Abstract
A prospective study was performed to determine the value of direct molecular testing of whole blood for detecting the presence of culturable and unculturable bacteria and yeasts in patients with suspected bloodstream infections. A total of 464 adult and pediatric patients with positive blood cultures matched with 442 patients with negative blood cultures collected during the same period were recruited during a 10-month study. PCR amplification coupled with electrospray ionization mass spectrometry (PCR-ESI-MS) plus blood culture reached an overall agreement of 78.6% in the detection and species-level identification of bacterial and candidal pathogens. Of 33 culture-negative/PCR-ESI-MS-positive specimens, 31 (93.9%) were judged to be truly bacteremic and/or candidemic based on a medical chart review and analytical metrics. Among the 15 culture-positive specimens in which PCR-ESI-MS detected additional bacterial or yeast species, 66.7% and 20.0% of the additional positive specimens by PCR-ESI-MS were judged to be truly or possibly bacteremic and/or candidemic, respectively. Direct analysis of blood samples by PCR-ESI-MS rapidly detects bacterial and yeast pathogens in patients with bloodstream infections. When used in conjunction with blood culture, PCR-ESI-MS enhances the diagnostics of septicemia by shortening test turnaround time and improving yields.
INTRODUCTION
Bacteremia and candidemia are associated with significant morbidity and mortality. Sepsis is estimated to cause >200,000 deaths annually in the United States alone (1, 2). Although various medical interventions are available, the initiation of early antimicrobial therapy remains a major factor in favorable outcomes in cases of sepsis (2, 3). Currently, patients meeting the clinical criteria for sepsis are placed on empirical antimicrobial therapy before any pathogen is identified. This practice is largely driven by studies demonstrating that sepsis-related morbidity and mortality are dramatically increased when antibiotic therapy is delayed (2, 4, 5).
Currently, the gold standard microbial detection method is the time-consuming process of microbiological culture. In general, culture detects bacterial pathogens within 12 to 48 h of inoculation (6, 7). Precise microbial identification and antibiotic susceptibility determination require an additional 6 to 24 h (7). Thus, a patient often is treated with broad-spectrum antibiotics for several days prior to the establishment of more focused therapy. Frequent and extended uses of broad-spectrum antibiotics are contributing factors in the recent increases in antibiotic-resistant organisms (8). Another limitation of culture is its decreased sensitivity for unculturable or fastidious organisms and in instances when the sample is collected from a patient who has already started antimicrobial therapy (9, 10). Blood cultures are reported to be negative in >50% of cases where true bacterial or yeast sepsis is believed to exist based upon other analytical measurements and clinical presentation (11, 12). This has driven the recommendation by the International Sepsis Committee that decisions regarding antibiotic administration, changes in therapy, or discontinuation of antibiotic therapy be based on clinical judgment rather than culture results (10, 11).
More rapid and sensitive methods to identify bloodstream infections are urgently needed (9, 13). Molecular methods that detect microbes utilizing PCR do so by directly detecting and amplifying microbial nucleic acids that are present in the blood (14, 15). A recently developed molecular detection method analyzes the PCR amplification products by electrospray ionization mass spectrometry (ESI-MS) (16). ESI-MS can measure the mass of PCR amplicons with sufficient accuracy to enable the calculation of the base composition of the amplicon. By comparing the base composition of the detected organism to the base compositions of known organisms, the pathogens present in the sample can be identified within 6 to 8 h of obtaining it (13, 17). Accurate PCR-ESI-MS detections would allow for the initial antimicrobial therapy to be based on the organism(s) present, resulting in more optimal outcomes, reduced toxicity, lower costs, and the preservation of existing antimicrobials from bacterial resistance.
In a previous study, we demonstrated the usefulness of PCR-ESI-MS for rapid and accurate detection of Ehrlichia species in patients who were clinically suspected to have ehrlichiosis. In addition, we were able to identify additional bacterial pathogens with matched clinical manifestations directly in blood specimens (17). This prospective study evaluates the use of PCR-ESI-MS as an adjunct to traditional blood culture to enhance the speed and yield of bacteremia and candidemia diagnostics.
(This study was presented in part at the 111th Annual Meeting of the American Society for Microbiology, New Orleans, LA, 21 to 24 May 2011.)
MATERIALS AND METHODS
Patient samples.
Whole-blood samples were collected at the Vanderbilt University Medical Center (VUMC) between 18 January and 13 November 2010. The samples were representative of those submitted to the VUMC microbiology laboratory for blood culture. When a patient culture was positive, refrigerated surplus whole-blood samples that were drawn within the same 8-h period for other diagnostic assays were collected as cases for PCR-ESI-MS analysis. In addition, refrigerated surplus blood samples from patients whose blood culture result remained negative were selected as controls, matched with the cases by age, sex, and incubation time. Only one whole-blood specimen per patient was included in the study and was aliquoted and stored at −80°C prior to nucleic acid extraction.
Blood culture, pathogen identification, and antimicrobial susceptibility.
Patient whole-blood samples were processed with the Bactec 9240 blood culture system (Becton, Dickinson, Sparks, MD). When the positive blood cultures were confirmed by Gram stain, the blood culture bottle contents were subcultured, and identification and differentiation were carried out by a battery of phenotypic methods, including the API commercial biochemical identification strips (bioMérieux, Inc., Durham, NC), which were used according to the manufacturer's instructions (18–20).
Molecular analysis.
Samples for molecular analysis were analyzed using the BAC Spectrum assay (Ibis Biosciences) on the PCR-ESI-MS system (PLEX-ID; Abbott Laboratories), as described previously (17, 21–23). The details of the primer sequences, gene targets, primer coverage of the bacterial and fungal domains, and the organization and multiplexing configuration of the primers on the microtiter plates are described in Table S1 in the supplemental material.
Lysis and DNA isolation.
A 1.25-ml whole-blood sample was mechanically lysed by bead beating in the presence of SDS and proteinase K. Following bead beating, the tube was incubated for 15 min at 56°C to facilitate enzymatic proteolysis. The DNA was purified on a KingFisher system using the UltraClean DNA isolation kit (22, 23). Briefly, DNA was captured on magnetic silica microparticles in the presence of guanidine isothiocyanate and ethanol and washed once in the same buffer. Three washes in aqueous ethanol were followed by elution in 250 μl of water at 70°C.
PCR amplification.
Whole-blood DNA was amplified in 16 separate wells in the assay plates. The 16 wells contained a set of 18 primer pairs optimized for the efficient detection and identification of bacterial and Candida species, as well as mecA, vanA, vanB, and Klebsiella pneumoniae carbapenemase (KPC) antibiotic resistance elements (see Table S1 in the supplemental material). All PCR cycling was carried out on an Eppendorf thermal cycler in plates that were heat sealed with a piercable foil. Loading of the KingFisher trays and the PCR plates was performed robotically on the PLEX-ID fluid handler (17, 22, 23).
ESI-MS.
Following amplification, the contents of each well were desalted by a process that included the binding of amplicons to magnetic microparticles, washing, and elution in a volatile buffer. The desalted amplicons were injected into a time of flight mass spectrometer by electrospray ionization. This process separates the two DNA strands of the amplicon without fragmentation, enabling the molecular masses of both strands to be determined separately (17, 22, 23).
Microbial species identification.
All data analyses were conducted in an automated fashion on the PCR-ESI-MS instrument. The algorithm triangulates to determine a maximum likelihood estimate of the population of microbial species present in the test sample (16). The measured base composition signatures were compared against a very large database, which contained the complete amplicon signatures for 612 reportable clinically relevant bacterial and Candida species. Additional amplicon information is fully populated for another 299 species for which complete genomic signatures exist (GenBank, NCBI, Bethesda, MD) but there is not sufficient literature supporting their clinical relevance. These organism base count signatures are utilized by the software algorithm; however, they are not reportable by name. Instead, the user is informed with a message saying, “Bacteria detected—no ID can be provided.”
Data analysis.
Along with organism identification, the ESI-MS analysis includes a level and a Q score. The level indicates the amount of amplified DNA present in the sample. This is reported as genome equivalents/well and is calculated with reference to the internal calibrant, as previously described (24). The Q score, which is a rating between 0 (low) and 1 (high), represents a relative measure of the strength of the data supporting an identification; only Q scores of ≥0.90 were reported.
Clinical relevance determination.
The patient medical charts were reviewed by an investigator (C.W.S.) and fell into one of two categories: (i) two or more pathogens were detected and identified by the Ibis PLEX-ID BAC in the case group, and (ii) pathogens were detected and identified by the Ibis PLEX-ID BAC in the control group. The clinical relevance of these findings was determined for each patient by the following criteria: (i) clinical manifestations, (ii) the results of laboratory tests, such as white blood cell count and differentiation and C-reactive proteins, (iii) blood culture results from this and other specimens collected from the same patient, and (iv) whether specific therapy was initiated and the results of such therapy.
RESULTS
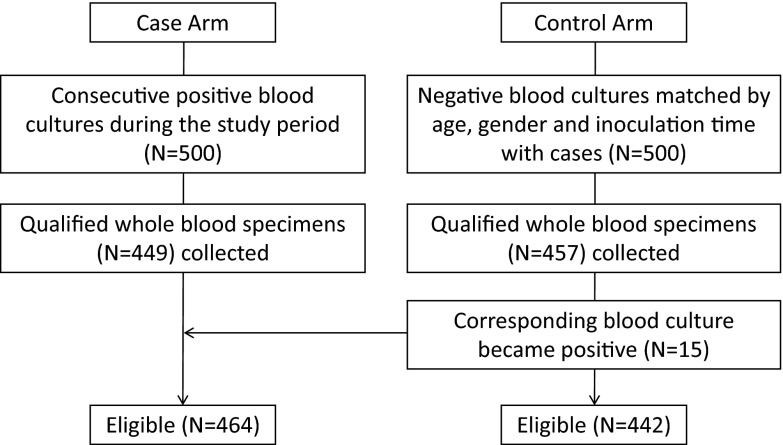
We performed a prospective study in which we applied a multilocus broad-range PCR amplification coupled with electrospray ionization mass spectrometry (PCR-ESI-MS) to directly identify microorganisms in whole-blood specimens collected from patients with clinically suspected septicemia at the Vanderbilt University Medical Center. During the study period, 500 consecutive cases with positive blood culture results and 500 controls with negative blood culture results matched by age, gender, and incubation time were selected for the study. Soon after the cases and controls were defined, 906 surplus whole-blood samples were collected from these selected patients within an 8-h period for use in other diagnostic assays. Fifteen whole-blood specimens from the control arm were transferred to the case arm, as their blood cultures turned positive later during the incubation period. A total of 464 culture-positive (cases) and 442 culture-negative (control) whole-blood samples were obtained and analyzed by PCR-ESI-MS (Fig. 1). The average age ± the standard deviation and the percentage of male participants were 52.10 ± 17.75 years and 60.3% for cases and 51.74 ± 17.53 years and 59.9% for controls, respectively.
Fig 1.
Accountability of specimens collected. See Materials and Methods for inclusion and exclusion criteria.
Among the total 906 samples, 173 were positive for coagulase-negative staphylococci (CoNS), including 119 detected by culture, 16 detected by PCR-ESI-MS only, and 38 detected by both methods. CoNS are more often than not seen in single-blood culture sets as a result of contamination. Repeatability across multiple sets can be used to increase confidence in the clinical significance of CoNS-positive cultures, but in the absence of this or other evidence favoring a different conclusion, statistics supports the interpretation of a single CoNS result as the result of a stochastic contamination event (25). In this study, no attempt was made to acquire multiple samples, and therefore these 173 samples were excluded from further analysis.
Of the 733 included specimens, there were 576 specimens (178 in cases and 398 in controls) where culture and PCR-ESI-MS were in agreement on the primary detection, resulting in an overall agreement of 78.6% (Table 1). In 155 of the case specimens, there was perfect concordance at the species level, including in 6 reported dual detections (Table 2). For another 23 samples, the primary identification matched between the two methods, but PCR-ESI-MS found an additional organism in 15 instances, culture found a second organism in six instances, and in 2 instances, each method had a secondary detection that did not match (Table 3). In 97 instances, the culture was positive but no detection was reported by PCR-ESI-MS (Table 4). While a small number of these organisms were outliers, the distribution of the organisms in this group was similar to that shown in Table 2.
Table 1.
Summary of results for PCR-ESI-MS and culture
| Category | Data (no.) by culture result |
|
|---|---|---|
| Positive | Negative | |
| PCR-ESI-MS and culture were in agreement in the no. of detections and species identity | 155a | 398 |
| Culture and PCR-ESI-MS were in agreement on primary pathogen identification with unmatched secondary detections in either method | 23b | 0 |
| Culture positive, PCR-ESI-MS negative | 97c | 0 |
| PCR-ESI-MS positive, culture negative | 0 | 33d |
Table 2.
Concordant detections in PCR-ESI-MS and culture
| Organism(s) detected | No. of samples in which organisms were detected |
|---|---|
| Single organism | |
| Staphylococcus aureus | 64 |
| Enterococcus faecalis | 15 |
| Escherichia coli | 14 |
| Klebsiella pneumoniae | 11 |
| Enterococcus faecium | 6 |
| Serratia marcescens | 4 |
| Candida glabrata | 3 |
| Enterobacter cloacae complex | 3 |
| Proteus mirabilis | 3 |
| Streptococcus agalactiae | 3 |
| Acinetobacter baumannii | 2 |
| Candida albicans | 2 |
| Candida parapsilosis | 2 |
| Enterobacter aerogenes | 2 |
| Pseudomonas aeruginosa | 2 |
| Staphylococcus lugdunensis | 2 |
| Stenotrophomonas maltophilia | 2 |
| Streptococcus mitis/Streptococcus pneumoniae | 2 |
| Candida tropicalis | 1 |
| Enterobacter sp. | 1 |
| Citrobacter braakii | 1 |
| Klebsiella oxytoca | 1 |
| Salmonella enterica | 1 |
| Streptococcus pyogenes | 1 |
| Streptococcus vestibularis | 1 |
| Multiple organisms | |
| Staphylococcus aureus and K. pneumoniae | 1 |
| E. coli and K. pneumoniae | 1 |
| S. aureus and P. aeruginosa | 1 |
| E. coli and E. aerogenes | 1 |
| K. pneumoniae and Aeromonas hydrophila | 1 |
| E. faecium and C. albicans | 1 |
| Total no. of organisms | 155 |
Table 3.
Concordant primary pathogen identification with unmatched secondary detections
| Primary organism(s) detected in concordant PCR-ESI-MS and culture results | Secondary detection result by: |
|
|---|---|---|
| PCR-ESI-MS | Culture | |
| Acinetobacter baumannii | Serratia marcescens | None |
| A. baumannii | Proteus mirabilis | Stenotrophomonas maltophilia |
| Candida albicans | Escherichia coli | None |
| Candida parapsilosis | None | Enterococcus faecium |
| Enterobacter cloacae complex | Enterococcus faecalis | None |
| E. cloacae complex | Leclercia adecarboxylata | None |
| E. cloacae complex | None | Citrobacter freundii |
| E. cloacae complex | None | E. faecalis |
| E. faecalis | E. cloacae complex | None |
| E. faecalis | Enterococcus durans or Enterococcus hirae | None |
| E. coli | None | Klebsiella oxytoca |
| K oxytoca | Citrobacter koseri | None |
| Klebsiella pneumoniae | Enterobacter aerogenes | None |
| K. pneumoniae | Citrobacter amalonaticus | None |
| K. pneumoniae | S. maltophilia | None |
| K. pneumoniae | C. albicans | P. mirabilis |
| Staphylococcus aureus | Candida guilliermondii | None |
| S. aureus | E. coli | None |
| S. aureus | Lactobacillus casei | None |
| S. aureus | Pseudomonas aeruginosa | None |
| S. aureus | None | E. faecalis |
| S. aureus, Streptococcus agalactiae | None | E. faecalis |
| Streptococcus pneumoniae | Streptococcus suis | None |
Table 4.
Culture-positive and PCR-ESI-MS-negative detections
| Culture-reported organism(s) when PCR-ESI-MS was negative | No. of culture-positive, PCR-ESI-MS-negative samples |
|---|---|
| Staphylococcus aureus | 28 |
| Streptococcus spp. | 13 |
| Escherichia coli | 13 |
| Enterococcus faecium | 6 |
| Acinetobacter spp. | 5 |
| Enterobacter cloacae | 4 |
| Klebsiella pneumoniae | 3 |
| Pseudomonas aeruginosa | 3 |
| Bacillus spp. | 2 |
| Citrobacter koseri | 2 |
| Enterococcus faecalis | 2 |
| Klebsiella oxytoca | 2 |
| Proteus mirabilis | 2 |
| Burkholderia cepacia | 1 |
| Candida albicans | 1 |
| C. albicans + E. faecalis | 1 |
| Diphtheroids (aerobic Corynebacterium sp.) | 1 |
| E. faecalis + Staphylococcus gallinarum | 1 |
| Enterococcus sp. + E. faecium | 1 |
| Stenotrophomonas maltophilia | 1 |
| Veillonella spp. (growth after 4 days) | 1 |
| Rhodotorula spp. | 1 |
| Zygosaccharomyces fermentati | 1 |
| Fusarium spp. (growth after 5 days) | 1 |
| Positive culture with no organism name provided | 1 |
| Total no. of organisms | 97 |
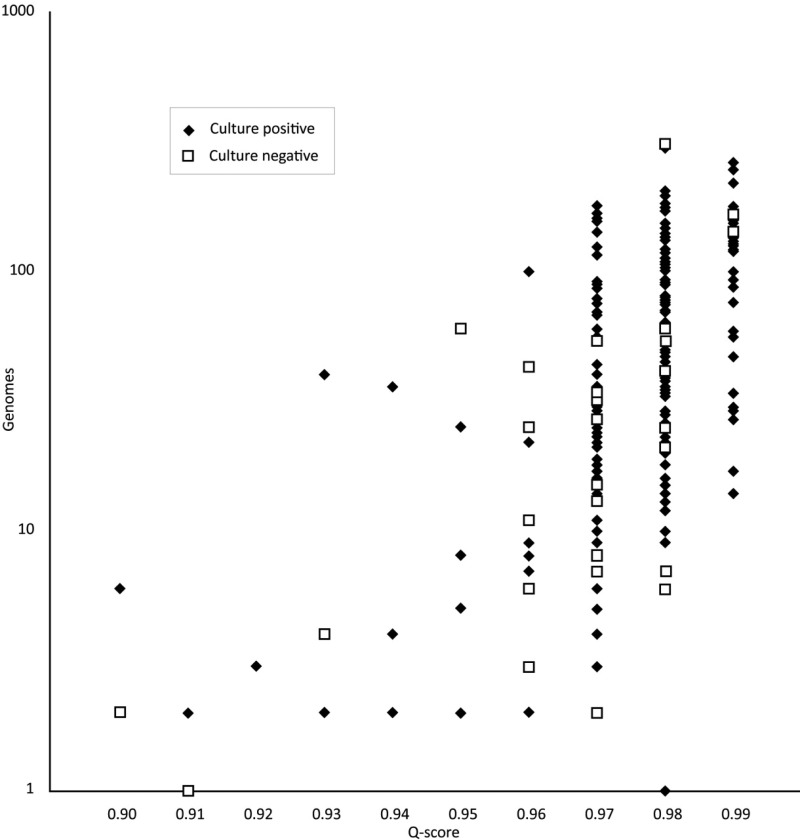
Finally, PCR-ESI-MS detected an organism in culture-negative samples in 33 instances (Table 5). The reliability and relevance of these results were further explored by a comparison of the analytical metrics (Fig. 2) and a review of patient medical charts (Table 6). The distribution of the analytic metrics of Q scores and levels for the culture-negative/PCR-ESI-MS-positive samples are illustrated in Fig. 2. The PCR-ESI-MS-positive/culture-negative results are distributed similarly to the results that had a positive corroborative culture, except for two samples that are at the lower limits of the acceptable Q score and genome level. Detailed clinical chart review results on 33 PCR-ESI-MS-positive/culture-negative and 15 PCR-ESI-MS-multipositive/culture-single-positive specimens (Table 2) are included in Table S2 in the supplemental material. Of the 33 PCR-ESI-MS-positive/culture-negative specimens, 31 (93.9%) were judged to be truly bacteremic and/or candidemic with or without identification of the correct pathogen (see Table S2a in the supplemental material). While there were no ambiguous results identified, two (6.1%) were judged to be false positives, most likely due to skin contamination, and this corresponded perfectly with the two specimens with low Q scores in Fig. 2. Of the 15 PCR-ESI-MS-multipositive/culture-single-positive specimens, a medical chart review indicated that 10 (66.7%) specimens were truly or possibly bacteremic and/or candidemic with or without identification of the correct pathogen in the medical record (see Table S2b in the supplemental material).
Table 5.
PCR-ESI-MS-positive and culture-negative detections
| Organism(s) detected | No. of PCR-ESI-MS-positive, culture-negative samples |
|---|---|
| Acinetobacter baumannii | 1 |
| Acinetobacter calcoaceticus | 1 |
| Brevundimonas diminuta | 1 |
| Corynebacterium kroppenstedtii | 2 |
| Enterococcus faecalis | 2 |
| Escherichia coli | 5 |
| Klebsiella pneumoniae | 1 |
| Lactobacillus gasseri | 1 |
| Mycoplasma hominis | 1 |
| Pseudomonas aeruginosa | 1 |
| Staphylococcus aureus | 6 |
| Staphylococcus lugdunensis | 1 |
| Streptococcus mitis/Streptococcus pneumoniae | 1 |
| Streptococcus pyogenes | 1 |
| Streptococcus salivarius/Streptococcus thermophilus | 1 |
| Actinomyces viscosus and P. aeruginosa | 1 |
| Corynebacterium striatum and E. faecalis | 1 |
| Enterobacter cloacae complex and Leclercia adecarboxylata | 2 |
| Streptococcus equi and S. salivarius/S. thermophilus | 1 |
| Citrobacter amalonaticus, E. faecalis, and Escherichia vulneris | 1 |
| Clostridium perfringens, Clostridium septicum/Clostridium tertium, and Klebsiella oxytoca | 1 |
| Total no. of organisms | 33 |
Fig 2.
Distribution of Q scores and genome levels for the culture-negative PCR-ESI-MS-positive samples. A Q score of 0.9 is required for reporting a positive detection, and higher Q scores are indicative of higher confidence results.
Table 6.
Clinical relevance of PCR/ESI-MS detections in culture-negative samples
| Clinical relevance of detections | No. (%) of samples with results: |
|
|---|---|---|
| PCR-ESI-MS positive, culture negative (n = 33)a | PCR-ESI-MS multipositive, culture single-positive (n = 15) | |
| True bacteremia and/or candidemia with correct pathogen identification | 10 (30.3) | 3 (20.0) |
| True bacteremia and/or candidemia | 21 (63.6) | 7 (46.7) |
| Possible bacteremia and/or candidemia | 0 (0.0) | 3 (20.0) |
| False positive most likely due to skin contamination | 2 (6.1) | 2 (13.3) |
See Table 5 for pathogen identification by PCR-ESI-MS.
DISCUSSION
Continuously monitored blood culture is the current gold standard for identifying pathogens associated with bloodstream infections. This study demonstrates that PCR-ESI-MS analysis done directly on blood specimens is a useful adjunct to continuously monitored blood cultures and can provide rapid and helpful microbial information to clinicians in the setting of the suspected bloodstream infection. The majority of the additional positive results provided by PCR-ESI-MS were clinically relevant.
In this study, among the 906 whole-blood specimens collected from patients with clinically suspected bloodstream infections, 78.6% overall agreement was observed between the blood culture and PCR-ESI-MS results. In 33 of 431 (about 7.7%) of the culture-negative samples, pathogen DNA was detected by PCR-ESI-MS. When 8% was used as the overall positive blood culture rate at the Vanderbilt University Medical Center (19), the 464 culture-positive samples represent the culture-positive yield from a total population of 5,800 blood cultures taken, of which 5,336 were negative by culture. If all 5,800 samples had been evaluated, an additional 409 (33/431 × 5,336) would have tested positive using the PCR-ESI-MS method. Using positivity by either culture or PCR-ESI-MS as a standard, the sum of all the culture positives and the projected PCR-ESI-MS positives is 711. Of these, 97 would be positive by culture only, 202 would be positive by both methods, and 409 samples would be positive by PCR-ESI-MS in the projected culture-negative population. The estimated sensitivities of PCR-ESI-MS and culture were 85.9% [(202 + 409)/711] and 41.2% [(202 + 91)/711], respectively. Our data support previous assessments that culture is believed to miss half of the cases of bacteremia (11, 12).
The PCR-ESI-MS results in the culture-negative cases are not corroborated by any direct analytical method. This conundrum is difficult to resolve. In the PCR-ESI-MS assay, nine primer pairs were used to amplify relatively short (∼100 bp) regions of the bacterial genome, and amplicons were not required from all the primer pairs in order to report a positive result (17, 22, 23). Nonetheless, each positive result depended on multiple positive PCRs. Corroboration by an independent 16S amplification followed by sequencing is a possible strategy for discrepancy resolution, but this requires that a single PCR generate an amplicon of >1,000 bp, which is inherently less sensitive than PCR generating shorter amplicons. In addition, if more than one organism is present, cloning or use of a next-generation sequencing technology would be required for identification. Here, we used two indirect methods to corroborate the PCR-ESI-MS-positive results: comparison of the analytical metrics obtained in the PCR-ESI-MS and patient chart review. In 33 PCR-ESI-MS-positive but culture-negative specimens, medical chart review suggested true bacteremia in 31 cases. The medical chart review findings correlated well with the PCR-ESI-MS analytical metrics. Figure 2 is an illustration of the system-reported Q score and the estimated microbial genome level (based on comparison to an internal calibration standard) for each PCR-ESI-MS-positive sample. The two samples considered to be false positives from the patient chart review show relatively lower Q score and microbial genome levels, suggesting that the remaining 31 cases detected by the PCR-ESI-MS method that were negative by culture are clinically significant.
There were three notable limitations of this study. First, the whole-blood specimens analyzed by PCR-ESI-MS were not collected at the same time and in the same manner as those used for blood culture. Although we strictly limited the study period to 8 h, the organism distributions and quantification in the samples evaluated by culture and by PCR-ESI-MS might have varied. Additional processing of these specimens in other laboratories might induce additional errors and/or contamination. Second, the study was performed at the Vanderbilt University Medical Center, where the false-contamination rate in blood culture has been high, mainly due to the use of persons other than phlebotomists for blood collection (19). Samples in which CoNS was detected were excluded from the data analysis to minimize the effect of contaminated samples. Finally, PCR-ESI-MS was performed on a single 1.25-ml specimen of whole blood from each patient without repeats. Numerous studies have addressed the issue of reproducibility of culture results (26–28), and the data suggest that two or three sequential consecutive samplings of blood are needed to obtain acceptable results, primarily due to sampling error (29, 30). The 97 specimens for which culture was positive with negative PCR-ESI-MS results showed an organism distribution very similar to the cases in which the detection matched between the two methods. This suggests there was nothing specific about the nature of the organisms to result in missed detections, but perhaps a combination of one or more of the above limitations caused them. Future molecular studies should analyze multiple serial samples of blood in higher volumes, as is done routinely with culture, to minimize errors and get a better estimate of clinical sensitivity.
Using the PCR-ESI-MS assay described here, the majority of pathogens can be detected and identified within 8 h directly from whole-blood specimens, which can significantly shorten the time between empirical therapy and evidence-based practice. In addition, the PCR-ESI-MS assay detected an additional 7% (31 in 431) of clinically relevant organisms in blood that yielded negative culture results. Direct analysis of patient blood samples by PCR-ESI-MS thus has the potential to enhance the diagnostic yield of bacteremia and candidemia in patients with bloodstream infections.
Supplementary Material
ACKNOWLEDGMENTS
We thank medical technologists from the Clinical Microbiology Laboratory and Molecular Infectious Diseases Laboratory at the Vanderbilt University Medical Center for specimen collections and technical assistance.
Thomas G. Laffler, Lendell L. Cummins, Michelle A. Toro, Heather E. Carolan, Donna M. Toleno, Megan A. Rounds, Mark Eshoo, Ranga Sampath, Lawrence B. Blyn, and David J. Ecker are employees of Ibis Biosciences, the commercial manufacturer of the PCR-ESI-MS assay. The study was supported in part by a research contract between the Vanderbilt University Medical Center and the Ibis Biosciences awarded to Yi-Wei Tang (no. VUMC 36118).
Footnotes
Published ahead of print 21 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00876-13.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 34:1589–1596 [DOI] [PubMed] [Google Scholar]
- 3.Fraser A, Paul M, Almanasreh N, Tacconelli E, Frank U, Cauda R, Borok S, Cohen M, Andreassen S, Nielsen AD, Leibovici L, TREAT Study Group 2006. Benefit of appropriate empirical antibiotic treatment: thirty-day mortality and duration of hospital stay. Am. J. Med. 119:970–976 [DOI] [PubMed] [Google Scholar]
- 4.Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez FJ, Perez-Paredes C, Ortiz-Leyba C. 2003. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit. Care Med. 31:2742–2751 [DOI] [PubMed] [Google Scholar]
- 5.Kollef MH, Sherman G, Ward S, Fraser VJ. 1999. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462–474 [DOI] [PubMed] [Google Scholar]
- 6.Reinhart K, Bauer M, Riedemann NC, Hartog CS. 2012. New approaches to sepsis: molecular diagnostics and biomarkers. Clin. Microbiol. Rev. 25:609–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Struelens MJ. 2010. Detection of microbial DNAemia: does it matter for sepsis management? Intensive Care Med. 36:193–195 [DOI] [PubMed] [Google Scholar]
- 8.Dark PM, Dean P, Warhurst G. 2009. Bench-to-bedside review: the promise of rapid infection diagnosis during sepsis using polymerase chain reaction-based pathogen detection. Crit. Care 13:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancini N, Carletti S, Ghidoli N, Cichero P, Burioni R, Clementi M. 2010. The era of molecular and other non-culture-based methods in diagnosis of sepsis. Clin. Microbiol. Rev. 23:235–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thirumala R, Ramaswamy M, Chawla S. 2010. Diagnosis and management of infectious complications in critically ill patients with cancer. Crit. Care Clin. 26:59–91 [DOI] [PubMed] [Google Scholar]
- 11.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. 2008. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 36:296–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenollar F, Raoult D. 2007. Molecular diagnosis of bloodstream infections caused by non-cultivable bacteria. Int. J. Antimicrob. Agents 30:S7–S15 [DOI] [PubMed] [Google Scholar]
- 13.Ecker DJ, Sampath R, Li H, Massire C, Matthews HE, Toleno D, Hall TA, Blyn LB, Eshoo MW, Ranken R, Hofstadler SA, Tang YW. 2010. New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev. Mol. Diagn. 10:399–415 [DOI] [PubMed] [Google Scholar]
- 14.Lehmann LE, Alvarez J, Hunfeld KP, Goglio A, Kost GJ, Louie RF, Raglio A, Regueiro BJ, Wissing H, Stuber F. 2009. Potential clinical utility of polymerase chain reaction in microbiological testing for sepsis. Crit. Care Med. 37:3085–3090 [DOI] [PubMed] [Google Scholar]
- 15.Wellinghausen N, Kochem AJ, Disqué C, Mühl H, Gebert S, Winter J, Matten J, Sakka SG. 2009. Diagnosis of bacteremia in whole-blood samples by use of a commercial universal 16S rRNA gene-based PCR and sequence analysis. J. Clin. Microbiol. 47:2759–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ecker DJ, Sampath R, Massire C, Blyn LB, Hall TA, Eshoo MW, Hofstadler SA. 2008. Ibis T5000: a universal biosensor approach for microbiology. Nat. Rev. Microbiol. 6:553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eshoo MW, Crowder CD, Li H, Matthews HE, Meng S, Sefers SE, Sampath R, Stratton CW, Blyn LB, Ecker DJ, Tang YW. 2009. Detection and identification of Ehrlichia species in blood using PCR and electrospray ionization mass spectrometry. J. Clin. Microbiol. 48:472–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilic A, Li H, Mellmann A, Basustaoglu AC, Kul M, Senses Z, Aydogan H, Stratton CW, Harmsen D, Tang YW. 2008. Acinetobacter septicus sp. nov. association with a nosocomial outbreak of bacteremia in a neonatal intensive care unit. J. Clin. Microbiol. 46:902–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang YW, Kilic A, Yang Q, McAllister SK, Li H, Miller RS, McCormac M, Tracy KD, Stratton CW, Han J, Limbago B. 2007. StaphPlex system for rapid and simultaneous identification of antibiotic resistance determinants and Panton-Valentine leukocidin detection of staphylococci from positive blood cultures. J. Clin. Microbiol. 45:1867–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan Y, He Y, Maier T, Quinn C, Shi G, Li H, Stratton CW, Kostrzewa M, Tang YW. 2011. Improved identification of yeast species directly from positive blood culture media by combining Sepsityper specimen processing and Microflex analysis with the matrix-assisted laser desorption ionization Biotyper system. J. Clin. Microbiol. 49:2528–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkman CL, Vergidis P, Uhl JR, Pritt BS, Cockerill FR, Steckelberg JM, Baddour LM, Maleszewski JJ, Edwards WD, Sampath R, Patel R. 2013. PCR-electrospray ionization mass spectrometry for direct detection of pathogens and antimicrobial resistance from heart valves in patients with infective endocarditis. J. Clin. Microbiol. 51:2040–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeng K, Gaydos CA, Blyn LB, Yang S, Won H, Matthews H, Toleno D, Hsieh YH, Carroll KC, Hardick J, Masek B, Kecojevic A, Sampath R, Peterson S, Rothman RE. 2012. Comparative analysis of two broad-range PCR assays for pathogen diagnosis in positive blood culture bottles: PCR-high-resolution melting analysis versus PCR-mass spectrometry. J. Clin. Microbiol. 50:3287–3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaleta EJ, Clark AE, Cherkaoui A, Wysocki VH, Ingram EL, Schrenzel J, Wolk DM. 2011. Comparative analysis of PCR-electrospray ionization/mass spectrometry (MS) and MALDI-TOF/MS for the identification of bacteria and yeast from positive blood culture bottles. Clin. Chem. 57:1057–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofstadler SA, Sampath R, Blyn LB, Eshoo MW, Hall TA, Jiang Y, Drader JJ, Hannis JC, Sannes-Lowery KA, Cummins LL, Libby B, Walcott DJ, Schink A, Massire C, Ranken R, Gutierrez J, Manalili S, Ivy C, Melton R, Levene H, Barrett-Wilt G, Li F, Zapp V, White N, Samant V, McNeil JA, Knize D, Robbins D, Rudnik K, Desai A, Moradi E, Ecker DJ. 2005. TIGER: the universal biosensor. Int. J. Mass Spectrom. 242:23–41 [Google Scholar]
- 25.Weinstein MP. 2003. Blood culture contamination: persisting problems and partial progress. J. Clin. Microbiol. 41:2275–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel R, Vetter EA, Harmsen WS, Schleck CD, Fadel HJ, Cockerill FR., III 2011. Optimized pathogen detection with 30- compared to 20-milliliter blood culture draws. J. Clin. Microbiol. 49:4047–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reimer LG, Wilson ML, Weinstein MP. 1997. Update on detection of bacteremia and fungemia. Clin. Microbiol. Rev. 10:444–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Washington JA, Jr, Ilstrup DM. 1986. Blood cultures: issues and controversies. Rev. Infect. Dis. 8:792–802 [DOI] [PubMed] [Google Scholar]
- 29.Lee A, Mirrett S, Reller LB, Weinstein MP. 2007. Detection of bloodstream infections in adults: how many blood cultures are needed? J. Clin. Microbiol. 45:3546–3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinstein MP. 1996. Current blood culture methods and systems: clinical concepts, technology, and interpretation of results. Clin. Infect. Dis. 23:40–46 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.