Abstract
Background
Both being overweight and exposure to indoor pollutants, which have been associated with worse health of asthmatic patients, are common in urban minority populations. Whether being overweight is a risk factor for the effects of indoor pollutant exposure on asthma health is unknown.
Objectives
We sought to examine the effect of weight on the relationship between indoor pollutant exposure and asthma health in urban minority children.
Methods
One hundred forty-eight children (age, 5–17 years) with persistent asthma were followed for 1 year. Asthma symptoms, health care use, lung function, pulmonary inflammation, and indoor pollutants were assessed every 3 months. Weight category was based on body mass index percentile.
Results
Participants were predominantly African American (91%) and had public health insurance (85%). Four percent were underweight, 52% were normal weight, 16% were overweight, and 28% were obese. Overweight or obese participants had more symptoms associated with exposure to fine particulate matter measuring less than 2.5 μm in diameter (PM2.5) than normal-weight participants across a range of asthma symptoms. Overweight or obese participants also had more asthma symptoms associated with nitrogen dioxide (NO2) exposure than normal-weight participants, although this was not observed across all types of asthma symptoms. Weight did not affect the relationship between exposure to coarse particulate matter measuring between 2.5 and 10 μm in diameter and asthma symptoms. Relationships between indoor pollutant exposure and health care use, lung function, or pulmonary inflammation did not differ by weight.
Conclusion
Being overweight or obese can increase susceptibility to indoor PM2.5 and NO2 in urban children with asthma. Interventions aimed at weight loss might reduce asthma symptom responses to PM2.5 and NO2, and interventions aimed at reducing indoor pollutant levels might be particularly beneficial in overweight children.
Keywords: Asthma, overweight, obesity, indoor pollutants childhood asthma, inner-city asthma
The prevalence of both asthma and overweight status has increased dramatically over the past 20 years, suggesting that these 2 conditions might be linked.1,2 In addition, African Americans, who have greater asthma morbidity than other racial/ethnic groups, also have a higher prevalence of being over-weight or obese.1,2 Several recent studies suggest that being overweight is a risk factor for both having a diagnosis of asthma and asthma morbidity.3–7 Although relationships between being overweight and asthma have been examined in adult asthmatic populations, there are less data in children, particularly in high-risk populations, such as urban minority children, who might be most affected by both being overweight and having asthma.
A few studies suggest that the association between being overweight and asthma morbidity might be explained, in part, by resistance to the treatment effects of corticosteroids.8,9 However, being overweight might confer susceptibility to worse asthma health through mechanisms other than corticosteroid resistance. For example, being overweight is associated with an underlying state of oxidative stress and inflammation, potentially reducing the capacity to defend against oxidative and proinflammatory exposures, such as particulate and gaseous pollutants.10,11 In addition, fine-particle deposition in the lungs is greater in over-weight children, so that lung exposure to a given airborne particulate matter concentration is greater in overweight children than in normal-weight children.12
Because fine particulate matter measuring less than 2.5 μm in diameter (PM2.5), coarse particulate matter measuring between 2.5 and 10 μm in diameter (PM2.5–10), and nitrogen dioxide (NO2) are all associated with respiratory symptoms and rescue medication use in asthmatic patients,13–19 it is plausible that the combination of a high prevalence of overweight status and higher indoor pollutant exposure among urban minority populations contributes to the disproportionate asthma morbidity in this population. We therefore hypothesized that overweight and obese urban children and adolescents with asthma are more susceptible to the effects of indoor pollutants than their normal-weight counterparts. To test our hypothesis, we examined relationships among weight status, asthma health, and indoor pollution in a population of urban, predominantly African American, asthmatic 5- to 17-year-olds.
RESULTS
Study population
The study population was predominantly male, African American, and low income (Table I). At baseline, 4% of the participants were underweight, 52% were normal weight, 16% were overweight, and 28% were obese. The population was highly atopic because 91% had at least 1 positive skin test response and total IgE levels were increased. The population also had a mean of more than 4 days per 2 weeks of rescue medication use, and more than 75% of participants had an emergency department visit for asthma in the previous year (Table II).
TABLE I.
Baseline sociodemographic characteristics (n = 148)
| Age (y), median (range) | 11.2 (5.0–17.8) |
| Male sex, no. (%) | 85 (57) |
| Black/African American, no. (%) | 135 (91) |
| Household annual income <$30,000 no. (%)* | 86 (64) |
| Primary caregiver educational attainment, no. (%) | |
| Less than high school | 42 (28) |
| High school graduate | 51 (34) |
| Some college or more | 55 (37) |
| Public health insurance, no. (%) | 126 (85) |
| Households with smokers, no. (%) | 84 (57) |
n = 134.
TABLE II.
Baseline clinical characteristics (n= 148)
| Allergic characteristics | No. (%) |
| Atopic (≥1 positive SPT response)* | 134 (91) |
| Skin test sensitivities* | |
| Cat | 96 (65) |
| Cockroach | 91 (62) |
| Dust mite | 85 (58) |
| Mouse | 78 (53) |
| Dog | 26 (18) |
| Total IgE (kU/L), median (IQR)† | 190 (56–458) |
| Lung function | Mean ± SD |
| FVC (% predicted)‡ | 100.5 ± 14.9 |
| FEV1 (% predicted)‡ | 93.7 ± 17.9 |
| FEV1/FVC ratio‡ | 80.6 ± 9.6 |
| Feno (ppb), median (IQR)§ | 33 (16–62) |
| Asthma-related health care use (12 mo before enrollment) | No. (%) |
| Hospitalization | 29 (20) |
| Emergency department visit | 121 (82) |
| Unscheduled doctor's office visit | 61 (41) |
| Controller medication use | 106 (72) |
| Days of short-acting β-agonist use/2 wk, mean ± SD | 4.2 ± 5.0 |
| BMI category | No. (%) |
| Underweight (<5th percentile) | 6 (4) |
| Normal weight (5th–<85th percentile) | 77 (52) |
| Overweight (85th–<95th percentile) | 23 (16) |
| Obese (≥95th percentile) | 42 (28) |
FVC, Forced vital capacity; SPT, skin prick test.
n = 147.
n = 145.
n = 132.
n = 131.
BMI and asthma characteristics
Overweight and obese participants had more days of exercise-induced and nocturnal asthma symptoms (over a 2-week period) than normal-weight participants (Fig 1 and Table III). For example, participants who were overweight or obese had approximately 0.7 more days of exercise-related symptoms than normal-weight participants (2.1 ± 3.6 vs 1.4 ± 2.6 [mean ± SD], respectively; P = .05). Overweight or obese participants also had approximately 0.6 more nights of wakening caused by asthma symptoms than normal-weight participants (1.7 ± 3.1 vs 1.1±2.2 [mean±SD], respectively; P=.04). However, there was little difference among BMI categories in other asthma symptoms, such as cough, wheeze, or chest tightness and short-acting β-agonist use. Overall, there were no differences in asthma-related health care use, lung function, or number of positive skin test responses among BMI categories. However, overweight or obese participants tended to have lower Feno values and total IgE levels (see Table E1 in this article's Online Repository at www.jacionline.org).
FIG 1.
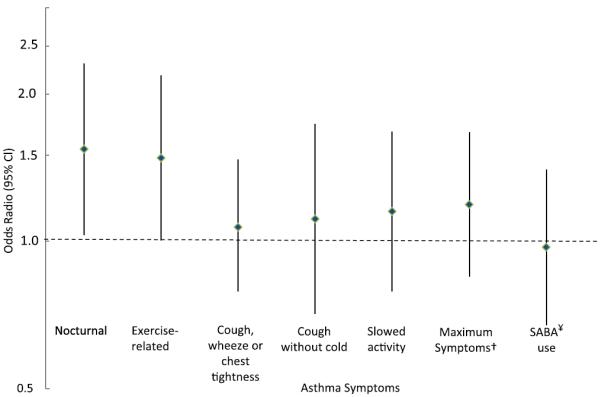
Relative odds of a day of asthma symptoms for overweight or obese participants versus normal-weight participants. Odds ratios and 95% CIs are generated from binomial models adjusted for age and sex; generalized estimating equations were used to account for repeated outcome measures. †Maximum days of slowed activity or exercise-related or nocturnal symptoms. ¥SABA, Short-acting β-agonist.
TABLE III.
Days of asthma symptoms by BMI category (n = 147)
| Symptoms | Normal weight (82 participants,* 376 observations) | Overweight (23 participants,* 104 observations) | Obese (42 participants,* 172 observations) | Pvalue† |
|---|---|---|---|---|
| Cough, wheeze, or chest tightness | 2.6 ± 3.4 | 2.9 ± 3.8 | 3.0 ± 3.7 | .65 |
| Nocturnal | 1.1 ± 2.2 | 1.5 ± 3.3 | 1.8 ± 2.9 | .02 |
| Exercise related | 1.4 ± 2.6 | 2.0 ± 3.6 | 2.2 ± 3.6 | .07 |
| Cough without cold | 1.4 ± 3.0 | 1.6 ± 3.3 | 1.8 ± 3.7 | .48 |
| Slowed activity | 1.9 ± 3.3 | 2.4 ± 3.7 | 2.1 ± 3.4 | .44 |
| Maximum symptom‡ | 2.6 ± 3.7 | 3.1 ± 4.3 | 3.3 ± 4.3 | .17 |
| SABA use | 3.4 ± 4.6 | 3.2 ± 3.9 | 3.5 ± 4.4 | .96 |
Values are presented as means ± SDs.
Number of participants in each BMI category reflects who contributed at least once in each categorical analysis because there were participants who crossed into multiple categories.
Results were derived from binomial models adjusted for age and sex; generalized estimating equations were used for repeated outcome measures. P values shown represent trends across BMI categories, and statistically significant associations are indicated in boldface.
Maximum days of slowed activity or exercise-related or nocturnal symptoms.
TABLE E1.
Lung function, asthma-related health care use, and inflammatory outcomes by BMI category*
| Outcome | Normal | Overweight | Obese |
|---|---|---|---|
| Lung function (mean ± SD) | |||
| FEV1 (% predicted) | 91.7 ± 15.8 | 95.5 ± 16.0 | 97.7 ± 19.5 |
| FEV1/FVC ratio (%) | 80.3 ± 9.3 | 79.7 ± 9.3 | 81.2 ± 7.7 |
| Asthma-related health care use | |||
| Acute care visits (% ever during the 1-y study) | 58 | 54 | 54 |
| Inflammatory outcomes, media | (IQR) | ||
| Feno (ppb) | 43 (19–73) | 20 (13–39) | 24 (13–47) |
| Total IgE (kU/L) | 213 (65–773) | 65 (24–223) | 170 (64–302) |
FVC, Forced vital capacity.
There were no statistically significant differences between any of the pairwise comparisons across BMI categories. Comparisons were made by using binomial models adjusted for age and sex; generalized estimating equations were used for repeated outcome measures.
BMI, indoor pollutant exposure, and asthma symptoms
Relationships between indoor pollutants and symptom outcomes were examined across BMI categories, and data were captured from clinical and home assessments that were performed every 3 months for 1 year. Median PM2.5-10, PM2.5, and NO2 levels were as follows: 13.2 μg/m3 (interquartile range [IQR], 7.9–19.9 μg/m3), 20.8 μg/m3 (IQR, 12.9–34.3 μg/m3), and 20.7 ppb (IQR, 13.7–31.3 ppb), respectively (see Table E2 in this article's Online Repository at www.jacionline.org).
TABLE E2.
Indoor pollutant concentrations
| Median (IQR) | Range | |
|---|---|---|
| PM2.5 (μg/m3) | 20.8 (12.9–34.3) | 1.7–300.3 |
| PM2.5-10 (μg/m3) | 13.2 (7.9–19.9) | 0.3–173.3 |
| NO2 (ppb) | 20.7 (13.7–31.3) | 0.1–351.3 |
There were positive and statistically significant associations between PM2.5 levels and most asthma symptom outcomes among overweight and obese participants, whereas associations among normal-weight participants were either absent or substantially weaker (Fig 2 and Table IV). For example, every 10-fold increase in PM2.5 level was associated with a 3- to 5-fold increase in the odds of cough without a cold among overweight and obese participants but only a 2-fold increase among normal-weight participants (interaction P = .02). Similarly, there was a 2.5- to 2.9-fold increase in the odds of slowed activity with every 10-fold increase in PM2.5 levels among overweight and obese participants compared with a 1.6-fold increase in the odds among normal-weight participants (interaction P =.08). There were no differences seen in relationships between PM2.5 levels and asthma-related health care use, lung function, or Feno values among normal-weight, overweight, and obese participants.
FIG 2.
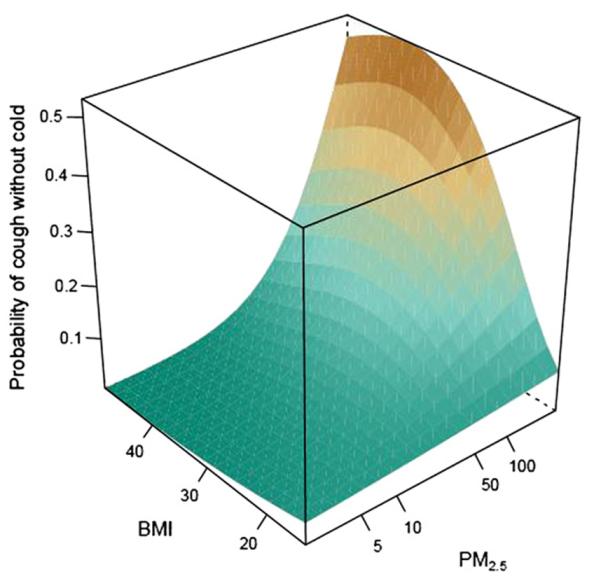
Three-dimensional representation of the predicted probability of cough without cold by BMI and indoor PM2.5 exposure. The figure is derived from a binomial model adjusted for age and sex; generalized estimating equations were used for repeated outcome measures. BMI is expressed in kilograms per square meter, and PM2.5 is expressed in micrograms per cubic meter.
TABLE IV.
Relationships between PM2.5 and symptoms stratified by BMI category (n = 141)*
| Outcome | Normal weight, OR (95% CI) | Overweight, OR (95% CI) | Obese, OR (95% CI) | P value, interaction* |
|---|---|---|---|---|
| Cough, wheeze, or chest tightness | 2.19 (1.18–4.08) | 2.84 (1.16–6.95) | 2.23 (0.99–5.05) | .05 |
| Nocturnal | 1.04 (0.52–2.07) | 5.59 (1.57–19.94) | 3.02 (0.88–10.39) | .24 |
| Exercise related | 1.70 (0.74–3.93) | 4.36 (1.14–16.71) | 3.06 (0.87–10.73) | .16 |
| Cough without cold | 2.06 (0.79–5.37) | 3.17 (1.33–7.57) | 5.00 (1.37–18.22) | .02 |
| Slowed activity | 1.58 (0.75–3.33) | 2.88 (1.15–7.23) | 2.45 (0.78–7.65) | .08 |
| Maximum symptom† | 1.53 (0.79–2.99) | 4.83 (1.49–15.64) | 2.18 (0.71–6.65) | .14 |
| SABA use | 1.97 (1.01–3.84) | 3.31 (1.37–8.03) | 2.26 (0.84–6.03) | .57 |
OR, Odds ratio; SABA, short-acting β-agonist.
Results are from binomial models adjusted for age and sex and account for repeated assessment of the symptom outcome. The P values were generated from models including a nonlinear interaction term for the interaction between BMI and pollutant exposure, and statistically significant associations are indicated in boldface.
Maximum days of slowed activity or exercise-related or nocturnal symptoms.
For PM2.5-10 levels, although associations with some of the symptom outcomes were greater among overweight participants, obese participants, or both than among normal-weight participants, none of the associations between PM2.5-10 levels and symptom outcomes within BMI categories were statistically significant, and none of the interactions between PM2.5-10 levels and BMI were statistically significant (Table V). There were also no differences seen in relationships between PM2.5-10 levels and asthma-related health care use, lung function, or Feno values among BMI categories.
TABLE V.
Relationships between PM2.5-10 and symptoms stratified by BMI category (n = 141)*
| Outcome | Normal weight, OR (95% CI) | Overweight, OR (95% CI) | Obese, OR (95% CI) | P value, interaction |
|---|---|---|---|---|
| Cough, wheeze, or chest tightness | 1.40 (0.83–2.36) | 2.00 (0.31–13.01) | 2.33 (0.96–5.64) | .33 |
| Nocturnal | 1.21 (0.75–1.94) | 1.31 (0.10–17.21) | 1.32 (0.59–2.96) | .55 |
| Exercise related | 1.23 (0.74–2.04) | 0.87 (0.09–8.53) | 2.17 (0.90–5.23) | .62 |
| Cough without cold | 1.71 (0.97–3.04) | 0.96 (0.14–6.52) | 4.67 (0.93–23.50) | .15 |
| Slowed activity | 0.83 (0.49–1.41) | 1.07 (0.20–5.73) | 1.71 (0.86–3.39) | .14 |
| Maximum symptom† | 1.06 (0.65–1.74) | 1.43 (0.24–8.72) | 1.88 (0.88–4.03) | .25 |
| SABA use | 0.97 (0.66–1.44) | 1.19 (0.44–3.21) | 1.83 (0.64–5.27) | .47 |
OR, Odds ratio; SABA, short-acting β-agonist.
Results are from binomial models adjusted for age and sex and account for repeated assessment of the symptom outcome. The P values were generated from models including a nonlinear interaction term for the interaction between BMI and pollutant exposure.
Maximum days of slowed activity or exercise-related or nocturnal symptoms.
There were no associations between NO2 levels and any of the asthma symptom outcomes among normal-weight participants (Table VI). However, NO2 levels were associated with some asthma symptom outcomes among overweight and obese participants. For example, for every 10-fold increase in indoor NO2 levels, overweight and obese participants had a 2.6- to 4.5-fold increased odds of nocturnal symptoms, whereas no association was seen among normal-weight participants. However, BMI did not modify the effect of NO2 exposure on nocturnal symptoms (interaction P =.29). Additionally, overweight and obese participants had a 2.9- to 4.3-fold increase in the odds of exercise-related symptoms for each 10-fold increase in indoor NO2 exposure compared with a 1.5-fold increase in normal-weight participants (interaction P =.05). Similarly, overweight and obese participants had a 1.3- to 2.0-fold increase in the odds of short-acting β-agonist use for each 10-fold increase in indoor NO2 exposure compared with association between NO2 levels and short-acting β-agonist use in normal-weight participants (interaction P = .07). There were no differences seen in relationships between NO2 levels and the other symptom outcomes, asthma-related health care use, lung function, and Feno values among BMI categories (data not shown).
TABLE VI.
Relationships between NO2 and symptoms stratified by BMI category (n = 142)*
| Outcome | Normal weight, OR (95% CI) | Overweight, OR (95% CI) | Obese, OR (95% CI) | P value, interaction |
|---|---|---|---|---|
| Cough, wheeze, or chest tightness | 1.34 (0.83–2.14) | 1.42 (0.65–3.11) | 1.93 (0.77–4.82) | .65 |
| Nocturnal | 0.91 (0.49–1.69) | 4.50 (1.31–15.44) | 2.56 (1.09–5.99) | .29 |
| Exercise related | 1.51 (0.82–2.76) | 2.87 (0.87–9.51) | 4.26 (1.24–14.65) | .05 |
| Cough without cold | 0.71 (0.40–1.25) | 2.56 (1.05–6.23) | 3.18 (0.57–17.66) | .24 |
| Slowed activity | 1.13 (0.65–1.99) | 2.21 (0.84–5.83) | 1.11 (0.30–4.06) | .41 |
| Maximum symptom† | 1.08 (0.66–1.76) | 2.37 (0.85–6.60) | 1.47 (0.42–5.12) | .43 |
| SABA use | 0.89 (0.59–1.35) | 1.31 (0.74–2.31) | 2.05 (0.75–5.61) | .07 |
OR, Odds ratio; SABA, short-acting β-agonist.
Results are from binomial models adjusted for age and sex and account for repeated assessment of the symptom outcome. The P values were generated from models including a nonlinear interaction term for the interaction between BMI and pollutant exposure, and statistically significant associations are indicated in boldface.
Maximum days of slowed activity or exercise-related or nocturnal symptoms.
DISCUSSION
In this urban, predominantly African American population, the effect of indoor PM2.5 exposure on asthma symptoms was greater in overweight and obese children compared with that seen in normal-weight children. We also found a similar pattern of more asthma symptoms associated with indoor NO2 exposure among overweight or obese children than their normal-weight counterparts. Our results suggest that being overweight might increase susceptibility to the pulmonary effects of indoor PM2.5 and NO2 and that the combination of a high prevalence of overweight status and high indoor pollutant exposure in urban children with asthma could explain some of the disproportionate asthma morbidity seen in this population.
Although exposure to indoor pollutants, including PM2.5 and NO2, is known to be associated with worse asthma health, the effect of being overweight on the relationships between indoor pollutant exposure and asthma health has not previously been examined to our knowledge. In fact, there are biologically plausible mechanisms by which being overweight could confer susceptibility to the pulmonary effects of pollutants in asthmatic patients. First, being overweight is associated with an underlying state of oxidative stress and inflammation, potentially reducing the capacity of overweight persons to defend against oxidative and proinflammatory exposures, such as pollutants. Second, overweight and obese children have greater pulmonary deposition of fine particles than normal-weight children, so that lower airways exposure to a given airborne particulate pollution concentration is greater in overweight than in normal-weight children. Third, it is possible that corticosteroid resistance, which has been reported in overweight or obese children and adults with asthma,8,9 could account for greater respiratory effects of indoor pollutant exposure in overweight asthmatic participants. Interestingly, BMI did not modify the relationship between PM2.5-10 levels and asthma symptoms, as was observed for PM2.5 and NO2 levels. Although the reasons for this finding are not clear, it is possible that overweight children have greater fine-particle deposition in the lungs but that coarse-particle deposition, which occurs in the more proximal airways, is less affected by weight.
In contrast to other studies that found associations between weight and asthma morbidity,26,27 we found that although overweight and obese urban children and adolescents with asthma appear more likely to have nocturnal and exercise-related symptoms than normal-weight children and adolescents, other measures of asthma morbidity did not vary by weight. However, our findings are more consistent with findings from an urban minority adult population in which there was no association between BMI and asthma control.28 Because the only asthma symptoms that were increased in the overweight and obese participants were nocturnal and exercise-related symptoms, it is possible that these symptoms are a manifestation of obesity-related comorbidities, such as sleep-disordered breathing and deconditioning, respectively, rather than poor asthma control. We considered whether the overweight and obese children had been given an incorrect diagnosis of asthma; however, the overweight and obese participants had features consistent with asthma, such as decreased FEV1/forced vital capacity ratios and higher Feno values. In addition, the degrees of pulmonary obstruction and inflammation observed in the overweight and obese participants were similar to those observed in normal-weight participants and other pediatric asthma populations.9 The positive association between BMI category and FEV1 percentage, although unexpected and not well understood, has been described in at least 1 other pediatric population.29 Therefore these findings suggest that future studies should determine whether overweight children and adolescents with asthma are at risk for underrecognition and undertreatment of overweight-associated comorbidities, the symptoms of which are incorrectly attributed to asthma.
Because our study population was composed predominantly of African American children and adolescents living in Baltimore City, our findings might not be generalizable to all children and adolescents with asthma. However, our study population is similar to pediatric asthmatic populations in other large urban centers in the Northeastern and Midwestern United States, so that being overweight might confer susceptibility to the effects of indoor pollutants in other urban minority populations. Secondhand smoke exposure is known to be a major contributor to indoor PM2.5. Although our results suggest that being overweight might increase susceptibility to the pulmonary effects of indoor PM2.5, the contribution of secondhand smoke exposure to this finding is unclear. In addition, although the associations between risk of symptoms and pollutant concentrations were expressed per 10-fold increase in the pollutant levels, we did observe 10-fold differences in pollutant concentrations in our study population. Our findings of the association between weight status and the effect of indoor pollutant exposure on childhood asthma were limited to symptom outcomes. This is not surprising because symptoms are the most robust asthma outcomes in this patient population, particularly with respect to pollutant exposure.14,15,18,19,23 Although the symptom outcomes are self-reported, symptoms were captured by using questions that have been used successfully for more than a decade in other inner-city populations.23,30 In addition, lung function outcomes in particular are known to be less sensitive than other measures of disease burden in pediatric populations.31–33 Larger studies that provide greater statistical power are needed to determine whether weight status has similar effects on these other measures of asthma health.
Even though multiple symptom outcomes were assessed, we did not apply statistical methods to correct for multiple analyses because they are overly conservative, particularly when the multiple outcomes assessed are related to one another.34 In addition, the threshold of a P value of less than .1 that was used for interactions differs from the P value threshold of .05 typically used for main effects, but it is a commonly used threshold for interactions.35,36 However, it is notable that although only 1 of the interactions met a more conservative statistical significance threshold (P < .05), there were consistent relationships across symptom outcomes, and the consistency in the pattern provides additional evidence for an interaction. Larger studies that provide greater statistical power are needed to confirm the effects we have observed of weight status on relationships between pollutant exposure and asthma symptoms.
In this urban, predominantly African American population, the effects of indoor PM2.5 and NO2 exposure on asthma symptoms are greater in overweight and obese than normal-weight children and adolescents. Children and adolescents with asthma in communities with a high prevalence of overweight status and indoor pollutant exposure might have greater asthma morbidity because of the interaction between these 2 factors. These findings suggest that weight loss might reduce symptom responses to these indoor pollutants and that overweight and obese children and adolescents might benefit from indoor pollutant reduction to a greater extent than normal-weight children and adolescents.
METHODS
Lung function testing
Lung function, including FEV1 and forced vital capacity, was assessed by using forced expiratory spirometry. Predicted values and percent predicted values were determined by using the standards of Hankinson et al.E1 All pulmonary function testing maneuvers were overread to determinevalidity of the data.
Questionnaires
A structured questionnaire that included symptom questions that have been successful in inner-city populations for more than a decade was administered.E2 The asthma symptom outcomes were captured from the following questions:
-
●
“In the last 2 weeks, how many days did [child] have wheezing, coughing or tightness in the chest?”
-
●
“In the last 2 weeks, how many days did [child] have to slow down or stop his/her activities while at home or playing with other children because of asthma, wheezing or tightness in the chest or cough?”
-
●
“In the last 2 weeks, how many days has [child]'s wheezing ever been so bad that he/she could only speak one or two words at a time between breaths?”
-
●
“In the last 2 weeks, has [child] had wheezing, coughing, tightness in the chest when running or going up stairs?”
-
●
“In the last 2 weeks, has [child] had a cough that wasn't from a cold?' If yes, how many days?”
-
●
“In the last 2 weeks, did [child] wake up at night with cough, wheeze, shortness of breath or tightness in chest?”
Recruitment
Participants were recruited primarily from patients seen for asthma in the Pediatric Emergency Department at Johns Hopkins Hospital. In addition, participants were recruited from subspecialty clinics, including the Pediatric Allergy/Immunology Clinic at Johns Hopkins and the University of Maryland, and an institutional review board-approved database of past study participants who had consented to being contacted for future studies.
Clinical implications: Being overweight might increase susceptibility to the pulmonary effects of indoor PM2.5 and NO2 exposure in urban children with asthma. Interventions aimed at weight loss might reduce asthma symptom responses to indoor PM2.5 and NO2. Interventions aimed at reducing indoor pollutant levels could be particularly beneficial in overweight children and adolescents.
Acknowledgments
Supported by the National Institute of Environmental Health Sciences (P50ES015903, P01ES018176), the Environmental Protection Agency (R832139), the National Institute of Allergy and Infectious Diseases (R01AI070630), and the Johns Hopkins University School of Medicine General Clinical Research Center (grant no. M01-RR00052) from the National Center for Research Resources/National Institutes of Health.
P. N. Breysse has received a grant from the National Institutes of Health (NIH). G. B. Diette has received grants from the NIH, the National Institute of Environmental Health Sciences (NIEHS), and the US Environmental Protection Agency and has consultant arrangements with GlaxoSmithKline and Fenzian. R. D. Peng has received grants from the NIH and receives royalties from Springer Publishing. M. C. McCormack has received a grant from the NIEHS and has consultant arrangements with Alexza Pharmaceuticals. E. C. Matsui has received a grant from the NIH and received the Phadia Research Foundation Award.
We thank Mary Beth Bollinger, DO (University of Maryland), for her support in recruiting patients for this study.
Abbreviations used
- BMI
Body mass index
- Feno
Fraction of exhaled nitric oxide
- IQR
Interquartile range
- NO2
Nitrogen dioxide
- PM2.5
Fine particulate matter measuring less than 2.5 μm in diameter
- PM2.5–10
Coarse particulate matter measuring between 2.5 and 10 μm in diameter
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. Surveillance for asthma—United States, 1980–2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–6. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor B, Mannino D, Brown C, Crocker D, Twum-Baah N, Holguin F. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008;63:14–20. doi: 10.1136/thx.2007.082784. [DOI] [PubMed] [Google Scholar]
- 5.Grammer LC, Weiss KB, Pedicano JB, Kimmel LG, Curtis LS, Catrambone CD, et al. Obesity and asthma morbidity in a community-based adult cohort in a large urban area: the Chicago Initiative to Raise Asthma Health Equity (CHIRAH) J Asthma. 2010;47:491–5. doi: 10.3109/02770901003801980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll CL, Bhandari A, Zucker AR, Schramm CM. Childhood obesity increases duration of therapy during severe asthma exacerbations. Pediatr Crit Care Med. 2006;7:527–31. doi: 10.1097/01.PCC.0000243749.14555.E8. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland ER, Lehman EB, Teodorescu M, Wechsler ME. National Heart, Lung, and Blood Institute's Asthma Clinical Research Network. Body mass index and phenotype in subjects with mild-to-moderate persistent asthma. J Allergy Clin Immunol. 2009;123:1328–34.e1. doi: 10.1016/j.jaci.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutherland E, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178:682–7. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedón JC, et al. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127:741–9. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holguin F, Fitzpatrick A. Obesity, asthma, and oxidative stress. J Appl Physiol. 2010;108:754–9. doi: 10.1152/japplphysiol.00702.2009. [DOI] [PubMed] [Google Scholar]
- 11.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–9. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 12.Bennett WD, Zeman KL. Effect of body size on breathing pattern and fine-particle deposition in children. J Appl Physiol. 2004;97:821–6. doi: 10.1152/japplphysiol.01403.2003. [DOI] [PubMed] [Google Scholar]
- 13.Breysse PN, Diette GB, Matsui EC, Butz AM, Hansel NN, McCormack MC. Indoor air pollution and asthma in children. Proc Am Thorac Soc. 2010;7:102–6. doi: 10.1513/pats.200908-083RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect. 2010;118:449–57. doi: 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormack MC, Breysse PN, Matsui EC, Hansel NN, Williams D, Curtin-Brosnan J, et al. In-home particle concentrations and childhood asthma morbidity. Environ Health Perspect. 2009;117:294–8. doi: 10.1289/ehp.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delfino RJ, Quintana PJ, Floro J, Gastañaga VM, Samimi BS, Kleinman MT, et al. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environ Health Perspect. 2004;112:932–41. doi: 10.1289/ehp.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delfino RJ, Zeiger RS, Seltzer JM, Street DH, McLaren CE. Association of asthma symptoms with peak particulate air pollution and effect modification by anti-inflammatory medication use. Environ Health Perspect. 2002;110:A607–17. doi: 10.1289/ehp.021100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabinovitch N, Strand M, Gelfand EW. Particulate levels are associated with early asthma worsening in children with persistent disease. Am J Respir Crit Care Med. 2006;173:1098–105. doi: 10.1164/rccm.200509-1393OC. [DOI] [PubMed] [Google Scholar]
- 19.Hansel NN, Breysse PN, McCormack MC, Matsui EC, Curtin-Brosnan J, Williams DL, et al. A longitudinal study of indoor nitrogen dioxide levels and respiratory symptoms in inner-city children with asthma. Environ Health Perspect. 2008;116:1428–32. doi: 10.1289/ehp.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric references values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society/European Respiratory Society ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 23.Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans R, 3rd, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 24. [Accessed September 2011];CDC BMI percentile calculator for child and teen. Available at: http://apps.nccd.cdc.gov/dnpabmi/.
- 25.Breysse PN, Buckley TJ, Williams D, Beck CM, Jo SJ, Merriman B, et al. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. Environ Res. 2005;98:167–76. doi: 10.1016/j.envres.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Quinto KB, Zuraw BL, Poon KY, Chen W, Schatz M, Christiansen SC. The association of obesity and asthma severity and control in children. J Allergy Clin Immunol. 2011;128:964–9. doi: 10.1016/j.jaci.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 27.Mosen DM, Schatz M, Magid DJ, Camargo CA., Jr The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol. 2008;122:507–11. doi: 10.1016/j.jaci.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Clerisme-Beaty EM, Karam S, Rand C, Patino CM, Bilderback A, Riekert KA, et al. Does higher body mass index contribute to worse asthma control in an urban population? J Allergy Clin Immunol. 2009;124:207–12. doi: 10.1016/j.jaci.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL. Childhood Asthma Management Program Research Group. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003;58:1036–41. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 31.Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF, Jr, Sorkness CA. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170:426–32. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins HA, Cherniack R, Szefler SJ, Covar R, Gelfand EW, Spahn JD. A comparison of the clinical characteristics of children and adults with severe asthma. Chest. 2003;124:1318–24. doi: 10.1378/chest.124.4.1318. [DOI] [PubMed] [Google Scholar]
- 33.Lang AM, Konradsen J, Carlsen KH, Sachs-Olsen C, Mowinckel P, Hedlin G, et al. Identifying problematic severe asthma in the individual child—does lung function matter? Acta Paediatr. 2010;99:404–10. doi: 10.1111/j.1651-2227.2009.01625.x. [DOI] [PubMed] [Google Scholar]
- 34.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 35.Fessler MB, Massing MW, Spruell B, Jaramillo R, Draper DW, Madenspacher JH, et al. Novel relationship of serum cholesterol with asthma and wheeze in the United States. J Allergy Clin Immunol. 2009;124:967–74. doi: 10.1016/j.jaci.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visness CM, London SJ, Daniel JL, Kaufman JS, Yeatts KB, Siega-Riz AM, et al. Association of obesity with IgE levels and allergy symptoms in children and adolescents: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2009;123:1163–9. doi: 10.1016/j.jaci.2008.12.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric references values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- E2.Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans R, 3rd, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]


