Abstract
PCR coupled with electrospray ionization mass spectrometry (PCR–ESI-MS) is a novel technology that has recently been used to identify pathogens from clinical specimens or after culture within about 6 h. We evaluated the MDR-TB (multidrug-resistant tuberculosis) assay, which uses PCR–ESI-MS for detection and identification of Mycobacterium spp. and Mycobacterium tuberculosis complex (MTBC) resistance determinants from solid and broth Middlebrook culture media. The performance of the MDR-TB assay was compared to identification using nucleic acid hybridization probes and 16S rRNA gene sequencing for 68 MTBC and 97 nontuberculous mycobacterial (NTM) isolates grown on agar and 107 cultures grown in Bactec MGIT broth. MTBC resistance profiles from the MDR-TB assay were compared to results with the agar proportion method. The PCR–ESI-MS system correctly identified all MTBC isolates and 97.9% and 95.8% of the NTM isolates from characterized agar cultures and MGIT broth cultures to the species level, respectively. In comparison to the agar proportion method, the sensitivity and specificity for the detection of drug resistance using the MDR-TB assay were 100% and 92.3% for rifampin, 100% and 93.8% for isoniazid, 91.6% and 94.4% for ethambutol, and 100% and 100% for fluoroquinolones, respectively. The MDR-TB assay appears to be a rapid and accurate method for the simultaneous detection and identification of mycobacterial species and resistance determinants of MTBC from culture.
INTRODUCTION
Tuberculosis (TB) persists as a global health concern, with 8.7 million new cases and 1.4 million deaths in 2011 (1). Further, drug-resistant and multidrug-resistant TB is established throughout the world (2). Since pulmonary TB is highly transmissible, rapid diagnosis and infection control make up essential elements of control. The poor sensitivity of smear microscopy and the untimely nature of culture (requiring up to 6 weeks) have hindered diagnoses (3). Thus, the development of rapid and accurate diagnostic tests is critical to help establish appropriate clinical management and infection control measures to further prevent transmission and the amplification of resistance (2).
Over the past decade, there have been increasing efforts, funding, and advances in diagnostic technologies for detecting Mycobacterium tuberculosis complex (MTBC) and its determinants of drug resistance. These new technologies include liquid media for culture and drug susceptibility testing (DST), line probe assays, and real-time PCR technologies (4). Currently, the detection of MTBC and characterization of drug resistance markers using molecular methods is largely limited to the use of separate assays for individual markers (e.g., 16S rRNA, rpoB, katG, etc). Exceptions include the real-time PCR GeneXpert MTB/RIF assay (MTBC and rifampin) and the InnoLiPA Rif.TB (MTBC and rifampin), GenoType MTBDRPlus (MTBC, rifampin, and isoniazid) and GenoType MTBDRsl (MTBC, fluoroquinolone, amikacin-capreomycin, and ethambutol) line probe assays. Recently, whole-gene sequencing for determining TB drug resistance mutations in a research setting has been reported, but it remains to be seen if this can be reasonably adapted to clinical laboratories (5). Another novel technology couples PCR with electrospray ionization mass spectrometry (PCR–ESI-MS) to permit the identification of microorganisms from culture or directly from clinical specimens within 6 h (6, 7).
In this study, we evaluated the ability of the MDR-TB (multidrug-resistant tuberculosis) assay (Ibis Biosciences, Carlsbad, CA), which utilizes PCR–ESI-MS, to simultaneously detect M. tuberculosis complex and nontuberculous mycobacteria from positive cultures (11). In addition, the MDR-TB assay was evaluated to determine its ability to detect resistance determinants in MTBC for isoniazid (INH), rifampin (RIF), ethambutol (EMB), and the fluoroquinolones (FQ).
MATERIALS AND METHODS
Mycobacteria.
The ability of the MDR-TB assay to accurately identify previously characterized mycobacterial isolates was evaluated by testing 68 previously characterized MTBC and 97 previously characterized nontuberculous mycobacterial (NTM) isolates. Isolates included ATCC strains and culture isolates identified using either the AccuProbe nucleic acid hybridization probes (GenProbe, San Diego, CA) or 16S rRNA gene sequencing as previously described (8). In addition, 57 positive and 50 negative Bactec MGIT broth cultures from patients suspected of having a mycobacterial infection and for which routine mycobacterial testing was requested were also tested with the MDR-TB assay using the AccuProbes or 16S sequencing as the reference methods. The ability of the MDR-TB assay to determine genetic markers of drug resistance of MTBC was evaluated using a subset of 48 well-characterized MTBC isolates described above for which agar proportion results were available for use as the reference method. The genetic markers detected by the MDR-TB assay include katG and the promoter regions of inhA and ahpC for INH, rpoB for RIF, embB for EMB, and gyrA for the FQ.
(i) Mycobacterial culture isolates.
The isolates were freshly subcultured onto Middlebrook 7H10 (BBL, Sparks, MD) and were lysed by placing a loopful of the organism into a 2.0-ml tube containing 500 μl of sterilized water, 50 μl of 0.1-mm silica glass beads, and 100 μl of 2.4-mm zirconia beads (BioSpec Products, Inc., Bartlesville, OK). The tubes were heated at 95°C for 5 min and then placed on a Disruptor Genie instrument (Scientific Industries, Bohemia, NY) for 2 min to mechanically lyse the organisms and release the nucleic acid. From the stock lysates, a 1:20 dilution was performed by adding 20 μl of lysate into 180 μl of sterile water. The diluted lysates were used as the template for the PCRs (10).
(ii) MGIT broth cultures.
After routine culture identification and susceptibility testing was completed, 1 ml of the MGIT culture was added to 2.0-ml tube containing 50 μl of 0.1-mm silica glass beads and 100 μl of 2.4-mm zirconia beads (BioSpec Products, Inc., Bartlesville, OK) and centrifuged at 17,900 × g for 10 min. The supernatant was removed, and the pellet was resuspended in 300 μl of sterile water. The tubes were then heated at 95°C for 5 min and then placed on a Disruptor Genie instrument (Scientific Industries, Bohemia, NY) for 2 min to mechanically lyse the organisms and release the nucleic acid. The lysates were used as the template for the PCRs.
PCR–ESI-MS analysis.
PCR plates (Broad Fungal Assay; Abbott Laboratories) were thawed and centrifuged at 1,800 × g (IEC Centra MP4R; Thermo Scientific) for 1 min. PCR plates were loaded with extracted DNA using a CAS-1200 precision liquid-handling system (Corbett Research [Qiagen], Valencia, CA). Eight wells containing two primer sets per well were filled with 10 μl of extracted sample, allowing testing of 12 samples per 96-well plate. Primer pairs were previously described by Massire et al. (11). Following sample loading, the plates were sealed with Easy Pierce 20-μm heat-sealing tape (catalog no. AB-1720; Thermo Scientific) at 175°C for 1.5 s (ThermoSci ALPS 50V; Thermo Scientific), centrifuged at 1,800 × g for 1 min, and loaded on to the Eppendorf Mastercycler proS thermocycler (Eppendorf, Hamburg, Germany. The PCR was carried out using the manufacturer's recommended thermocycler protocol as previously reported (10).
Sample analysis.
Sample analysis was performed following PCR amplification by loading the plate onto the Plex-ID system (Abbott Molecular, Des Plaines, IL), where the PCR product was desalted and analyzed using electrospray ionization mass spectrometry (ESI-MS). The base count of each amplicon was determined for each primer pair and compared to database version NFDU.415.455.349. The MDR-TB assay utilizes signal thresholds (cutoffs) designed to limit reporting of irreproducible detections. Cutoffs are applied to two measurements, the level and the Q score, as previously described (9, 10). The level is an indication of the amount of the amplicon present in the sample in comparison to a calibrant, and the Q score is a rating between 0 (low) and 1 (high) which represents the strength of the data supporting identification. For the MDR-TB assay, a Q score of ≥0.85 is considered a reportable result. Results included the Mycobacterium species identification and resistance determinants for INH, RIF, EMB, and FQ if MTBC was identified.
Work flow.
The overall work flow to process 12 samples (one MDR-TB assay plate) on the PCR–ESI-MS system from start to finish is approximately 6 h, with approximately 1 h of hands-on time. Following a strict unidirectional workflow, the overall turnaround time (TAT) to reporting of results was 24 h, since the plates were placed on the PCR–ESI-MS system at the end of the work day and the data were analyzed the following morning, as previously described (10).
RESULTS
Mycobacterial culture isolates.
PCR–ESI-MS identified all (100%; 68/68) M. tuberculosis complex culture isolates as MTBC (Table 1). To challenge the specificity of the MDR-TB assay to identify NTMs, we evaluated 97 previously characterized NTM isolates (Table 2). Of the 97 NTM isolates, PCR–ESI-MS was able to identify 99.0% (96/97) and 96.9% (94/97) of the isolates to the genus and species levels, respectively. Only one misidentification occurred, where M. rhodesiae was identified as M. aichiense. M. alvei was not detected by the MDR-TB assay, and an M. xenopi isolate was identified as Mycobacterium species JDM601. Three isolates were correctly identified but also had a second NTM identified (M. austroafricanum was identified as M. austroafricanum/M. vanbaalenii, while M. murale and M. tokaiense were each identified as M. murale/ M. tokaiense). The three results with multiple NTM matches reported were counted as correct identifications, but it should be recognized that the MDR-TB assay cannot separate these species. M. murale and M. tokaiense are also indistinguishable by 16S rRNA gene sequencing.
Table 1.
Identification of previously characterized M. tuberculosis complex isolates grown on agar medium and resistance profiles determined by the agar proportion method compared to those determined by the MDR-TB assaya
| No. of isolatesb | Agar proportion result |
MDR-TB assay result |
||||||
|---|---|---|---|---|---|---|---|---|
| INH | RIF | EMB | OFL | INH | RIF | EMB | FQ | |
| 26 | S | S | S | S | S | S | S | S |
| 4 | R | S | S | S | R | S | S | S |
| 3 | R | R | R | S | R | R | R | S |
| 2 | S | S | R | S | S | S | R | S |
| 2 | R | R | R | R | R | R | R | R |
| 1 | R | S | R | S | R | S | R | S |
| 1 | S | R | S | S | S | R | S | S |
| 1 | R | S | R | S | R | R | R | S |
| 1 | S | S | S | S | R | S | S | S |
| 1 | S | S | S | S | S | R | S | S |
| 1 | R | R | R | S | R | R | S | S |
| 1 | S | S | S | S | R | S | R | S |
| 1 | R | S | R | S | R | S | S | S |
| 1 | S | R | R | S | S | R | R | S |
| 1 | R | S | S | S | R | R | S | S |
| 1 | R | R | S | S | R | R | R | S |
S, susceptible; R, resistant; INH, isoniazid; RIF, rifampin; EMB, ethambutol; OFL, ofloxacin. Discordant results are in bold.
All isolates were appropriately identified as M. tuberculosis complex by the MDR-TB assay. An additional 20 isolates were appropriately identified by PCR–ESI-MS but are not included in this table because agar proportion method susceptibility results were not available for comparison.
Table 2.
Previously characterized nontuberculous mycobacteria identified by AccuProbes and/or 16S sequencing and comparison with results of MDR-TB assaya

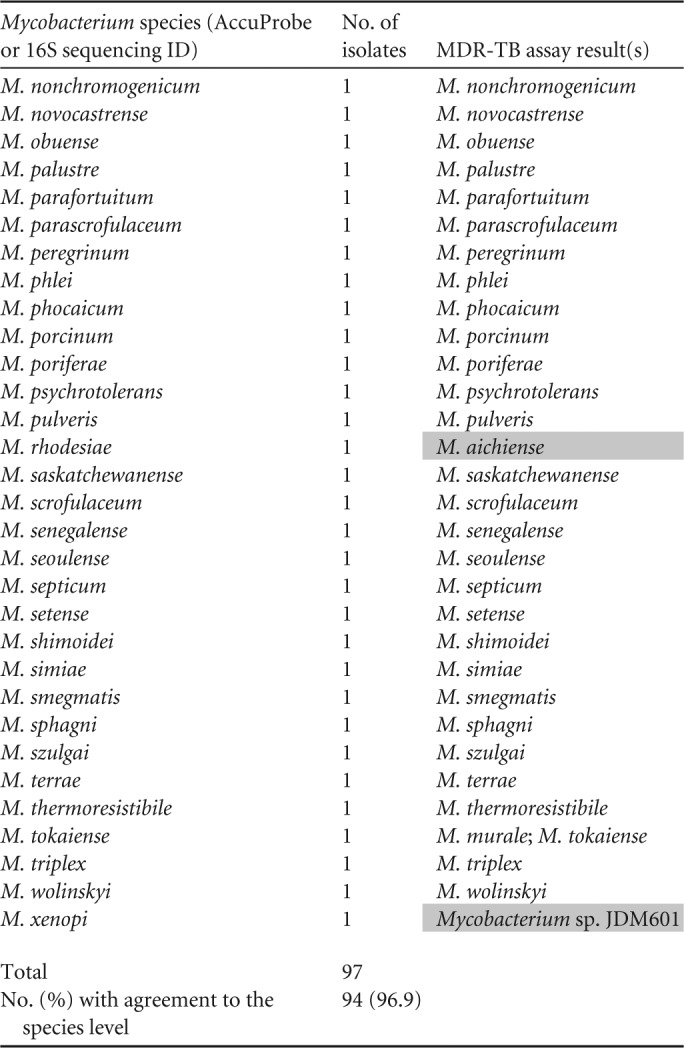
Shaded areas are discordant results.
MGIT broth cultures.
The accuracy of the MDR-TB assay to identify MTBC and NTM directly from positive MGIT broth cultures was compared to identification using the AccuProbes for MTBC, M. avium complex, and M. gordonae, while 16S rRNA gene sequencing was used for identification of all other species. Fifty-seven positive and 50 negative MGIT tube broth cultures were tested. Of the positive MGIT broth cultures, 9 of 9 (100%) MTBC isolates were identified correctly (Table 3). In addition, all 9 isolates were found to be susceptible to the first-line agents EMB, INH, and RIF by broth susceptibility on the VersaTREK system (Trek Diagnostics, Cleveland, OH) and by the MDR-TB genotypic assay. For the NTM-positive MGIT broth cultures, the MDR-TB assay identified 100% (48) and 95.8% (46/48) of the NTM isolates correctly to the genus and species levels, respectively. Two M. gordonae isolates were identified by the MDR-TB assay as Mycobacterium species. One M. avium complex isolate identified by AccuProbe had a multiple detection of M. avium (Q score, 1; level, 666) and M. gordonae (Q score, 0.92; level, 37) by the MDR-TB assay. Sequencing of the MGIT tube lysate confirmed the presence of M. avium complex. Of the 50 MGIT tube-negative cultures, all were negative except 1, which was identified as M. gordonae (Q score, 1; level, 379). Sequencing of the lysate was negative and confirmed the negative culture results.
Table 3.
Identification of Mycobacterium species from Bactec MGIT broth positive bottles by AccuProbes and/or 16S sequencing compared with identification by MDR-TB assay
| Mycobacterium species or group | No. of isolates | MDR-TB result |
|---|---|---|
| M. avium complex | 17 | M. intracellulare |
| M. tuberculosis complex | 9 | M. tuberculosis |
| M. avium complex | 8 | M. avium |
| M. gordonae | 5 | M. gordonae |
| M. abscessus group | 3 | M. abscessus |
| M. kansasii | 2 | M. kansasii |
| M. chelonae | 2 | M. chelonae |
| M. gordonae | 2 | Mycobacterium sp. |
| M. fortuitum | 1 | M. fortuitum |
| M. avium complex | 1 | M. avium/M. gordonae |
| M. xenopi | 1 | M. xenopi |
| M. scrofulaceum | 1 | M. scrofulaceum |
| M. mucogenicum/phocaicum | 1 | M. phocaicum |
| M. abscessus group | 1 | M. massiliense |
| M. abscessus group | 1 | M. bolletii |
| M. obuense | 1 | M. obuense |
| M. simiae | 1 | M. simiae |
| Total | 57 | |
| No. (%) with agreement to the species level | 55 (96.5) |
Genetic markers of drug resistance.
The second aim of the study was to evaluate the ability of the MDR-TB assay to determine genetic markers of drug resistance of MTBC using a subset of 48 well-characterized isolates (Table 1). The sensitivities and specificities for each antimicrobial agent, comparing the MDR-TB assay results to the reference standard method of agar proportion, are summarized in Table 4. The MDR-TB assay had a sensitivity and specificity of 100% and 93.8% for the detection of INH resistance. Two major errors were detected in which a katG S315T nucleotide substitution which would suggest INH resistance was detected, but the isolates were susceptible by the agar proportion method. The MDR-TB assay had a sensitivity and specificity of 100% and 92.3% for detection of RIF resistance. Three discordant results were observed. All three were determined to be susceptible by agar proportion, but previously described resistance substitutions were detected to be encoded in rpoB, L511P, D516G, and D516F, by PCR–ESI-MS. The MDR-TB assay also demonstrated a sensitivity and specificity of 91.6% and 94.4% for the detection of EMB resistance. Two major errors occurred (embB, M306V and M306I), and one very major error occurred (no resistance mutations were detected in an isolate identified as resistant by the agar proportion method). The MDR-TB assay had a sensitivity and specificity of 100% for the detection of FQ resistance. Notably, the negative predictive value (NPV) for INH, RIF, and FQ resistance was 100%, and that for EMB resistance was 97.1%.
Table 4.
Comparison of M. tuberculosis complex resistance profiles determined by agar proportion and MDR-TB assay
| Drug and MDR-TB assay result | No. of isolates with result by agar proportion method |
Performance of MDR-TB assay |
||
|---|---|---|---|---|
| Resistant | Susceptible | % sensitivity | % specificity | |
| Isoniazid | 100 | 93.8 | ||
| Resistant | 16 | 2 | ||
| Susceptible | 0 | 30 | ||
| Rifampin | 100 | 92.3 | ||
| Resistant | 9 | 3 | ||
| Susceptible | 0 | 36 | ||
| Ethambutol | 91.6 | 94.4 | ||
| Resistant | 11 | 2 | ||
| Susceptible | 1 | 34 | ||
| Fluoroquinolones | 100 | 100 | ||
| Resistant | 2 | 0 | ||
| Susceptible | 0 | 46 | ||
DISCUSSION
There are only two published studies of the MDR-TB assay using the PCR–ESI-MS system. The first report, by Massire et al., described the development of the assay by Ibis Biosciences (11). The second article, by Wang et al., described the molecular characterization of drug resistance profiles in 96 Mycobacterium tuberculosis isolates circulating in China (7). This is the first study evaluating the clinical utility of the MDR-TB assay for simultaneously detecting MTBC, NTM, and MTBC resistance determinants in comparison to conventional laboratory methods.
The first aim of this study was to determine the accuracy of the MDR-TB assay in correctly identifying previously characterized MTBC and NTM isolates. The MDR-TB assay correctly identified 100% of the MTBC and 96.9% of the NTM culture isolates to the species level. One M. alvei isolate was not detected, one M. xenopi isolate was identified as Mycobacterium species, and one misidentification of M. rhodesiae as M. aichiense was observed. The accuracy of the MDR-TB assay was further challenged by comparing results from AFB-positive MGIT tube broth cultures to those of standard methods used in the clinical microbiology laboratory. As seen with the isolates cultured on solid Middlebrook agar, 100% of MTBC and 95.8% of NTM isolates were identified correctly to the species level from positive MGIT broths. Two M. gordonae isolates were identified to the genus level only as Mycobacterium species. These results are similar to those described by Massire et al., where all MTBC and 96.3% of NTM isolates from positive MGIT broth cultures were appropriately identified by the MDR-TB assay (11). Most important, none of the MTBC isolates from our study were incorrectly identified as NTM or vice versa. The MDR-TB assay detected M. gordonae isolates in one positive M. avium complex MGIT tube and one negative MGIT tube that were not detected by culture. Sequencing was performed on both lysates, and M. gordonae was not detected. This suggests the possibility that a reagent may have been contaminated with M. gordonae DNA. The MDR-TB assay can reliably detect MTBC and does not appear to “cross-react” with NTM species, which is important in a setting with a high NTM prevalence.
The second aim of the study was to compare the ability of the MDR-TB assay to determine MTBC genetic markers of drug resistance compared to the gold standard agar proportion method. The study by Wang et al. characterized the resistance determinants of 96 MTBC isolates from China using the MDR-TB assay; however, no comparison with phenotypic DST or other comparator method was performed (7). In comparison to phenotypic DST, the sensitivity and specificity values for the detection of first-line and second-line drug resistance using the MDR-TB assay were 100% and 92.3% for RIF, 100% and 93.8% for INH, 91.6% and 94.4% for EMB, and 100% and 100% for FQ, respectively. These results are comparable to those with other rapid molecular methods, such as Sanger dideoxy sequencing, pyrosequencing, the Cepheid GeneXpert MTB/RIF assay, and the InnoLiPA Rif.TB, GenoType MTBDRPlus, and GenoType MTBDRsl line probe assays (12–15).
Fortunately, genotypic and phenotypic correlation for the most frequently used first-line drugs, INH and RIF, is quite reliable (15). Rifampin resistance was detected by the MDR-TB assay with a sensitivity and specificity of 100% and 92.3%, respectively. Mutations within the rpoB gene, which encodes the DNA-dependent RNA polymerase, generally correlate with phenotypic results and predict resistance to RIF in 90 to 95% of the isolates (16). Likewise, a study comparing sequencing of the rpoB gene to the agar proportion method revealed a sensitivity of 97% and specificity of 93.6% (17). Discordant results by the MDR-TB assay resulting in a decreased specificity of the assay included L511P, D516G, and D516F substitutions. These substitutions have been associated with discordant susceptibility tests in previous studies (17, 18). It has been proposed that they could confer low-level but clinically relevant resistance (18).
Resistance to INH has a high correlation with mutations present within two genes: katG (encoding a catalase-peroxidase enzyme required for isoniazid activation) and inhA (promoter region). Mutations within the katG gene provide high-level resistance and account for the majority of phenotypic resistance (∼85%) (15). Isoniazid resistance was detected by the MDR-TB assay with a sensitivity and specificity of 100% and 93.8%, respectively. The MDR-TB assay includes a third target for the detection of INH resistance, the promoter region of ahpC. The ahpC gene encodes an alkyl hydroperoxide reductase, which is involved in the cellular response to oxidative stress (19). Studies have demonstrated an association of mutations in the ahpC promoter region with an INH resistance phenotype (20). However, the inclusion of the ahpC gene in molecular studies has been questioned because there are limited data on the role in INH resistance (12). None of the isolates that were resistant to INH in this study had mutations detected in the promoter region of the ahpC gene. Based on our results, the ahpC target does not appear to play a substantial role in increasing the sensitivity of the assay to detect INH resistance. However, the number of INH-resistant isolates tested in this study was small (n = 16), so further testing with a larger cohort of INH-resistant isolates is required to substantiate the role of ahpC. The decreased specificity of the MDR-TB assay was owing to two isolates with katG S315T nucleotide substitutions that were found to be susceptible by the agar proportion method. Generally, mutations at codon 315 within katG are the most common providing resistance to INH.
Although the resistance-determining region of embB (arabinosyl transferase) has been described and used as the primary molecular target for EMB resistance, it represents only ∼60% of phenotypic resistance (21). On the other hand, conventional DST for EMB is notoriously problematic due to a narrow range between the MICs of susceptible and resistant isolates (12, 22). The MDR-TB assay exploits the resistance-determining region of embB to predict EMB susceptibility with a sensitivity and specificity of 91.6% and 94.4%, respectively. These results are in line with those of other studies demonstrating both false-positivity and false-negativity issues with embB as the sole genetic marker for EMB resistance in comparison to phenotypic DST (15, 17, 22).
Interestingly, the resistance-determining region of gyrA covered by the MDR-TB assay provided 100% sensitivity and 100% specificity for determining phenotypic susceptibilities to the FQ. Our study supports the high specificity of the gyrA marker but contrasts with other studies that found that the resistance-determining region of gyrA accounts for only 80% of phenotypic resistance (17). These differences could be due to the region of the target gene used in each assay, differences in strains tested, and the small number of FQ-resistant strains in our cohort.
In recent years, several techniques that simultaneously detect MTBC and drug resistance markers for MTBC have been described. These methods are generally nucleic acid amplification (NAAT) tests, including line probe assays, real-time PCR assays, and sequencing methods. A comparison of these methodologies with the PCR–ESI-MS method described in this article is presented in Table 5. PCR–ESI-MS has the advantage over the current NAAT methods of being able to identify both MTBC and NTM species, and if MTCB is detected, it will simultaneously provide markers for drug resistance in a single assay. The line probe assays are the NAAT method which comes closest to providing this information, but at least two distinct strips/assays are needed at this time. Another advantage of the PCR–ESI-MS method is the ability to test for a wide variety of organisms on the same platform, including bacteria, viruses, fungi, and parasites (6, 10, 23, 24). This can be accomplished by some of the current NAAT tests (PCR and sequencing) but generally requires the performance of a variety of PCR assays and the use of multiple sequencing targets. However, the PCR–ESI-MS technology does not come without limitations, including the open system format, the cost of supplies and labor, and the requirement for experienced personnel, as previously described (10). Most important, the high instrument costs of the PCR–ESI-MS system (∼$500,000) may hamper the implementation of this technology in small clinical microbiology laboratories, and therefore this technology may be limited to use in reference laboratories or state health laboratories.
Table 5.
Comparison of methodologies for simultaneous detection and identification of MTBC, NTM, and MTBC resistance determinantsa
| Methodology | Detection of MTBC | Detection of NTMs | Detection of 1st–line agents | Detection of 2nd–line agents | Advantages | Limitations |
|---|---|---|---|---|---|---|
| GeneXpert MTB/RIF (2, 15) | Yes | No | rpoB only | No | Direct from sputum | Cost of cartridge and instrumentation (2) |
| Automated closed system | Only one marker for resistance included | |||||
| Random access | ||||||
| Highly trained staff not required | ||||||
| WHO endorsed (25) | ||||||
| Rapid [∼2 h] | ||||||
| Line probe assaysb (2, 15) | Yes | Yes | Yes | Yes | Validated from specimens and culture isolates | Multiple different line probes required for simultaneous detection of mycobacterial species and resistance determinants |
| WHO endorsed (26) | Cost/strip (26) | |||||
| Rapid [∼4–6 h] | Cost of instrumentation [thermocycler and automated washer] | |||||
| Open system | ||||||
| Labor-intensive | ||||||
| Sensitivity decreases if performed direct from smear-negative specimens (15) | ||||||
| Pyrosequencing (12, 27) | Yes (27) | Yes (27) | rpoB, katG, embB, inhA (12) | rrs, gyrA, eis (12) | Moderately rapid [∼1 day] | Short sequence reads |
| High throughput [12 samples/96 well plate] | Not yet performed on direct specimens | |||||
| Multiple targets needed | ||||||
| Cost/isolate | ||||||
| Cost of instrumentation | ||||||
| Experienced personnel required | ||||||
| Sanger sequencing for speciation and for all known resistance-determining regions (15) | Yes | Yes | rpoB, inhA, katG, embB, pncA | gyrA, rrs, eis, tlyA | Referenced against a large database of isolates | Labor-intensive |
| Ability to identify novel mutations associated with resistance | Cost/gene | |||||
| Performed from smear positive specimens and culture isolates | Cost of instrumentation | |||||
| Provides a resistance marker for PZA | Most suitable for reference laboratories | |||||
| Performed at CDC | Experienced personnel required | |||||
| Moderately rapid [∼1 day] | ||||||
| Next-generation sequencing (5) | Not at this time; only use of known positive MTBC isolates (5) | Not at this time | katG, rpoB, pncA | rrs, gyrA | Full-length characterization of genes | Not yet performed on direct specimens |
| Ability to identify novel mutations associated with resistance | Cost/sample | |||||
| Moderately rapid [∼2 days] | Cost of instrumentation | |||||
| Provides a resistance marker for PZA | Labor-intensive | |||||
| Experienced personnel required | ||||||
| Most suitable for reference laboratories | ||||||
| PCR–ESI-MS; current study (7, 11) | Yes | Yes | katG, inhA, ahpC, rpoB, embB | gyrA | Simultaneous detection of mycobacterial species [MTBC or NTMs] and MTBC drug resistance markers from culture isolates | Short sequence reads |
| High throughput [12 samples/MDR-TB panel] | Not yet performed on direct specimens | |||||
| Moderately rapid [∼1 day] | Open system | |||||
| Cost/sample | ||||||
| Cost of instrumentation (10) | ||||||
| Labor intensive | ||||||
| Experienced personnel required | ||||||
| Most suitable for reference laboratories |
References are given in parentheses.
Commercial tests include INNO-LiPA Rif.TB (MTBC and rifampin), GenoType MTBDRPlus (MTBC, rifampin, and isoniazid) and GenoType MTBDRsl (MTBC, fluoroquinolone, amikacin-capreomycin and ethambutol), INNO-LiPA Mycobacteria v2 assay (mycobacterial species; MTBC and NTM), GenoType Mycobacterium CM/AS (mycobacterial species; NTMs).
Overall, the MDR-TB assay appears to be a rapid and accurate method for the simultaneous detection and identification of mycobacterial species and resistance determinants to MTBC directly from culture (solid or broth) compared to standard laboratory methods (nucleic acid hybridization probes or 16S rRNA gene sequencing). Implementation of this assay in a clinical microbiology laboratory could provide a turnaround time for identification of MTBC and NTM isolates that rivals that of Sanger dideoxy sequencing while simultaneously providing genotypic drug resistance patterns to physicians, allowing them to tailor the treatment regimens for M. tuberculosis prior to receiving phenotypic susceptibility results. Future studies evaluating the use of the MDR-TB assay directly with specimens will be important to determine whether the assay performs as well without waiting for growth in culture.
ACKNOWLEDGMENT
The equipment and reagents for this study were provided by Abbott Molecular, Des Plaines, IL.
Footnotes
Published ahead of print 14 August 2013
REFERENCES
- 1.World Health Organization 2013. Fact sheet on Tuberculosis. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs104/en/index.html [Google Scholar]
- 2.O'Grady J, Maeurer M, Mwaba P, Kapata N, Bates M, Hoelscher M, Zumla A. 2011. New and improved diagnostics for detection of drug-resistant pulmonary tuberculosis. Curr. Opin. Pulm. Med. 17:134–141 [DOI] [PubMed] [Google Scholar]
- 3.Reid MJ, Shah NS. 2009. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect. Dis. 9:173–184 [DOI] [PubMed] [Google Scholar]
- 4.McNerney R, Maeurer M, Abubakar I, Marais B, McHugh TD, Ford N, Weyer K, Lawn S, Grobusch MP, Memish Z, Squire SB, Pantaleo G, Chakaya J, Casenghi M, Migliori GB, Mwaba P, Zijenah L, Hoelscher M, Cox H, Swaminathan S, Kim PS, Schito M, Harari A, Bates M, Schwank S, O'Grady J, Pletschette M, Ditui L, Atun R, Zumla A. 2012. Tuberculosis diagnostics and biomarkers: needs, challenges, recent advances, and opportunities. J. Infect. Dis. 205(Suppl 2):S147–S158 [DOI] [PubMed] [Google Scholar]
- 5.Daum LT, Rodriguez JD, Worthy SA, Ismail NA, Omar SV, Dreyer AW, Fourie PB, Hoosen AA, Chambers JP, Fischer GW. 2012. Next-generation ion torrent sequencing of drug resistance mutations in Mycobacterium tuberculosis strains. J. Clin. Microbiol. 50:3831–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolk DM, Kaleta EJ, Wysocki VH. 2012. PCR-electrospray ionization mass spectrometry: the potential to change infectious disease diagnostics in clinical and public health laboratories. J. Mol. Diagn. 14:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang F, Massire C, Li H, Cummins LL, Li F, Jin J, Fan X, Wang S, Shao L, Zhang S, Meng S, Wu J, Lu C, Blyn LB, Sampath R, Ecker DJ, Zhang W, Tang Y-W. 2011. Molecular characterization of drug-resistant Mycobacterium tuberculosis isolates circulating in China by multilocus PCR and electrospray ionization mass spectrometry. J. Clin. Microbiol. 49:2719–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall L, Doerr KA, Wohlfiel SL, Roberts GD. 2003. Evaluation of the MicroSeq system for identification of mycobacteria by 16S ribosomal DNA sequencing and its integration into a routine clinical mycobacteriology laboratory. J. Clin. Microbiol. 41:1447–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofstadler SA, Sampath R, Blyn LB, Eshoo MW, Hall TA, Jiang Y, Drader JJ, Hanns JC, Sannes-Lowery KA, Cummins LL, Libby B, Walcott DJ, Schink A, Massire C, Ranken R, Gutierrez J, Manalili S, Ivy C, Meltion R, Levene H, Barrett-Wilt G, Li F, Zapp V, White N, Samant V, McNeil JA, Knize D, Robbins D, Rudnick K, Desai A, Moradi E, Eckert DJ. 2005. TIGER: the universal biosensor. Int. J. Mass Spectrom. 242:23–41 [Google Scholar]
- 10.Simner PJ, Uhl JR, Hall L, Weber MM, Walchak RC, Buckwalter S, Wengenack NL. 2013. Broad-range direct detection and identification of fungi using the PLEX-ID PCR-electrospray ionization mass spectrometry (ESI-MS) system. J. Clin. Microbiol. 51:1699–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massire C, Ivy CA, Lovari R, Kurepina N, Li H, Blyn LB, Hofstadler SA, Khechinashvili G, Stratton CW, Sampath R, Tang Y-W, Ecker DJ, Kreiswirth BN. 2011. Simultaneous identification of Mycobacterial isolates to the species level and determination of tuberculosis drug resistance by PCR followed by electrospray ionization mass spectrometry. J. Clin. Microbiol. 49:908–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engstrom A, Morcillo N, Imperiale B, Hoffner SE, Jureen P. 2012. Detection of first- and second-line drug resistance in Mycobacterium tuberculosis clinical isolates by pyrosequencing. J. Clin. Microbiol. 50:2026–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Roa P, Ruiz-Serrano MJ, Alcala L, Garcia-Escribano Raez N, Garcia de Viedma D, Bouza E. 2012. Susceptibility testing to second-line drugs and ethambutol by GenoType MTBDRsl and Bactec MGIT 960 comparing with agar proportion method. Tuberculosis (Edinb.) 92:417–421 [DOI] [PubMed] [Google Scholar]
- 14.Chryssanthou E, Angeby K. 2012. The GenoType® MTBDRplus assay for detection of drug resistance in Mycobacterium tuberculosis in Sweden. APMIS 120:405–409 [DOI] [PubMed] [Google Scholar]
- 15.Heysell SK, Houpt ER. 2012. The future of molecular diagnostics for drug-resistant tuberculosis. Expert Rev. Mol. Diagn. 12:395–405 [DOI] [PubMed] [Google Scholar]
- 16.Watterson SA, Wilson SM, Yates MD, Drobniewski FA. 1998. Comparison of three molecular assays for rapid detection of rifampin resistance in Mycobacterium tuberculosis. J. Clin. Microbiol. 36:1969–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell PJ, Morlock GP, Sikes RD, Dalton TL, Metchock B, Starks AM, Hooks DP, Cowan LS, Plikaytis BB, Posey JE. 2011. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 55:2032–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Deun A, Barrera L, Bastian I, Fattorini L, Hoffmann H, Kam KM, Rigouts L, Rusch-Gerdes S, Wright A. 2009. Mycobacterium tuberculosis strains with highly discordant rifampin susceptibility test results. J. Clin. Microbiol. 47:3501–3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiepiela P, Bishop KS, Smith AN, Roux L, York DF. 2000. Genomic mutations in the katG, inhA and aphC genes are useful for the prediction of isoniazid resistance in Mycobacterium tuberculosis isolates from Kwazulu Natal, South Africa. Tuber. Lung Dis. 80:47–56 [DOI] [PubMed] [Google Scholar]
- 20.Sreevatsan S, Pan X, Zhang Y, Deretic V, Musser JM. 1997. Analysis of the oxyR-ahpC region in isoniazid-resistant and -susceptible Mycobacterium tuberculosis complex organisms recovered from diseased humans and animals in diverse localities. Antimicrob. Agents Chemother. 41:600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starks AM, Gumusboga A, Plikaytis BB, Shinnick TM, Posey JE. 2009. Mutations at embB codon 306 are an important molecular indicator of ethambutol resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 53:1061–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madison B, Robinson-Dunn B, George I, Gross W, Lipman H, Metchock B, Sloutsky A, Washabaugh G, Mazurek G, Ridderhof J. 2002. Multicenter evaluation of ethambutol susceptibility testing of mycobacterium tuberculosis by agar proportion and radiometric methods. J. Clin. Microbiol. 40:3976–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brinkman CL, Vergidis P, Uhl JR, Pritt BS, Cockerill FR, Steckelberg JM, Baddour LM, Maleszewski JJ, Edwards WD, Sampath R, Patel R. 2013. PCR-electrospray ionization mass spectrometry for direct detection of pathogens and antimicrobial resistance from heart valves in patients with infective endocarditis. J. Clin. Microbiol. 51:2040–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang YW, Lowery KS, Valsamakis A, Schaefer VC, Chappell JD, White-Abell J, Quinn CD, Li H, Washington CA, Cromwell J, Giamanco CM, Forman M, Holden J, Rothman RE, Parker ML, Ortenberg EV, Zhang L, Lin YL, Gaydos CA. 2013. Clinical accuracy of a PLEX-ID flu device for simultaneous detection and identification of influenza viruses A and B. J. Clin. Microbiol. 51:40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization 2011. Rapid implementation of the Xpert MTB/RIF diagnostic test, 2011. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2011/9789241501569_eng.pdf [Google Scholar]
- 26.World Health Organization 2008. Molecular line probe assays for rapid screening of patients at risk of multi-drug resistant tuberculosis (MDR-TB). World Health Organization, Geneva, Switzerland: http://www.who.int/tb/features_archive/policy_statement.pdf [Google Scholar]
- 27.Tuohy MJ, Hall GS, Sholtis M, Procop GW. 2005. Pyrosequencing as a tool for the identification of common isolates of Mycobacterium sp. Diagn. Microbiol. Infect. Dis. 51:245–250 [DOI] [PubMed] [Google Scholar]


