Abstract
An Irkut virus (IRKV) was recently isolated from a bat in China. The protective ability of rabies biologics available in the Chinese market and experimental biologics against the rabies virus (RABV) and IRKV were assessed in a hamster model via preexposure prophylaxis (PrEP) and postexposure prophylaxis (PEP) experiments. The results demonstrated that a single dose of rabies vaccine did not induce adequate protection against IRKV infection. However, routine PrEP with three doses of vaccine induced complete protection against IRKV infection. Higher doses of RABV immunoglobulins and alpha interferon were required during PEP to protect hamsters against IRKV versus RABV infection. Experimental recombinant vaccines containing IRKV glycoproteins induced more-reliable protection against IRKV than against RABV infection. Those findings may be explained by limited cross-neutralization of these viruses (confirmed via in vitro tests) in conjunction with antigenic distances between RABV and IRKV. These results indicate that the development and evaluation of new biologics for PrEP and PEP are required to ensure sufficient protection against IRKV infection in China and other territories where this virus is present.
INTRODUCTION
Rabies is an acute progressive encephalomyelitis caused by negative-sense single-stranded RNA viruses from the genus Lyssavirus, family Rhabdoviridae. At present, the genus includes 12 established viral species and 3 viruses awaiting taxonomic assessment (1–4). Of these, classic rabies virus (RABV) is the most broadly distributed, causing >99% of human lyssavirus cases worldwide (1). In China, RABV causes 2,000 to 3,000 reported human deaths per year. Almost all Chinese lyssavirus isolates belong to RABV species originating from carnivores (5). However, several human rabies cases of bat origin, in which the viruses were not identified, were reported (6). In 2012, for the first time, a lyssavirus was isolated from a greater tube-nosed bat (Murina leucogaster) in the Jilin Province of China. It was identified as Irkut virus (IRKV) (isolate IRKV-THChina12) (6). Phylogenetic analysis demonstrated that structural proteins of this virus shared high sequence identity (>98%) with IRKV isolate Ozernoe, from a human case of rabies following a bat bite in Russia in 2007 (7). Several studies have investigated the serological cross-reactivity of lyssaviruses and the protective ability of commercially available RABV biologics against non-RABV lyssaviruses. In general, it has been shown that rabies biologics provide sufficient protection against lyssaviruses from phylogroup I (8). However, a more recent study demonstrated that rabies biologics available in the United States elicited only partial protection against several non-RABV lyssaviruses, including IRKV (9). The need existed, therefore, to further assess the serological cross-reactivity between RABV and IRKV and to evaluate whether rabies biologics available in China and elsewhere provide reliable protection against IRKV infection. In this study, we conducted a series of preexposure prophylaxis (PrEP) and postexposure prophylaxis (PEP) experiments in an animal model, to evaluate the protective effects of several commercially available and experimental biologics against IRKV infection.
MATERIALS AND METHODS
Sequence comparisons of viral glycoproteins.
Multiple alignments of lyssavirus glycoprotein sequences were performed using the DNASTAR Lasergene program (DNASTAR, Madison, WI) and the BioEdit program (10).
Viruses and cells.
IRKV isolate IRKV-THChina12, which was obtained in 2012 from a Murina leucogaster bat in Tonghua county, Jilin Province (6), was amplified in a single intracerebral mouse passage. RABV isolate BD06, which was obtained in 2006 from a rabid dog in China, was maintained in dog brains via serial passages (11). The titers of IRKV-THChina12 and BD06 suspensions used in the study in the Syrian hamster model were 103.0 to 103.5 times the intramuscular (i.m.) 50% lethal dose (LD50)/ml. One hundred times the hamster LD50 (the lowest intramuscular 100% lethal dose) of IRKV-THChina12 and BD06 was used for challenge. RABV strain CVS-11 was grown in BHK-21 cells and used in the virus neutralization tests described below. BHK-21 and HEK-293 cells were maintained at 37°C in Dulbecco's minimum essential medium (DMEM) supplemented with 2% newborn calf serum, 100 U/ml penicillin G, and 100 μg/ml streptomycin sulfate, in a 5% CO2 humidified incubator.
Animals.
Two-month-old female adult Syrian hamsters weighing approximately 100 g were obtained from the Changchun Institute of Biological Products (China) and were randomly divided into 36 groups of 10 hamsters (groups 1 to 36) (Table 1). Following challenge, the hamsters were observed for 28 days. Animals that showed clinical signs of rabies and animals that survived 28 days of observation were euthanized by CO2 intoxication. Brains were removed and tested by direct fluorescent antibody (DFA) testing for the presence of RABV or IRKV antigens (12). All animal experiments described in this paper were conducted according to the Guideline on Humane Treatment of Laboratory Animals, stipulated by the Ministry of Science and Technology (MOST) of the People's Republic of China (13), and were approved by the Animal Welfare Committee of the Military Veterinary Research Institute (Changchun, China).
Table 1.
Design of experiments on preexposure and postexposure prophylaxis in a hamster model
| Treatment and group no. | Rabies biologic(s) | Inoculation amount/dose | Challenge virus |
|---|---|---|---|
| PrEP | |||
| 1 | Human vaccine | 1 dose | BD06 |
| 2 | Human vaccine | 1 dose | IRKV-THChina12 |
| 3 | Human vaccine | 3 dosesa | BD06 |
| 4 | Human vaccine | 3 dosesa | IRKV-THChina12 |
| 5 | Veterinary vaccine | 1 dose | BD06 |
| 6 | Veterinary vaccine | 1 dose | IRKV-THChina12 |
| 7 | rHAd5-TH12-G | 1 dose | BD06 |
| 8 | rHAd5-TH12-G | 1 dose | IRKV-THChina12 |
| 9 | rHAd5-TH12-G | 3 dosesa | BD06 |
| 10 | rHAd5-TH12-G | 3 dosesa | IRKV-THChina12 |
| 11 | rHAd5-BD06-G | 1 dose | BD06 |
| 12 | rHAd5-BD06-G | 1 dose | IRKV-THChina12 |
| 13 | rHAd5-BD06-G | 3 dosesa | BD06 |
| 14 | rHAd5-BD06-G | 3 dosesa | IRKV-THChina12 |
| 15 | Negative control (PBS) | 1 dose | BD06 |
| 16 | Negative control (PBS) | 1 dose | IRKV-THChina12 |
| PEP | |||
| 17 | HRIG | 20 IU/kg | BD06 |
| 18 | HRIG | 20 IU/kg | IRKV-THChina12 |
| 19 | HRIG | 200 IU/kg | BD06 |
| 20 | HRIG | 200 IU/kg | IRKV-THChina12 |
| 21 | Human vaccine | 4 dosesb | BD06 |
| 22 | Human vaccine | 4 dosesb | IRKV-THChina12 |
| 23 | HRIG + human vaccine | 200 IU/kg + 4 dosesb | BD06 |
| 24 | HRIG + human vaccine | 200 IU/kg + 4 dosesb | IRKV-THChina12 |
| 25 | HRIG + rHAd5-TH12-G | 200 IU/kg + 4 dosesb | BD06 |
| 26 | HRIG + rHAd5-TH12-G | 200 IU/kg + 4 dosesb | IRKV-THChina12 |
| 27 | HRIG + rHAd5-BD06-G | 200 IU/kg + 4 dosesb | BD06 |
| 28 | HRIG + rHAd5-BD06-G | 200 IU/kg + 4 dosesb | IRKV-THChina12 |
| 29 | IFN-α2a | 3 dosesc | BD06 |
| 30 | IFN-α2a | 3 dosesc | IRKV-THChina12 |
| 31 | HRIG + IFN-α2a | 200 IU/kg + 3 dosesc | BD06 |
| 32 | HRIG + IFN-α2a | 200 IU/kg + 3 dosesc | IRKV-THChina12 |
| 33 | Human vaccine + IFN-α2a | 4 dosesb + 3 dosesc | BD06 |
| 34 | Human vaccine + IFN-α2a | 4 dosesb + 3 dosesc | IRKV-THChina12 |
| 35 | Negative control (PBS) | 1 dose | BD06 |
| 36 | Negative control (PBS) | 1 dose | IRKV-THChina12 |
Hamsters were inoculated with a priming dose, followed by boosters 7 and 28 days later (50 μl/hamster), before exposure.
Hamsters were inoculated with vaccine on days 0 (100 μl/hamster), 7 (50 μl/hamster), and 21 (50 μl/hamster) after exposure.
Hamsters were injected with IFN-α2a on days 0, 1, and 2 (100,000 IU/hamster) after exposure.
Rabies biologics.
The following commercial rabies biologics were used in this study: a locally produced Vero cell vaccine for human use (strain PV2061, Chengda Suda, 0.5 ml/dose, lot 201209276; Liaoning Chengda Biotechnology Co.), an imported veterinary vaccine (strain Pasteur RIV, Nobivac Rabies, 1 ml/dose, lot A154A01; Intervet International), a commercially available human RABV immunoglobulin (HRIG) (Wusheng, 20 IU/kg, lot 20111101; Wuhan Institute of Biological Products Co.), and recombinant human alpha interferon 2a (IFN-α2a) (5,000,000 IU/ml, lot 20110443; Changchun Institute of Biological Products Co.).
Construction of recombinant human adenovirus type 5 expressing RABV and IRKV glycoproteins.
To compare the cross-immunogenicity of the viral glycoproteins of IRKV and RABV, the recombinant products recombinant human adenovirus type 5 (rHAd5)-THChina12-G and rHAd5-BD06-G were generated using an E1- and E3-deleted cytomegalovirus (CMV) adenoviral expression system (RAPAd; Cell Biolabs, Inc., CA) (14). In a typical procedure, BD06 and IRKV-THChina12 glycoprotein genes were inserted into multiple cloning sites of the shuttle vector pacCMVK-NpA. Then, HEK-293 cells were cotransfected with HAd5 backbone vector pacAd5 9.2–100 (devoid of the left inverted terminal repeat [ITR] and the packaging signal) using Lipofectamine (Invitrogen, CA) and were passaged until a typical cytopathic effect was observed (without the need to perform multiple plaque isolations). Expression of the lyssavirus glycoproteins in the transfected HEK-293 cells was determined using Light Diagnostics rabies FITC-globulin conjugate (EMD Millipore Corp., MA) (6).
Evaluation of the efficacy of rabies biologics against RABV and IRKV infection in animal models.
For preexposure prophylaxis (PrEP) experiments (http://www.who.int/rabies/human/WHO_strategy_prepost_exposure/en/index.html), 14 groups (groups 1 to 14) received intramuscular injections (in the gastrocnemius muscle) of 50 μl of commercial human or veterinary vaccines or experimental recombinant vaccines (rHAd5-TH12-G and rHAd5-BD06-G) (Table 1). Negative controls (groups 15 and 16) received only phosphate-buffered saline (PBS) (Table 1). Four weeks after the last vaccination, the hamsters were challenged i.m. with BD06 or IRKV-THChina12 (100 times the hamster i.m. LD50) (Table 1). Each hamster was immobilized and a blood sample was taken from the retro-orbital plexus before challenge. Serum samples were collected by centrifugation at 5,000 × g for 10 min and were subjected to fluorescent antibody virus neutralization (FAVN) testing based on RABV strain CVS-11 (15).
In the postexposure prophylaxis (PEP) experiments, 20 groups (groups 17 to 36) were challenged in the gastrocnemius muscle in the left hind leg with BD06 or IRKV-THChina12 (100 times the hamster LD50) (Table 1). Four hours after challenge, HRIG (20 or 200 IU/kg) was administered i.m. at the site of virus challenge (Table 1). The human vaccine was administered in the gastrocnemius muscle in the right hind leg, via the 2-1-1 regimen, on days 0 (4 hours after challenge, in the triceps brachii muscle in the right foreleg and the gastrocnemius muscle), 7, and 21 (Table 1) (http://www.who.int/rabies/human/WHO_strategy_prepost_exposure/en/index.html). IFN-α2a was administered in the triceps brachii muscle in the left foreleg on days 0 (4 hours after challenge), 1, and 2 (Table 1). Negative controls (groups 35 and 36) received PBS postchallenge (Table 1). On day 3 after challenge, a serum sample was taken from each hamster using the same method as described above, for FAVN testing based on RABV strain CVS-11 (15).
Statistical analyses.
The 95% confidence intervals for virus titers indicated in the text were calculated by the Neoprobit method (16). Fisher's exact test was used to compare the survival rates for animals in the groups challenged with different viruses and subjected to different prophylactic treatments. Variance analyses were performed to determine statistically significant differences in antibody titers by one-way analysis of variance (ANOVA). The analyses were performed using the SPSS 16.0 package (SPSS Inc., Chicago, IL). The results of the comparisons between groups were considered significant at P < 0.05.
RESULTS
Glycoprotein sequence comparisons.
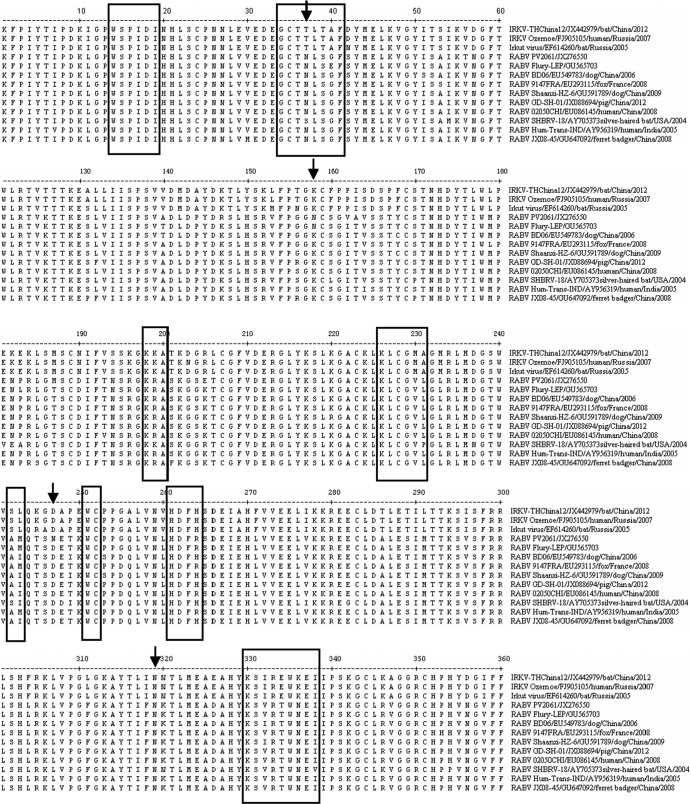
Comparison of the deduced amino acid (aa) sequences revealed that the ectodomain of RABV contains N-linked glycosylation sites at positions 37, 158 (PV2061 and Flury-LEP), 247, and 319, with two at positions 247 and 319 being present in IRKV (Fig. 1). Comparison of major antigenic sites (17–19) demonstrated that antigenic sites I (aa 226 to 231), II (aa 34 to 42 and 198 to 200), III (aa 330 to 338), and G1 (aa 242 to 243) in the ectodomain were different in IRKV than in RABV; however, antigenic sites IV (aa 251), G5 (aa 261 to 264), and VI (aa 264) and a B-cell epitope (aa 14 to 19) were fully conserved (Fig. 1).
Fig 1.
Comparative amino acid sequence alignment of glycoprotein ectodomains of IRKV and RABV. Numbers, amino acid positions. Boxes, antigenic sites, i.e., site I (aa 226 to 231), site II (aa 34 to 42 and 198 to 200), site III (aa 330 to 338), site IV (aa 251), site G1 (aa 242 to 243), site G5 (aa 261 to 264), and site VI (aa 264) and a novel B-cell epitope (aa 14 to 19). Arrows, four putative N-glycosylation sites.
Protective effects of rabies biologics against IRKV infection.
In the experiment with one-dose vaccination, all hamsters (except two immunized with rHAd5-TH12-G) survived the challenge with RABV; however, only 80% of the hamsters survived the challenge with IRKV (Table 2). In the conventional PrEP experiment, all hamsters immunized with 3 doses of human rabies vaccines survived challenges with RABV and IRKV (Table 2). All hamsters developed significant titers of virus-neutralizing antibodies (VNAs) in the serum, and the VNA titers of serum samples from hamsters immunized with 3 doses of vaccine were greater than those of hamsters immunized with one dose of vaccine.
Table 2.
Protection against RABV and IRKV elicited in hamsters by vaccines via preexposure prophylaxis
| Groups | Prechallenge VNA titer (mean ± SD) (IU/ml)a | No. of surviving hamsters/no. challenged with: |
|
|---|---|---|---|
| RABV BD06 | IRKV-THChina12 | ||
| 1 and 2 | 7.47 ± 2.06 | 10/10b | 8/10b |
| 3 and 4 | 94.07 ± 21.06 | 10/10 | 10/10 |
| 5 and 6 | 6.02 ± 3.63 | 10/10 | 8/10 |
| 7 and 8 | 0 | 2/10c | 8/10c |
| 9 and 10 | 1.17 ± 0.32 | 10/10 | 10/10 |
| 11 and 12 | 2.51 ± 2.43 | 8/10 | 2/10 |
| 13 and 14 | 27.22 ± 11.84 | 10/10 | 10/10 |
| 15 and 16 | 0 | 0/10 | 0/10 |
VNA titers were determined in FAVN tests against the CVS-11 RABV strain on the day of challenge.
P > 0.05.
P < 0.05.
The recombinant vaccines rHAd5-TH12-G and rHAd5-BD06-G were less efficient. No survival rates of 100% were achieved with one-dose vaccination, although these vaccines protected animals better against infection from a homologous virus (80%) than against a heterologous virus (20%) (P < 0.05). However, all hamsters immunized with 3 doses of recombinant vaccines survived challenges with RABV and IRKV (Table 2). In FAVN testing, virus neutralization titers of serum samples from hamsters immunized with 3 doses of rHAd5-BD06-G were about 27 times higher than those of hamsters immunized with 3 doses of rHAd5-TH12-G (Table 2).
In the PEP experiment (Table 3), 20 IU/kg of HRIG injected at the challenge site did not protect hamsters against IRKV infection, whereas 80% of hamsters survived RABV challenge. After treatment with 200 IU/kg of HRIG, the protection against IRKV infection increased to 60% (P < 0.05). The difference in VNA titers between groups that received 20 IU/kg and 200 IU/kg of HRIG (0.45 ± 0.21 and 3.50 ± 0.95 IU/ml, respectively) was statistically significant (P < 0.05). Furthermore, the combination of HRIG and IFN-α2a improved the survival rate (P > 0.05). The addition of human vaccines to prophylactic regimens decreased VNA titers and survival rates for animals in the groups challenged with IRKV (P < 0.05); however, the addition of recombinant vaccines did not significantly decrease VNA titers or survival rates. Incubation periods for hamsters challenged with RABV were 8 to 13 days and those for hamsters challenged with IRKV were 6 to 13 days. Following the incubation periods, rabies-like symptoms, such as lethargy, muscle weakness, and progressive paralysis of one or both hind limbs, were observed.
Table 3.
Protection against RABV and IRKV elicited in hamsters in postexposure prophylaxis experiments
| Groups | VNA titer (mean ± SD) (IU/ml)a | No. of surviving hamsters/no. challenged with: |
|
|---|---|---|---|
| RABV BD06 | IRKV-THChina12 | ||
| 17 and 18 | 0.45 ± 0.21 | 8/10b | 0/10b |
| 19 and 20 | 3.50 ± 0.95 | 10/10c | 6/10c |
| 21 and 22 | 0 | 0/10 | 0/10 |
| 23 and 24 | 1.10 ± 0.42 | 10/10 | 2/10 |
| 25 and 26 | 2.39 ± 1.15 | 10/10 | 6/10 |
| 27 and 28 | 1.90 ± 1.32 | 10/10b | 4/10b |
| 29 and 30 | 0 | 4/10 | 4/10 |
| 31 and 32 | 3.23 ± 1.09 | 10/10 | 8/10 |
| 33 and 34 | 0 | 3/10 | 3/10 |
| 35 and 36 | 0 | 0/10 | 0/10 |
Antibody titers were determined in FAVN tests against the CVS-11 RABV strain on day 3 after challenge with the viruses.
P < 0.05.
P > 0.05.
DISCUSSION
For the first time, an IRKV was recently isolated from a bat in China through the active rabies surveillance program. Although Chinese IRKV belongs to the same phylogroup, 1, as the widely distributed RABV, it is more closely related phylogenetically to European bat lyssavirus type 1 and Duvenhage virus than to RABV (6). Phylogenetic relationships and genetic distances between lyssaviruses partially reflect the extent of serological cross-reactivity. For example, it was suggested that 72 to 74% amino acid sequence identity within glycoprotein ectodomains provides sufficient cross-neutralization between lyssaviruses (8). The same can be inferred for the protection elicited by rabies biologics (which all are based on several well-characterized RABV strains) against non-RABV lyssaviruses. Commercial rabies biologics have been fully efficacious against a phylogenetically related Australian bat lyssavirus (20). However, the protection elicited by several rabies biologics against IRKV in animal models was limited (9).
Additional attention should be paid to conservation of antigenic sites on lyssavirus glycoproteins. This is particularly important as several virus-neutralizing monoclonal antibodies (MAbs) were offered for replacement of conventional HRIG in human rabies PEP (21–28). For example, a linear epitope with the key residues LCGV within antigenic site I serves for binding of MAbs CR57 and 62071-3 (22, 23). Similarly, conserved conformational antigenic sites II and III contain binding epitopes for several other MAbs (24–28). Amino acid differences in these epitopes may alter MAb binding and thus abolish neutralization of non-RABV lyssaviruses, including IRKV. Therefore, MAbs selected for antibody cocktails for use in PEP must be scrutinized for their ability to neutralize non-RABV lyssaviruses.
In this study, the efficacy of rabies biologics available in China against IRKV was determined in routine and modified PrEP and PEP experiments, as described previously (9). Although a single dose of rabies vaccine did not induce adequate protection against IRKV infection, routine PrEP (three vaccine doses, on days 0, 7, and 28) induced strong protection against RABV and IRKV infection.
In the PEP experiments, however, only very high doses of RABV immunoglobulins conferred partial protection of animals against IRKV infection, whereas the routine PEP regimen (20 IU/kg body weight of HRIG injected only once, followed by the 2-1-1 vaccine regimen) did not protect animals against IRKV infection at all. Moreover, combination of HRIG with vaccines decreased the VNA titers and survival rates of hamsters, compared with the groups that were given HRIG only, likely because of interference between the vaccines and the immunoglobulins. Short incubation periods in a hamster model may not provide sufficient time for the development of active immune responses, with protection against infection being based solely on antibodies delivered passively. These antibodies may fail to protect animals against IRKV infection based on limited cross-neutralization activity (Tables 2 and 3). It is possible that PEP models with longer incubation periods or situations involving real-life exposures would allow sufficient time for the development of active immune responses to vaccination and would protect animals and humans against IRKV infection, as may be inferred from our PrEP experiments. However, short incubation periods may occur after real-life exposures as well (29), and this increases the demand for the development of novel immunoglobulin preparations (including MAbs) for protection against IRKV and other non-RABV lyssaviruses.
Interferon can inhibit rabies virus activity in vivo and does not interfere with circulating VNAs (30). This was confirmed in our PEP experiment. Perhaps addition of interferon to PEP regimens would increase the protection of humans against non-RABV lyssaviruses, although this must be further evaluated in higher-mammalian models and clinical trials.
A replication-deficient vector derived from human adenovirus type 5 was chosen for the cross-immunogenicity experiments, since it has been well studied for expression of RABV proteins and has been evaluated in animal models (31). In our experiments, a recombinant vaccine expressing IRKV glycoproteins provided significantly better results in animals challenged with IRKV than did a recombinant vaccine expressing RABV glycoproteins. However, it did not provide reliable protection against RABV challenge. Chimeric lyssavirus glycoproteins from partial RABV and IRKV genes should be considered, to achieve protection against these two viruses (32, 33).
Surveillance for non-RABV lyssaviruses in China is very limited, as is diagnostic capacity across the country. The threat of IRKV to animals and humans is difficult to estimate. Although at least two bat-associated human rabies cases in China have been reported (6), IRKV was not identified in those cases, as the diagnoses were based on clinical signs and exposure history only.
In conclusion, commercially available rabies biologics do not provide protection against IRKV infection as reliable as that against RABV infection. Before the development of new biologics (e.g., chimeric vaccines and MAbs), the combination of higher doses of RABV immunoglobulins with interferon may be effective treatment for exposure to IRKV and other non-RABV lyssaviruses. In addition, active field surveys should be performed to investigate more thoroughly the prevalence and circulation patterns of IRKV in China, to assess properly the public health and veterinary implications.
ACKNOWLEDGMENTS
This project was funded by the Key Project (grant 30630049) and the Youth Program (grants 30900058 and 31001070) of the National Natural Science Foundation of China and the National 973 Project (grant 2011CB500705).
Footnotes
Published ahead of print 14 August 2013
REFERENCES
- 1.Kuzmin IV, Tordo N. 2012. Genus lyssavirus, p 37–57 In Dietzgen RD, Kuzmin IV. (ed), Rhabdoviruses: molecular taxonomy, evolution, genomics, ecology, host-vector interactions, cytopathology and control. Caister Academic Press, London, United Kingdom [Google Scholar]
- 2.Marston DA, Horton DL, Ngeleja C, Hampson K, McElhinney LM, Banyard AC, Haydon D, Cleaveland S, Rupprecht CE, Bigambo M, Fooks AR, Lembo T. 2012. Ikoma lyssavirus, highly divergent novel lyssavirus in an African civet. Emerg. Infect. Dis. 18:664–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freuling CM, Beer M, Conraths CD, Finke S, Hoffmann B, Keller B, Kliemt J, Mettenleiter TC, Mühlbach E, Teifke JP, Wohlsein P, Müller T. 2011. Novel lyssavirus in Natterer's bat, Germany. Emerg. Infect. Dis. 17:1519–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arechiga Ceballos N, Vazquez Morón S, Berciano JM, Nicolás O, Aznar López C, Juste J, Rodriguez Nevado C, Aguilar Setién A, Echevarría JE. 2013. Novel lyssavirus in bat, Spain. Emerg. Infect. Dis. 19:793–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu RL, Tang Q, Tang JR, Fooks AR. 2009. Rabies in China: an update. Vector Borne Zoonotic Dis. 9:1–12 [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Zhang SF, Zhao J, Zhang F, Hu RL. 2013. Isolation of Irkut virus from a Murina leucogaster bat in China. PLoS Negl. Trop. Dis. 7:e2097. 10.1371/journal.pntd.0002097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonova GN, Belikov SI, Kondratov IG, Krylova NV, Pavlenko EV, Romanova EV, Chentsova IV, Petukhova SA. 2009. A fatal case of bat lyssavirus infection in Primorye Territory of the Russian Far East. Rabies Bull. Eur. 33:5–8 [Google Scholar]
- 8.Badrane H, Bahloul C, Perrin P, Tordo N. 2001. Evidence of two lyssavirus phylogroups with distinct pathogenicity and immunogenicity. J. Virol. 75:3268–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanlon CA, Kuzmin IV, Blanton JD, Weldon WC, Manangan JS, Rupprecht CE. 2005. Efficacy of rabies biologics against new lyssaviruses from Eurasia. Virus Res. 111:44–54 [DOI] [PubMed] [Google Scholar]
- 10.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/95/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]
- 11.Liu Y, Zhang SF, Zhang F, Hu RL. 2012. Adaptation of a Chinese ferret badger strain of rabies virus to high-titered growth in BHK-21 cells for canine vaccine development. Arch. Virol. 157:2397–2403 [DOI] [PubMed] [Google Scholar]
- 12.Meslin F-X, Kaplan MM, Koprowski H. (ed). 1996. Laboratory techniques in rabies, 4th ed, p 80–168 World Health Organization, Geneva, Switzerland [Google Scholar]
- 13.Ministry of Science and Technology (MOST) of the People's Republic of China 2006. Guideline on humane treatment of laboratory animals. Ministry of Science and Technology, Beijing, China [Google Scholar]
- 14.Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. 2000. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 7:1034–1038 [DOI] [PubMed] [Google Scholar]
- 15.Cliquet F, Aubert M, Sagné L. 1998. Development of a fluorescent antibody virus neutralization test (FAVN test) for the quantitation of rabies-neutralizing antibody. J. Immunol. Methods 212:79–87 [DOI] [PubMed] [Google Scholar]
- 16.Aubert MFA. 1996. Methods of the calculation of titers, p 445–456 In Meslin FX, Kaplan MM, Koprowski H. (ed), Laboratory techniques in rabies, 4th ed. World Health Organization, Geneva, Switzerland [Google Scholar]
- 17.Houimel M, Dellagi K. 2009. Peptide mimotopes of rabies virus glycoprotein with immunogenic activity. Vaccine 27:4648–4655 [DOI] [PubMed] [Google Scholar]
- 18.Bunschoten H, Gore M, Claassen IJ, Uytdehaag FG, Dietzschold B, Wunner WH, Osterhaus AD. 1989. Characterization of a new virus-neutralizing epitope that denotes a sequential determinant on the rabies virus glycoprotein. J. Gen. Virol. 70:291–298 [DOI] [PubMed] [Google Scholar]
- 19.Benmansour A, Leblois H, Coulon P, Tuffereau C, Gaudin Y, Flamand A, Lafay F. 1991. Antigenicity of rabies virus glycoprotein. J. Virol. 65:4198–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torvaldsen S, Watson T. 1998. Rabies prophylaxis in Western Australia: the impact of Australian bat lyssavirus. Commun. Dis. Intell. 22:149–152 [PubMed] [Google Scholar]
- 21.Horton DL, McElhinney LM, Marston DA, Wood JL, Russell CA, Lewis N, Kuzmin IV, Fouchier RA, Osterhaus AD, Fooks AR, Smith DJ. 2010. Quantifying antigenic relationships among the lyssaviruses. J. Virol. 84:11841–11848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization 2002. Monoclonal antibody cocktail for rabies post exposure treatment: report of a WHO consultation, 23–24 May 2002. WHO document R2-370-15. World Health Organization, Geneva, Switzerland [Google Scholar]
- 23.Both L, van Dolleweerd C, Wright E, Banyard AC, Bulmer-Thomas B, Selden D, Altmann F, Fooks AR, Ma JK. 2013. Production, characterization, and antigen specificity of recombinant 62-71-3, a candidate monoclonal antibody for rabies prophylaxis in humans. FASEB J. 27:2055–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marissen WE, Kramer RA, Rice A, Weldon WC, Niezgoda M, Faber M, Slootstra JW, Meloen RH, Clijsters-van der Horst M, Visser TJ, Jongeneelen M, Thijsse S, Throsby M, de Kruif J, Rupprecht CE, Dietzschold B, Goudsmit J, Bakker AB. 2005. Novel rabies virus-neutralizing epitope recognized by human monoclonal antibody: fine mapping and escape mutant analysis. J. Virol. 79:4672–4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller T, Dietzschold B, Ertl H, Fooks AR, Freuling C, Fehlner-Gardiner C, Kliemt J, Meslin FX, Franka R, Rupprecht CE, Tordo N, Wanderler AI, Kieny MP. 2009. Development of a mouse monoclonal antibody cocktail for post-exposure rabies prophylaxis in humans. PLoS Negl. Trop. Dis. 3:e542. 10.1371/journal.pntd.0000542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakker AB, Marissen WE, Kramer RA, Rice AB, Weldon WC, Niezgoda M, Hanlon CA, Thijsse S, Backus HH, de Kruif J, Dietzschold B, Rupprecht CE, Goudsmit J. 2005. Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants. J. Virol. 79:9062–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Rowley KJ, Booth BJ, Sloan SE, Ambrosino DM, Babcock GJ. 2011. G glycoprotein amino acid residues required for human monoclonal antibody RAB1 neutralization are conserved in rabies virus street isolates. Antiviral Res. 91:187–194 [DOI] [PubMed] [Google Scholar]
- 28.Sloan SE, Hanlon C, Weldon W, Niezgoda M, Blanton J, Self J, Rowley KJ, Mandell RB, Babcock GJ, Thomas WD, Jr, Rupprecht CE, Ambrosino DM. 2007. Identification and characterization of a human monoclonal antibody that potently neutralizes a broad panel of rabies virus isolates. Vaccine 25:2800–2810 [DOI] [PubMed] [Google Scholar]
- 29.Wilde H, Sirikawin S, Sabcharoen A, Kingnate D, Tantawichien T, Harischandra PA, Chaiyabutr N, de Silva DG, Fernando L, Liyanage JB, Sitprija V. 1996. Failure of postexposure treatment of rabies in children. Clin. Infect. Dis. 22:228–232 [DOI] [PubMed] [Google Scholar]
- 30.Hilfenhaus J, Weinmann E, Majer M, Barth R, Jaeger O. 1977. Administration of human interferon to rabies virus-infected monkeys after exposure. J. Infect. Dis. 135:846–849 [PubMed] [Google Scholar]
- 31.Vos A, Neubert A, Pommerening E, Müller T, Döhner L, Neubert L, Hughes K. 2001. Immunogenicity of an E1-deleted recombinant human adenovirus against rabies by different routes of administration. J. Gen. Virol. 82:2191–2197 [DOI] [PubMed] [Google Scholar]
- 32.Bahloul C, Jacob Y, Tordo N, Perrin P. 1998. DNA-based immunization for exploring the enlargement of immunological cross-reactivity against the lyssaviruses. Vaccine 16:417–425 [DOI] [PubMed] [Google Scholar]
- 33.Jallet C, Jacob Y, Bahloul C, Drings A, Desmezieres E, Tordo N, Perrin P. 1999. Chimeric lyssavirus glycoprotein genes with increased immunological potential. J. Virol. 73:225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]