Abstract
Microbeam/3D human tissues/Arabidopsis/Bystander effects/Genomic instability
Recent developments in microbeam technology have made drastic improvements in particle delivery, focusing, image processing and precision to allow for rapid advances in our knowledge in radiation biology. The unequivocal demonstration that targeted cytoplasmic irradiation results in mutations in the nuclei of hit cells and the presence of non-targeted effects, all made possible using a charged particle microbeam, results in a paradigm shift in our basic understanding of the target theory and other radiation-induced low dose effects. The demonstration of a bystander effect in 3D human tissue and whole organisms have shown the potential relevance of the non-targeted response in human health. The demonstration of delayed mutations in the progeny of bystander cells suggest that genomic instability induced following ionizing radiation exposure is not dependent on direct damage to cell nucleus. The identification of specific signaling pathways provides mechanistic insight on the nature of the bystander process.
INTRODUCTION
The use of microbeam irradiation techniques in radiobiological studies dates back more than five decades ago to the work of Zirkle and Munro (please refer to the accompanied review article by Professor Barry Michael). While these earlier studies with microbeam were highly innovative, they are far from precise. For example, a polonium tipped syringe needle was used to deliver alpha particles to either the nucleus or cytoplasm of Chinese hamster fibroblasts.1) As such, neither the dose nor target could be precisely controlled. Nevertheless, some important observations were made regarding the differential sensitivity of the nucleus versus cytoplasm. The development of single particle microbeams, where a single cell and/or a subcellular compartment can be selectively irradiated with either one or multiple particles, has greatly facilitated our understanding of a variety of biological endpoints including cytoplasmic irradiation, bystander effects and genomic instability. With recent improvements made in particle delivery, focusing, image processing and detection in microbeam technology, significant advances in radiobiological sciences have been made that result in a paradigm shift in our basic understanding of radiation-sensitive targets and other delayed effects.
Biological consequence of targeted cytoplasmic irradiation
Generations of students in radiation biology have been taught that heritable biological effects induced by ionizing radiation are the consequence of a direct radiation-nuclear interaction. Using the Columbia University charged particle microbeam and the human hamster hybrid (AL) cell mutagenic assay, there is evidence that targeted cytoplasmic irradiation is mutagenic in mammalian cells.2) The shape of the dose response curves for mutation induced by cytoplasmic irradiation is quite different from that of nuclear irradiation. For the cytoplasmic irradiation, the mutation induction curve initially increased with the number of particle traversals reaching a peak of 125 ± 58 per 105 survivors at 8 particles, this represents an increase of ~3 fold over background. The curve showed a saturation effect with particle traversal higher than 8 whereas nuclear irradiation demonstrated a clear dose dependent induction. Furthermore, by comparing the mutant fractions induced by alpha particles striking the cell nucleus or just the cytoplasm at an equitoxic dose which results in 90% survival level, cytoplasmic irradiation was ~7 fold more mutagenic than nuclear targeting. These findings suggest that cytoplasmic traversal by alpha particles may be more harmful than nuclear traversal since the mutagenicity is accomplished by little or no killing of the target cells. Using multiplex polymerase chain reaction analyses, it has been shown that the types of mutations induced by cytoplasmic irradiation were completely different from those induced by direct nuclear irradiation indicating that different mutagenic mechanism was involved.2)
Cytoplasmic damage implies extranuclear targets
The development of single particle microbeams has greatly facilitated our understanding of the bystander phenomenon. To demonstrate the induction of a radiation induced bystander effect unequivocally, studies were conducted using a microbeam where a defined proportion of cells in a confluent monolayer were irradiated individually with a lethal dose of 20 alpha particles.3) Since dead cells could not produce mutants, the progeny of the irradiated population, in the absence of any interaction between the hit and non-hit cells, should result in a mutant fraction that was comparable to the non-hit cells, i.e. background levels. In actuality, when the experiments were completed, it was clear that the non-clonogenically viable cells had influenced the mutant fraction of the non-hit cells since the incidence among the progeny was 3–4 times higher than expected. This study provided the first clear-cut indication of a radiation-induced bystander phenomenon.
Using microbeam technology, a variety of biological endpoints in both human and other mammalian cell lines have firmly established the presence of a bystander effect under either confluent or sparsely populated culture conditions. Table 1 lists all the endpoints that have been examined thus far using microbeams with charged particles, protons or soft X-rays. In general, as few as one cell in a population that is targeted by a single particle has been shown to be sufficient in eliciting a bystander response.4) Furthermore, increasing the number of particle traversals per cells or the total dose delivered to the irradiated fraction does not increase the intensity of the bystander response. Thus, there is no evidence of a dose response in any of the biological endpoints examined thus far.
Table 1.
Biological endpoints that have been used to demonstrate the presence of a bystander effect
| Clonogenic survival |
| Mutation |
| Neoplastic transformation |
| γH2AX foci induction |
| Apoptosis |
| Chromosomal aberrations |
| Micronucleus |
| Intracellular oxidant levels |
| DNA damage signaling |
| Length and number of root hair in Arabidopsis |
Can cytoplasmic irradiation induce a bystander effect?
Since targeted cytoplasmic irradiation induces genotoxic damage to the nucleus of the hit cells, it is possible that the damage signals can result in a non-targeted response among neighboring, non-hit cells. Indeed, using a charged-particle microbeam to target a single alpha particle to individual glioma cells through the cytoplasm, there is evidence of a bystander response in the neighboring, non-irradiated glioma or fibroblasts such that the yield of micronuclei was increased by 36% for the glioma population and 78% for the bystander fibroblast population.4) Importantly, the yield of bystander-induced micronuclei was independent of whether the cytoplasm or nucleus of a cell was targeted. This finding shows that direct DNA damage is not required for switching on important cell-signaling mechanisms after low-dose irradiation and that, under these conditions, the whole cell should be considered a sensor of radiation exposure.
Non-targeted response in 3D human tissues
A reconstituted 3-dimensional human skin model has been used to ascertain the validity of the bystander effect in a simulated in vivo environment using microbeam irradiation.5) The MatTek EPI-200 is a skin tissue model constructed from neonatal foreskin derived epidermis that is multilayered, differentiated tissue consisting of basal, cornified layers similar to human epidermis. The tissue samples were irradiated from below through the membrane that forms the base of the culture with a repositioning accuracy of better than 2 µm. A given alpha-particle will traverse 5–10 cells as it penetrates the tissue. Un-irradiated cells up to 1 mm distant from irradiated cells showed a significant enhancement in bystander effect over background, with an average increase in effect of 1.7-fold for micronuclei and 2.8-fold for apoptosis. The surprisingly long range of bystander signals in human tissue suggests that bystander responses may be important in extrapolating radiation risk estimates from epidemiologically accessible doses down to very low doses where non-hit bystander cells will predominate.
Non-targeted response in whole organism
The non-targeted response indicated that the target size of the responding cells/tissues is much larger than the hit cells. However, it remains to be seen whether this response is limited to the specific organ irradiated, whether it spans a limited region of the body, or even extend throughout the whole body of the target. To determine whether long-distance bystander/abscopal effects exist in whole organisms and to clarify the problem of intercellular communication, the shoot apical meristem in Arabidopsis embryo was irradiated with a defined number of protons and examined for root development post-irradiation.6) The results showed that after direct damage to the shoot apical meristem from 1,000 proton traversals (proton energy 2.6 MeV with a beam size of 10 µm), root hair differentiation, primary root elongation and lateral root initiation were all inhibited significantly in postembryonic development, suggesting that radiation-induced long-distance bystander/abscopal responses exist in the whole organism. Furthermore, treatment with either 2,4-dichlorophenoxyacetic acid, a synthetic plant auxin, or DMSO, an effective reactive oxygen species (ROS) scavenger, could rescue the damaged response in the length of primary root in irradiated shoot apical meristem embryos, indicating that ROS or probably the ROS related auxin and auxin-dependent transcription process may be involved in radiation-induced long-distance bystander/abscopal effects.6)
Genomic instability in bystander cells
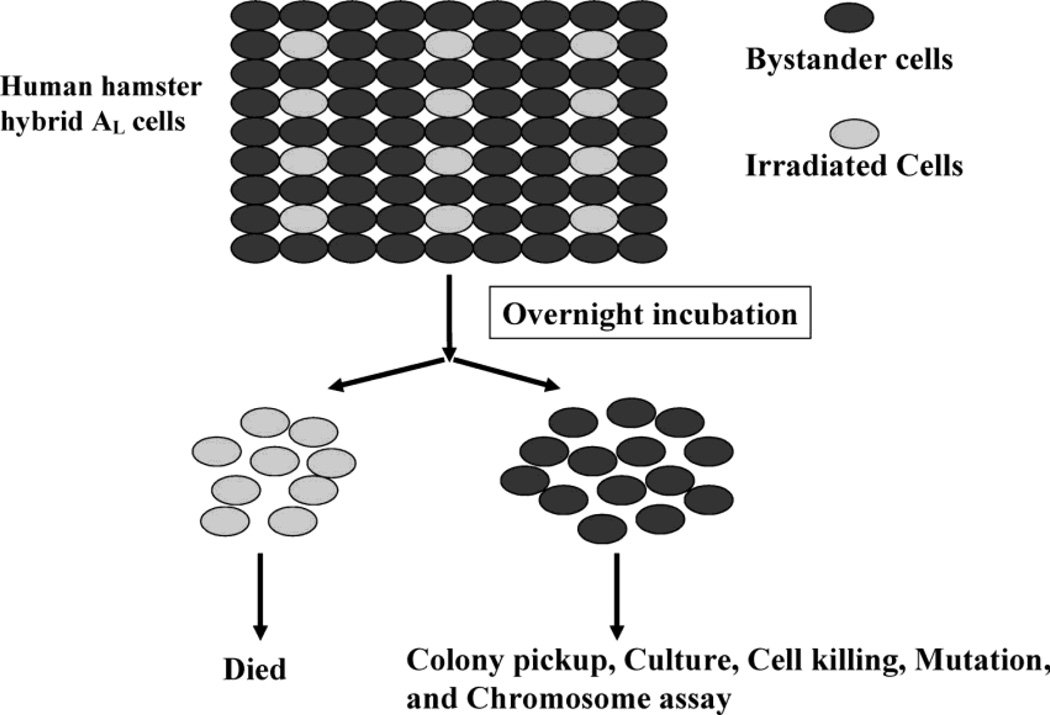
Although ionizing radiations have been widely studied as a human carcinogen, and are known to induce malignancies in a wide variety of tissues, the underlying mechanisms at the cellular and molecular level are not known with any certainty. An attractive hypothesis is that radiation induces “genomic instability” in a tissue or population of cells that is the source of the multiple mutational events that appear to be required in the transformation of a normal tissue into a metastasizing invasive tumor.7) Table 2 lists all the available biological endpoints used in measuring genomic instability under both in vitro and in vivo conditions. The notion is that a mutation may occur in a gene responsible for the stability of the genome and the fidelity of replication, resulting in what has been referred to as mutator phenotype, i.e. a single induced mutation followed by a cascade of further mutations. Support of this concept comes from the observation of microsatellite instability in a wide range of human tumors8,9) Genomic instability and the bystander effect have one thing in common, namely that both involve non-targeted effects in non-irradiated cells, exhibiting responses typically associated with direct radiation exposure, but occurring in one case in the progeny of irradiated cells and in the other case in the close neighbors of irradiated cells. The observations that 1) several cell cycle checkpoint genes such as cyclin B1 and RAD 51 have been shown to be over-expressed in radiation induced bystander cells,10) and 2) that DNA repair deficient cells have a higher bystander chromosomal aberration and mutagenic response11) provide a possible link between genomic instability and bystander response, though direct evidence has yet to be demonstrated. Recent unpublished studies obtained using the Columbia University charged particle microbeam and the highly sensitive human hamster hybrid (AL) cell mutagenic assay provided some clues between the two phenomena. As shown below in Fig. 1, studies were conducted to lethally irradiate 10% of the AL cell population with 30 α-particles. After overnight incubation, the bystander cells were individually cloned and the incidence of CD59− mutations and chromatid breaks based on G2 phase premature chromosome condensation assay were assessed over a period of 20–30 generations. Both the mutation incidence and the chromatid breaks per cells were found to be elevated in a biphasic fashion over a period of many generations post-irradiation, indicative of genomic instability among the progeny of the bystander cells.
Table 2.
Radiation induced genomic instability
|
Fig. 1.
Do progeny of bystander cells demonstrate genomic instability?
Mechanism of the bystander effect
The plethora of data now available concerning the bystander effect fall into two categories 1) in confluent cultures where physical contacts between irradiated and non-irradiated cells are made and where gap junctional communications have been shown to be essential for the process; 2) in sparsely populated cultures where bystander effects may be mediated by damage signals released into the culture medium by the irradiated cells. As a result, incubation of non-irradiated cells with conditioned medium from irradiated cultures may lead to biological effects in these bystander cells. Since the nature of the signaling molecules involved in the two bystander pathways are not known, their mechanisms are not mutually exclusive at this moment. In fact, it is likely that some common initiating or intermediate steps are involved in the two processes.12)
Role of gap junctional communication in the induction of bystander effect
The relationship between gap junctional activity and radiation-induced bystander mutagenicity was investigated in two ways: 1) the use of chemicals such as octanol and lindane to inhibit gap junction-mediated intercellular communication; and 2) using genetically engineered cells that lack gap junctions. In the first set of studies using microbeam-generated alpha particles, AL cells were treated with a non-toxic, and largely non-mutagenic dose of octanol (1 mM) beginning 2 hr before and up to 3 days after irradiation. Octanol reduced the yield of induced CD59− mutants from 92 ± 35 to 16 ± 3 per 105 survivors.3) Treatment of octanol alone resulted in a low but detectable mutant fraction of ~10 ± 4. Similar results were also obtained using tritiated thymidine labelled cells.13) Although these results indicate a role of gap junctions in the bystander mutagenic response, octanol and lindane are non-specific inhibitors of gap junctions, and can have wide ranging effects on other cellular structures and functions including membrane fluidity. Therefore, to investigate more specifically the role of gap junction mediated cell-to-cell communication with alpha particle-induced bystander mutagenicity, it is necessary to use cells in which gap junctional activity was suppressed by a dominant negative connexin construct. Cells containing the dominant negative connexin 43 vector showed little or no bystander mutagenesis.14,15) In contrast, cells containing the empty vector control showed little or no suppression of the bystander effect. These data clearly show that the connexin 43 vector is working well in the transfected cells and that gap junction intercellular communication is critical in mediating the bystander mutagenic process.
Nature of the signaling molecule(s): the million dollar question
To identify the signaling pathways involved in radiation-induced bystander effect, genes that are differentially expressed among the bystander versus control cells have been examined. A novel mylar ring design consisting of two concentric stainless steel rings fitted with mylar bottoms of different thickness has been used to study signaling cascade in bystander cells. The outer ring is fitted with a thin (6 µM) mylar bottom and the inner ring has thicker (38 µM) strips of mylar thereby creating a bystander population of cells located next to directly hit cells in a confluent culture. Experiments have been conducted with human lung fibroblasts (NHLF) to identify differentially expressed genes between directly irradiated and bystander cells.16) Among the 96 genes represented on the platform, the abundance of one message, cyclooxygenase-2 (COX-2), was found to be consistently higher by more than three-fold, while the RNA level of insulin-like growth factor binding protein-3 (IGFBP3) was found to be consistently lower by more than seven-fold in several analyses of multiple bystander samples. Semi-quantitative reverse transcription (RT) PCR was used to confirm the expression levels of these two genes, by comparing expression levels to the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene as an internal control. Expression of the COX-2 protein in the non-irradiated bystander cells was further confirmed by Western blotting. Addition of the COX-2 inhibitor NS-398 (50 µM) suppressed COX-2 activity in NHLF cells and bystander mutagenesis at the hypoxanthine guanine phosphoribosyl-transferase (HPRT) locus.16) These results indicated that expression of COX-2 is associated with the bystander effect.
A decrease in insulin-like growth factor binding protein-3 level increases the binding of insulin growth factor to cell surface receptors, which activates the downstream signaling events, including among others, mitogen activated protein kinase (MAPK). Activation of extracellular signal-related kinase (ERK) by phosphorylation is a critical upstream event preceding COX-2 expression. Previous studies have shown a strong up-regulation of phospho-ERK levels in bystander NHLF four hours after treatment and that the ratio of phos-phorylated ERK relative to native ERK increased from 2 to 13 among bystander cells.16) The observations that addition of PD 98059 (50 µM), a specific inhibitor of MEK-ERK, to the culture medium suppressed bystander effects provided further evidence of the role of ERK in the signaling scheme. Using the medium transfer approach, similar findings on the role of ERK signaling pathways have also been documented with immortalized human keratinocytes.17)
Nuclear factor kappa B as the center of the bystander signaling process
Since NFκB is an important transcription factor for many signaling genes including COX-2, it is likely that NFκB participates in the bystander response. There is clear evidence that alpha particle irradiation upregulates NFκB binding activity in both directly irradiated and bystander cells, while Bay 11–7082, a pharmacological inhibitor of IKK/NFκB, efficiently suppresses this up-regulation and also reduces levels below the basal amount.18) This inhibitor of NFκB activity also efficiently down-regulates COX-2 and iNOS-expression levels in both directly irradiated and bystander fibroblasts. Earlier studies using confluent human skin fibroblasts exposed to a low fluence of alpha particles show a rapid up-regulation of NFκB, JNK and ERK in the exposed population.19) and suggested activation of these stress inducible signaling pathways in bystander cells. Furthermore, addition of the antioxidant superoxide dismutase (SOD) was found to suppress the induction. Since induction of NFκB binding activity can be found in both directly irradiated and bystander cells, its role in the bystander response in this study is equivocal.
Role of reactive radical species in the bystander response
Reactive oxygen species including hydroxyl radical and superoxide anions have been implicated in various medium-mediated bystander responses using a variety of endpoints.12 for review) Since almost all reactive oxygen species have relatively short half-lives, it is likely that they are generated either very close to the target sites or are produced through a continuous cascade of events in order for them to be relevant. There is evidence that the radical generating scheme of nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) is involved in the bystander response19,20) Furthermore, these short-lived, highly reactive radical species have been postulated to be important in the secondary generation of long-lived organic radicals that cause mutations and transformation in human cells.21)
The role of reactive nitrogen species, particularly nitric oxide, in the bystander response using a variety of endpoints has been extensively investigated. Using a charged particle microbeam, there is evidence that in bystander cells treated with the nitric oxide scavenger, 2-(4-Carboxyphenyl)-4, 4, 5, 5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO), the induction of micronuclei4,22) was significantly reduced, suggesting the role of NO, particularly the constitutive form, in mediating bystander effects.
Mitochondria as an important contributor to the bystander signaling process
The observations that extracellularly applied antioxidant enzymes such as superoxide dismutase23) and catalase17) can inhibit the medium-mediated bystander response suggest a role for reactive radical species in the bystander process. Since mitochondria are the main source of energy production as well as generators of free radicals in cells, especially in pathological and stressful conditions, they are the prime target for the source of these radical species. There is recent evidence that mitochondrial function plays a critical role in modulating biological response of targeted cytoplasmic irradiation (Hei et al. unpublished observation). Using human fibroblasts that are devoid of mitochondrial DNA and, consequently, reduced mitochondrial DNA functions, there is evidence that mitochondria play an important role in the regulation of radiation-induced bystander effects.18)
SUMMARY AND FUTURE DIRECTION
For over a century since the discovery of X-rays, it has always been accepted that the deleterious effects of ionizing radiation such as mutation and carcinogenesis are due mainly to direct damage to DNA. With advances in microbeam technology, it has been made possible to demonstrate unequivocally that cells and tissues that are not directly exposed to radiation, but merely in the vicinity of ones that are, can contribute to the radiobiological response; this represents a major paradigm shift in our understanding of the target theory and other low dose phenomena. There is increasing evidence that the non-targeted response can equally be demonstrated in multicellular organism using a microbeam. The observations that gap junctional communication and the COX-2 signaling pathways are causally linked to the bystander process provide a mechanistic interpretation of the steps involved in the process. A better understanding of the mechanism of the bystander effect is important for an accurate assessment of cancer risk associated with low dose radiation exposure.
Although many of the non-targeted responses reported thus far using microbeams have been detrimental in nature, e.g. oncogenic transformation, mutations and chromosomal aberrations,7,12) there are reported protective effects as well, e.g induction of terminal differentiation24) and apoptosis of potentially damaged cells.25) On the other hand, and discussed previously, there is evidence that bystander cells also show increase in genomic instability, a predisposing factor for carcinogenesis. Hence the contribution of bystander/non-targeted effects in radiation risk assessment has to be evaluated in terms of tissue context, the phenotypic behavior of their progeny and the presence of other competing, low dose effects which include adaptive response, genomic instability and individual genetic susceptibility.
Thus far, most of the published data on the bystander effects have been largely phenomenological in nature. In the future, mechanistic based studies that can provide insight on the nature of the signaling molecule(s), the clinical relevance of the bystander effects and ways in which the bystander phenomenon can be manipulated to increase therapeutic gain in radiotherapy should be considered as top priority for investigators in the field.
ACKNOWLEDGEMENTS
Work supported by funding from the National Institutes of Health grants CA 49062, ES 12888, ES 11804, P41EB002033 and the Environmental Center grant ES 09089.
REFERENCES
- 1.Munro TR. The relative radiosensitivity of the nucleus and cytoplasm of Chinese hamster fibroblasts. Radiation Research. 1970;42:451–470. [PubMed] [Google Scholar]
- 2.Wu LJ, Randers-Pehrson G, Waldren CA, Geard CR, Yu ZY, Hei TK. Targeted cytoplasmic irradiation by alpha particles induces gene mutations. Proc. National Academy Science (U.S.A.) 1999;96:4959–4964. doi: 10.1073/pnas.96.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc. National Academy Science (U.S.A.) 2000;97:2099–2104. doi: 10.1073/pnas.030420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shao CL, Folkard M, Michael BD, Prise KM. Targeted cytoplasmic irradiation induces bystander responses. Proc. National Academy Science (U.S.A.) 2004;101:13495–13500. doi: 10.1073/pnas.0404930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belyakov OV, Mitchell SA, Parikh D, Randers-Pehrson G, Marino SA, Amundson SA, Geard CR, Brenner DJ. Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proc. National Academy Science (U.S.A.) 2005;102(40):14203–14208. doi: 10.1073/pnas.0505020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang G, Wu LJ, Chen LY, Pei B, Wang YG, Zhan F, Wu YJ, Yu ZY. Targeted irradiation of shoot apical meristem of Arabidopsis embryos induces long distance bystander/ abscopal effects. Radiation Research. 2007;167:298–305. doi: 10.1667/RR0710.1. [DOI] [PubMed] [Google Scholar]
- 7.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation I: Radiation induced genomic instability and bystander effects in vitro. Radiation Research. 2003;159:567–580. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Loeb LA, Loeb KR, Anderson JP. Multiple mutations and cancer. Proc. National Academy Science (U.S.A.) 2003;100(40):776–781. doi: 10.1073/pnas.0334858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azzam EI, deToledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiation Research. 1998;150:497–504. [PubMed] [Google Scholar]
- 11.Nagasawa H, Huo L, Little JB. Increased bystander mutagenic effect in DNA double-strand break repair deficient mammalian cells. Int. J. Radiation Biology. 2003;79:35–41. [PubMed] [Google Scholar]
- 12.Hei TK, Zhou H, Ivanov VN, Mei H, Lieberman HB, Brenner DJ, Amundson SA, Geard CR. Mechanism of radiation induced bystander effects: A unifying model. Journal of Pharmacy and Pharmacology. 2008;60:943–950. doi: 10.1211/jpp.60.8.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persaud R, Zhou H, Baker SE, Hei TK, Hall EJ. Assessment of low linear energy transfer radiation-induced bystander mutagenesis in a three-dimensional culture model. Cancer Research. 2005;65:9876–9882. doi: 10.1158/0008-5472.CAN-04-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou H, Suzuki M, Randers-Pehrson G, Vannais D, Trosko J, Waldren CA, Hei TK. Radiation risk at low doses may be greater than we thought. Proc. National Academy Science (U.S.A) 2001;98:14410–14415. doi: 10.1073/pnas.251524798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azzam EI, deToledo SM, Little JB. Direct evidence for the participation of gap junction mediated intercellular communication in the transmission of damage signals from alpha particle irradiated to non-irradiated cells. Proc. National Academy Science (U.S.A) 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, Yu Z, Lieberman HB, Hei TK. Mechanism of radiation-induced bystander effect: role of Cox-2 signaling pathway. Proc Natl Acad Sci (USA) 2005;102:14641–14646. doi: 10.1073/pnas.0505473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiation Research. 2006;165:400–409. doi: 10.1667/rr3527.1. [DOI] [PubMed] [Google Scholar]
- 18.Zhou H, Ivanov VN, Lien YC, Davidson M, Hei TK. Mitochondrial function and NFκB mediated signaling in radiation induced bystander effects. Cancer Research. 2008;68:2233–2240. doi: 10.1158/0008-5472.CAN-07-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azzam EI, deToledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62:5436–5442. [PubMed] [Google Scholar]
- 20.Narayanan PK, Goodwin EH, Lehnert BE. Alpha Particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Research. 1997;57:3963–3971. [PubMed] [Google Scholar]
- 21.Koyama S, Kodama K, Suzuki K, Matsumoto T, Miyazaki T, Watanabe M. Radiation induced long lived radicals which cause mutation and transformation. Mutation Research. 1998;421:45–54. doi: 10.1016/s0027-5107(98)00153-5. [DOI] [PubMed] [Google Scholar]
- 22.Shao C, Stewart V, Folkard M, Michael BD, Prise KM. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Research. 2003;63:8437–8442. [PubMed] [Google Scholar]
- 23.Yang H, Asaad N, Held KD. Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene. 2005;24:2096–2103. doi: 10.1038/sj.onc.1208439. [DOI] [PubMed] [Google Scholar]
- 24.Belyakov OV, Folkard M, Mothersill M, Prise KM, Michael BD. Bystander induced differentiation: A major response to targeted irradiation of a urothelial explant model. Mutation Research. 2006;597:43–49. doi: 10.1016/j.mrfmmm.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Coates PJ, Lorimore SA, Wright EG. Damaging and protective cell signaling in the untargeted effects of ionizing radiation. Mutation Research. 2004;568:5–20. doi: 10.1016/j.mrfmmm.2004.06.042. [DOI] [PubMed] [Google Scholar]