 1996;17:180–211. doi: 10.1006/frne.1996.0005. [DOI] [PubMed] [Google Scholar]
- 67.Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Gilabert-Juan J, Castillo-Gomez E, Guirado R, Moltó MD, Nacher J. Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Struct Funct. 2013;218:1591–1605. doi: 10.1007/s00429-012-0479-1. [DOI] [PubMed] [Google Scholar]
- 69.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 71.Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gómez-Nicola D, Fransen NL, Suzzi S, Perry VH. Regulation of microglial proliferation during chronic neurodegeneration. J Neurosci. 2013;33:2481–2493. doi: 10.1523/JNEUROSCI.4440-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goodwin RD, Gotlib IH. Gender differences in depression: the role of personality factors. Psychiatry Res. 2004;126:135–142. doi: 10.1016/j.psychres.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 74.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanson JL, Chung MK, Avants BB, Rudolph KD, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. J Neurosci. 2012;32:7917–7925. doi: 10.1523/JNEUROSCI.0307-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harris AP, Holmes MC, de Kloet ER, Chapman KE, Seckl JR. Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behaviour. Psychoneuroendocrinology. 2013;38:648–658. doi: 10.1016/j.psyneuen.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 78.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 79.Heine VM, Maslam S, Zareno J, Joëls M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- 80.Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21:1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- 81.Hercher C, Canetti L, Turecki G, Mechawar N. Anterior cingulate pyramidal neurons display altered dendritic branching in depressed suicides. J Psychiatr Res. 2010;44:286–293. doi: 10.1016/j.jpsychires.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 82.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 83.Heuser I, Bissette G, Dettling M, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Nemeroff CB, Holsboer F. Cerebrospinal fluid concentrations of corticotropin-releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety. 1998;8:71–79. [PubMed] [Google Scholar]
- 84.Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hinterberger M, Zehetmayer S, Jungwirth S, Huber K, Krugluger W, Leitha T, Krampla W, Tragl KH, Fischer P. High cortisol and low folate are the only routine blood tests predicting probable Alzheimer’s disease after age 75-results of the Vienna Transdanube Aging Study. J Am Geriatr Soc. 2013;61:648–651. doi: 10.1111/jgs.12178. [DOI] [PubMed] [Google Scholar]
- 86.Hinwood M, Morandini J, Day TA, Walker FR. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex. 2012;22:1442–1454. doi: 10.1093/cercor/bhr229. [DOI] [PubMed] [Google Scholar]
- 87.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 88.Hu P, Oomen C, van Dam AM, Wester J, Zhou JN, Joëls M, Lucassen PJ. A single-day treatment with mifepristone is sufficient to normalize chronic glucocorticoid induced suppression of hippocampal cell proliferation. PLoS ONE. 2012;7:e46224. doi: 10.1371/journal.pone.0046224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer’s disease. J Clin Neurosci. 2009;16:1283–1286. doi: 10.1016/j.jocn.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 90.Huang Y, Coupland NJ, Lebel RM, Carter R, Seres P, Wilman AH, Malykhin NV. Structural changes in hippocampal subfields in major depressive disorder: a high-field magnetic resonance imaging study. Biol Psychiatry. 2013;74:62–68. doi: 10.1016/j.biopsych.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 91.Jeong YH, Park CH, Yoo J, Shin KY, Ahn SM, Kim HS, Lee SH, Emson PC, Suh YH. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer’s disease model. FASEB J. 2006;20:729–731. doi: 10.1096/fj.05-4265fje. [DOI] [PubMed] [Google Scholar]
- 92.Jia Z, Huang X, Wu Q, Zhang T, Lui S, Zhang J, Amatya N, Kuang W, Chan RC, Kemp GJ, Mechelli A, Gong Q. High-field magnetic resonance imaging of suicidality in patients with major depressive disorder. Am J Psychiatry. 2010;167:1381–1390. doi: 10.1176/appi.ajp.2010.09101513. [DOI] [PubMed] [Google Scholar]
- 93.Joëls M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev. 2012;64:901–938. doi: 10.1124/pr.112.005892. [DOI] [PubMed] [Google Scholar]
- 94.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, Lepack A, Majik MS, Jeong LS, Banasr M, Son H, Duman RS. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- 96.Kempermann G, Krebs J, Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry. 2008;21:290–295. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- 97.Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, Williams SC. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 98.Kerr DS, Campbell LW, Applegate MD, Brodish A, Landfield PW. Chronic stress-induced acceleration of electrophysiologic and morphometric biomarkers of hippocampal aging. J Neurosci. 1991;11:1316–1324. doi: 10.1523/JNEUROSCI.11-05-01316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klok MD, Alt SR, Irurzun Lafitte AJ, Turner JD, Lakke EA, Huitinga I, Muller CP, Zitman FG, de Kloet ER, Derijk RH. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. J Psychiatr Res. 2011;45:871–878. doi: 10.1016/j.jpsychires.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 101.Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wöhr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 103.Kovács KJ, Miklós IH, Bali B. GABAergic mechanisms constraining the activity of the hypothalamo-pituitary-adrenocortical axis. Ann NY Acad Sci. 2004;1018:466–476. doi: 10.1196/annals.1296.057. [DOI] [PubMed] [Google Scholar]
- 104.Kretz O, Reichardt HM, Schütz G, Bock R. Corticotropin-releasing hormone expression is the major target for glucocorticoid feedback-control at the hypothalamic level. Brain Res. 1999;818:488–491. doi: 10.1016/s0006-8993(98)01277-3. [DOI] [PubMed] [Google Scholar]
- 105.Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:744–759. doi: 10.1016/j.pnpbp.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 106.Lambert KG, Gerecke KM, Quadros PS, Doudera E, Jasnow AM, Kinsley CH. Activity-stress increases density of GFAP-immunoreactive astrocytes in the rat hippocampus. Stress. 2000;3:275–284. doi: 10.3109/10253890009001133. [DOI] [PubMed] [Google Scholar]
- 107.Landfield PW, Baskin RK, Pitler TA. Brain aging correlates: retardation by hormonal-pharmacological treatments. Science. 1981;214:581–584. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- 108.Landgraf R, Kessler MS, Bunck M, Murgatroyd C, Spengler D, Zimbelmann M, Nussbaumer M, Czibere L, Turck CW, Singewald N, Rujescu D, Frank E. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase-I. Neurosci Biobehav Rev. 2007;31:89–102. doi: 10.1016/j.neubiorev.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 109.Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, Wüst S, Pruessner JC, Rietschel M, Deuschle M, Meyer-Lindenberg A. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- 110.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 111.Leventopoulos M, Ruedi-Bettschen D, Knuesel I, Feldon J, Pryce CR, Opacka-Juffry J. Long-term effects of early life deprivation on brain glia in Fischer rats. Brain Res. 2007;1142:119–126. doi: 10.1016/j.brainres.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 112.Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind MA, Peskind ER. Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J Neurosci. 1999;19:2356–2361. doi: 10.1523/JNEUROSCI.19-06-02356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin Y, Ter Horst GJ, Wichmann R, Bakker P, Liu A, Li X, Westenbroek C. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cereb Cortex. 2009;19:1978–1989. doi: 10.1093/cercor/bhn225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: a possible window of opportunity effect. Neurobiol Aging. 2008;29:95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 117.Lucassen PJ, Müller MB, Holsboer F, Bauer J, Holtrop A, Wouda J, Hoogendijk WJ, De Kloet ER, Swaab DF. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158:453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lucassen PJ, Fuchs E, Czéh B. Antidepressant treatment with tianeptine reduces apoptosis in the hippocampal dentate gyrus and temporal cortex. Biol Psychiatry. 2004;55:789–796. doi: 10.1016/j.biopsych.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 119.Lucassen PJ, Heine VM, Muller MB, van der Beek EM, Wiegant VM, De Kloet ER, Joels M, Fuchs E, Swaab DF, Czeh B. Stress, depression and hippocampal apoptosis. CNS Neurol Disord Drug Targets. 2006;5:531–546. doi: 10.2174/187152706778559273. [DOI] [PubMed] [Google Scholar]
- 120.Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, Oomen CA, Czéh B. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 121.Lucassen PJ, Stumpel MW, Wang Q, Aronica E. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology. 2010;58:940–949. doi: 10.1016/j.neuropharm.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 122.Lucassen PJ, Naninck EF, van Goudoever JB, Fitzsimons C, Joels M, Korosi A (2013) Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci. 2013 Aug 30. doi:10.1016/j.tins.2013.08.002 (Epub ahead of print) [DOI] [PubMed]
- 123.Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 124.Lupien SJ, Evans A, Lord C, Miles J, Pruessner M, Pike B, Pruessner JC. Hippocampal volume is as variable in young as in older adults: implications for the notion of hippocampal atrophy in humans. Neuroimage. 2007;34:479–485. doi: 10.1016/j.neuroimage.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 125.Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, Pruessner JC, Séguin JR. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA. 2011;108:14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 128.MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry. 2008;64:880–883. doi: 10.1016/j.biopsych.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 129.Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maller JJ, Daskalakis ZJ, Thomson RH, Daigle M, Barr MS, Fitzgerald PB. Hippocampal volumetrics in treatment-resistant depression and schizophrenia: the devil’s in de-tail. Hippocampus. 2012;22:9–16. doi: 10.1002/hipo.20873. [DOI] [PubMed] [Google Scholar]
- 131.Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, Maletic-Savatic M. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martin KP, Wellman CL. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex. 2011;21:2366–2373. doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castrén E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 134.McKernan DP, Dinan TG, Cryan JF. “Killing the Blues”: a role for cellular suicide (apoptosis) in depression and the antidepressant response? Prog Neurobiol. 2009;88:246–263. doi: 10.1016/j.pneurobio.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 135.Medina A, Seasholtz AF, Sharma V, Burke S, Bunney W, Jr, Myers RM, Schatzberg A, Akil H, Watson SJ. Glucocorticoid and mineralocorticoid receptor expression in the human hippocampus in major depressive disorder. J Psychiatr Res. 2013;47:307–314. doi: 10.1016/j.jpsychires.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meynen G, Unmehopa UA, van Heerikhuize JJ, Hofman MA, Swaab DF, Hoogendijk WJ. Increased arginine vasopressin mRNA expression in the human hypothalamus in depression: a preliminary report. Biol Psychiatry. 2006;60:892–895. doi: 10.1016/j.biopsych.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 137.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 139.Murgatroyd C, Spengler D. Epigenetic programming of the HPA axis: early life decides. Stress. 2011;14:581–589. doi: 10.3109/10253890.2011.602146. [DOI] [PubMed] [Google Scholar]
- 140.Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmühl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 141.Murialdo G, Barreca A, Nobili F, Rollero A, Timossi G, Gianelli MV, Copello F, Rodriguez G, Polleri A. Dexamethasone effects on cortisol secretion in Alzheimer’s disease: some clinical and hormonal features in suppressor and nonsuppressor patients. J Endocrinol Invest. 2000;23:178–186. doi: 10.1007/BF03343703. [DOI] [PubMed] [Google Scholar]
- 142.Müller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJ, Holsboer F, Swaab DF. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci. 2001;14:1603–1612. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- 143.Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, Bain EE, Charney DS, Drevets WC. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 144.Nichols NR, Osterburg HH, Masters JN, Millar SL, Finch CE. Messenger RNA for glial fibrillary acidic protein is decreased in rat brain following acute and chronic corticosterone treatment. Brain Res Mol Brain Res. 1990;7:1–7. doi: 10.1016/0169-328x(90)90066-m. [DOI] [PubMed] [Google Scholar]
- 145.O’Brien JT, Ames D, Schweitzer I, Mastwyk M, Colman P. Enhanced adrenal sensitivity to adrenocorticotrophic hormone (ACTH) is evidence of HPA axis hyperactivity in Alzheimer’s disease. Psychol Med. 1996;26:7–14. doi: 10.1017/s0033291700033675. [DOI] [PubMed] [Google Scholar]
- 146.Ohira K, Takeuchi R, Shoji H, Miyakawa T. Fluoxetine-induced cortical adult neurogenesis. Neuropsychopharmacology. 2013;38:909–920. doi: 10.1038/npp.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oliveira JF, Dias NS, Correia M, Gama-Pereira F, Sardinha VM, Lima A, Oliveira AF, Jacinto LR, Ferreira DS, Silva AM, Reis JS, Cerqueira JJ, Sousa N. Chronic stress disrupts neural coherence between cortico-limbic structures. Front Neural Circuits. 2013;7:10. doi: 10.3389/fncir.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, Joëls M, Lucassen PJ, Krugers H. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J Neurosci. 2010;30:6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306:1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pascucci T, Ventura R, Latagliata EC, Cabib S, Puglisi-Allegra S. The medial prefrontal cortex determines the accumbens dopamine response to stress through the opposing influences of norepinephrine and dopamine. Cereb Cortex. 2007;17:2796–2804. doi: 10.1093/cercor/bhm008. [DOI] [PubMed] [Google Scholar]
- 152.Patchev VK, Almeida OF. Gonadal steroids exert facilitating and “buffering” effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. J Neurosci. 1996;16:7077–7084. doi: 10.1523/JNEUROSCI.16-21-07077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33:360–367. doi: 10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pego JM, Sousa JC, Almeida OF, Sousa N. Stress and the neuroendocrinology of anxiety disorders. Curr Top Behav Neurosci. 2010;2:97–117. doi: 10.1007/7854_2009_13. [DOI] [PubMed] [Google Scholar]
- 155.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 156.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pravosudov VV, Omanska A. Prolonged moderate elevation of corticosterone does not affect hippocampal anatomy or cell proliferation rates in mountain chickadees (Poecile gambeli) J Neurobiol. 2005;62:82–91. doi: 10.1002/neu.20069. [DOI] [PubMed] [Google Scholar]
- 158.Pruessner JC, Collins DL, Pruessner M, Evans AC. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J Neurosci. 2001;21:194–200. doi: 10.1523/JNEUROSCI.21-01-00194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 160.Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Dagher A, Lupien SJ. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations—2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 161.Pryce CR, Aubert Y, Maier C, Pearce PC, Fuchs E. The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset. Psychopharmacology. 2011;214:33–53. doi: 10.1007/s00213-010-1989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qi XR, Kamphuis W, Wang S, Wang Q, Lucassen PJ, Zhou JN, Swaab DF. Aberrant stress hormone receptor balance in the human prefrontal cortex and hypothalamic paraventricular nucleus of depressed patients. Psychoneuroendocrinology. 2013;38:863–870. doi: 10.1016/j.psyneuen.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 163.Qian X, Droste SK, Lightman SL, Reul JM, Linthorst AC. Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology. 2012;153:4346–4353. doi: 10.1210/en.2012-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev. 2005;4:271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 165.Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 166.Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- 167.Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets. 2013;14:1225–1236. doi: 10.2174/13894501113149990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rajkowska G, Hughes J, Stockmeier CA, Javier Miguel-Hidalgo J, Maciag D. Coverage of blood vessels by astrocytic endfeet is reduced in major depressive disorder. Biol Psychiatry. 2013;73:613–621. doi: 10.1016/j.biopsych.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rasmuson S, Andrew R, Näsman B, Seckl JR, Walker BR, Olsson T. Increased glucocorticoid production and altered cortisol metabolism in women with mild to moderate Alzheimer’s disease. Biol Psychiatry. 2001;49:547–552. doi: 10.1016/s0006-3223(00)01015-5. [DOI] [PubMed] [Google Scholar]
- 170.Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 171.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 172.Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, Zink M, Hörtnagl H, Flor H, Henn FA, Schütz G, Gass P. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci. 2005;25:6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sánchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sandi C, Davies HA, Cordero MI, Rodriguez JJ, Popov VI, Stewart MG. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur J Neurosci. 2003;17:2447–2456. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- 177.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 178.Sapolsky RM. A mechanism for glucocorticoid toxicity in the hippocampus: increased neuronal vulnerability to metabolic insults. J Neurosci. 1985;5:1228–1232. doi: 10.1523/JNEUROSCI.05-05-01228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sapolsky RM. Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci USA. 2001;98:12320–12322. doi: 10.1073/pnas.231475998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sapolsky RM. Chickens, eggs and hippocampal atrophy. Nat Neurosci. 2002;5:1111–1113. doi: 10.1038/nn1102-1111. [DOI] [PubMed] [Google Scholar]
- 181.Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 183.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schermuly I, Wolf D, Lieb K, Stoeter P, Fellgiebel A. State dependent posterior hippocampal volume increases in patients with major depressive disorder. J Affect Disord. 2011;135:405–409. doi: 10.1016/j.jad.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 185.Schiavone S, Jaquet V, Sorce S, Dubois-Dauphin M, Hultqvist M, Bäckdahl L, Holmdahl R, Colaianna M, Cuomo V, Trabace L, Krause KH. NADPH oxidase elevations in pyramidal neurons drive psychosocial stress-induced neuropathology. Transl Psychiatry. 2012;2:e111. doi: 10.1038/tp.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 187.Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schüle C, Baghai TC, Eser D, Rupprecht R. Hypothalamic-pituitary-adrenocortical system dysregulation and new treatment strategies in depression. Expert Rev Neurother. 2009;9:1005–1019. doi: 10.1586/ern.09.52. [DOI] [PubMed] [Google Scholar]
- 189.Seckl JR, Dickson KL, Yates C, Fink G. Distribution of glucocorticoid and mineralocorticoid receptor messenger RNA expression in human postmortem hippocampus. Brain Res. 1991;561:332–337. doi: 10.1016/0006-8993(91)91612-5. [DOI] [PubMed] [Google Scholar]
- 190.Sinclair D, Tsai SY, Woon HG, Weickert CS. Abnormal glucocorticoid receptor mRNA and protein isoform expression in the prefrontal cortex in psychiatric illness. Neuropsychopharmacology. 2011;36:2698–2709. doi: 10.1038/npp.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sinclair D, Webster MJ, Wong J, Weickert CS. Dynamic molecular and anatomical changes in the glucocorticoid receptor in human cortical development. Mol Psychiatry. 2011;16:504–515. doi: 10.1038/mp.2010.28. [DOI] [PubMed] [Google Scholar]
- 192.Sinclair D, Fullerton JM, Webster MJ, Shannon Weickert C. Glucocorticoid receptor 1B and 1C mRNA transcript alterations in schizophrenia and bipolar disorder, and their possible regulation by GR gene variants. PLoS ONE. 2012;7:e31720. doi: 10.1371/journal.pone.0031720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sierksma AS, Prickaerts J, Chouliaras L, Rostamian S, Delbroek L, Rutten BP, Steinbusch HW, van den Hove DL. Behavioral and neurobiological effects of prenatal stress exposure in male and female APPswe/PS1dE9 mice. Neurobiol Aging. 2013;34:319–337. doi: 10.1016/j.neurobiolaging.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 194.Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–113. doi: 10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 196.Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, Mirnics K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. 2011;16:751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sloviter RS, Sollas AL, Dean E, Neubort S. Adrenalectomy-induced granule cell degeneration in the rat hippocampal dentate gyrus: characterization of an in vivo model of controlled neuronal death. J Comp Neurol. 1993;330:324–336. doi: 10.1002/cne.903300304. [DOI] [PubMed] [Google Scholar]
- 198.Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques F, Palha JA, Cerqueira JJ, Sousa N. Stress-induced changes in human decision-making are reversible. Transl Psychiatry. 2012;2:e131. doi: 10.1038/tp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sotiropoulos I, Catania C, Pinto LG, Silva R, Pollerberg GE, Takashima A, Sousa N, Almeida OF. Stress acts cumulatively to precipitate Alzheimer’s disease-like tau pathology and cognitive deficits. J Neurosci. 2011;31:7840–7847. doi: 10.1523/JNEUROSCI.0730-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sousa N, Almeida OF. Disconnection and reconnection: the morphological basis of (mal)adaptation to stress. Trends Neurosci. 2012;35:742–751. doi: 10.1016/j.tins.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 201.Sousa N, Almeida OF, Holsboer F, Paula-Barbosa MM, Madeira MD. Maintenance of hippocampal cell numbers in young and aged rats submitted to chronic unpredictable stress. Comparison with the effects of corticosterone treatment. Stress. 1998;2:237–249. doi: 10.3109/10253899809167288. [DOI] [PubMed] [Google Scholar]
- 202.Sousa N, Paula-Barbosa MM, Almeida OF. Ligand and subfield specificity of corticoid-induced neuronal loss in the rat hippocampal formation. Neuroscience. 1999;89:1079–1087. doi: 10.1016/s0306-4522(98)00311-x. [DOI] [PubMed] [Google Scholar]
- 203.Sousa N, Cerqueira JJ, Almeida OF. Corticosteroid receptors and neuroplasticity. Brain Res Rev. 2008;57:561–570. doi: 10.1016/j.brainresrev.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 204.Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Biol Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- 205.Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, Rodriguez JJ, Cordero MI, Donohue HS, Gabbott PL, Popov VI. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131:43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 206.Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Surget A, Wang Y, Leman S, Ibarguen-Vargas Y, Edgar N, Griebel G, Belzung C, Sibille E. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology. 2009;34:1363–1380. doi: 10.1038/npp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Swaab DF, Raadsheer FC, Endert E, Hofman MA, Kamphorst W, Ravid R. Increased cortisol levels in aging and Alzheimer’s disease in postmortem cerebrospinal fluid. J Neuroendocrinol. 1994;6:681–687. doi: 10.1111/j.1365-2826.1994.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 209.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 210.Symonds LL, Archibald SL, Grant I, Zisook S, Jernigan TL. Does an increase in sulcal or ventricular fluid predict where brain tissue is lost? J Neuroimaging. 1999;9:201–209. doi: 10.1111/jon199994201. [DOI] [PubMed] [Google Scholar]
- 211.Tait MJ, Saadoun S, Bell BA, Papadopoulos MC. Water movements in the brain: role of aquaporins. Trends Neurosci. 2008;31:37–43. doi: 10.1016/j.tins.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 212.Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14:398–406. doi: 10.3109/10253890.2011.586446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol. 2006;498:363–374. doi: 10.1002/cne.21071. [DOI] [PubMed] [Google Scholar]
- 214.Teffer K, Semendeferi K. Human prefrontal cortex: evolution, development, and pathology. Prog Brain Res. 2012;195:191–218. doi: 10.1016/B978-0-444-53860-4.00009-X. [DOI] [PubMed] [Google Scholar]
- 215.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA. 2012;109:E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, Walker FR. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol. 2013;126:75–91. doi: 10.1007/s00401-013-1102-0. [DOI] [PubMed] [Google Scholar]
- 217.Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 219.van der Beek EM, Wiegant VM, Schouten WG, van Eerdenburg FJ, Loijens LW, van der Plas C, Benning MA, de Vries H, de Kloet ER, Lucassen PJ. Neuronal number, volume, and apoptosis of the left dentate gyrus of chronically stressed pigs correlate negatively with basal saliva cortisol levels. Hippocampus. 2004;14:688–700. doi: 10.1002/hipo.10213. [DOI] [PubMed] [Google Scholar]
- 220.van Londen L, Goekoop JG, van Kempen GM, Frankhuijzen-Sierevogel AC, Wiegant VM, van der Velde EA, De Wied D. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology. 1997;17:284–292. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- 221.Ventura-Silva AP, Pêgo JM, Sousa JC, Marques AR, Rodrigues AJ, Marques F, Cerqueira JJ, Almeida OF, Sousa N. Stress shifts the response of the bed nucleus of the stria terminalis to an anxiogenic mode. Eur J Neurosci. 2012;36:3396–3406. doi: 10.1111/j.1460-9568.2012.08262.x. [DOI] [PubMed] [Google Scholar]
- 222.Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Vollmann-Honsdorf GK, Flügge G, Fuchs E. Chronic psychosocial stress does not affect the number of pyramidal neurons in tree shrew hippocampus. Neurosci Lett. 1997;233:121–124. doi: 10.1016/s0304-3940(97)00647-2. [DOI] [PubMed] [Google Scholar]
- 224.Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry. 2003;54:1035–1040. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- 225.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 226.Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Staib LH, Charney DS, Bremner JD. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 227.Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013;14:1262–1276. doi: 10.2174/13894501113149990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry. 2008;13(786–99):741. doi: 10.1038/mp.2008.38. [DOI] [PubMed] [Google Scholar]
- 229.Wang Q, Joels M, Swaab DF, Lucassen PJ. Hippocampal GR expression is increased in elderly depressed females. Neuropharmacology. 2012;62:527–533. doi: 10.1016/j.neuropharm.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 230.Wang L, Dai Z, Peng H, Tan L, Ding Y, He Z, Zhang Y, Xia M, Li Z, Li W, Cai Y, Lu S, Liao M, Zhang L, Wu W, He Y, Li L (2013a) Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum Brain Mapp. 2013 Feb 13. doi:10.1002/hbm.22241 (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 231.Wang Q, Van Heerikhuize J, Aronica E, Kawata M, Seress L, Joels M, Swaab DF, Lucassen PJ. Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol Aging. 2013;34:1662–1673. doi: 10.1016/j.neurobiolaging.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 232.Wang Q, Verweij EW, Krugers HJ, Joels M, Swaab DF, Lucassen PJ (2013c) Distribution of the glucocorticoid receptor in the human amygdala; changes in mood disorder patients. Brain Struct Funct. 2013 Jun 8 (Epub ahead of print) [DOI] [PubMed]
- 233.Webster MJ, Knable MB, O’Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry. 2002;7(985–94):924. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- 234.Wei Q, Hebda-Bauer EK, Pletsch A, Luo J, Hoversten MT, Osetek AJ, Evans SJ, Watson SJ, Seasholtz AF, Akil H. Overexpressing the glucocorticoid receptor in forebrain causes an aging-like neuroendocrine phenotype and mild cognitive dysfunction. J Neurosci. 2007;27:8836–8844. doi: 10.1523/JNEUROSCI.0910-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Winterburn JL, Pruessner JC, Chavez S, Schira MM, Lobaugh NJ, Voineskos AN, Chakravarty MM. A novel in vivo atlas of human hippocampal subfields using high-resolution 3 T magnetic resonance imaging. Neuroimage. 2013;74:254–265. doi: 10.1016/j.neuroimage.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 236.Wong EY, Herbert J. Raised circulating corticosterone inhibits neuronal differentiation of progenitor cells in the adult hippocampus. Neuroscience. 2006;137:83–92. doi: 10.1016/j.neuroscience.2005.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 238.Yao YY, Liu DM, Xu DF, Li WP. Memory and learning impairment induced by dexamethasone in senescent but not young mice. Eur J Pharmacol. 2007;574:20–28. doi: 10.1016/j.ejphar.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 239.Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(Suppl 17):41–46. [PubMed] [Google Scholar]
- 240.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 241.Yu S, Yang S, Holsboer F, Sousa N, Almeida OF. Glucocorticoid regulation of astrocytic fate and function. PLoS ONE. 2011;6:e22419. doi: 10.1371/journal.pone.0022419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zahr NM, Mayer D, Rohlfing T, Orduna J, Luong R, Sullivan EV, Pfefferbaum A. A mechanism of rapidly reversible cerebral ventricular enlargement independent of tissue atrophy. Neuropsychopharmacology. 2013;38:1121–1129. doi: 10.1038/npp.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zalachoras I, Houtman R, Atucha E, Devos R, Tijssen AM, Hu P, Lockey PM, Datson NA, Belanoff JK, Lucassen PJ, Joëls M, de Kloet ER, Roozendaal B, Hunt H, Meijer OC. Differential targeting of brain stress circuits with a selective glucocorticoid receptor modulator. Proc Natl Acad Sci USA. 2013;110:7910–7915. doi: 10.1073/pnas.1219411110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 245.Zhang J, Wang J, Wu Q, Kuang W, Huang X, He Y, Gong Q. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 246.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 247.Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, Thuret S, Price J, Pariante CM. Interleukin-1b: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37:939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]