Abstract
Doxycycline is a tetracycline that has been licensed for veterinary use in some countries, but no clinical breakpoints are available for veterinary pathogens. The objectives of this study were (i) to establish breakpoints for doxycycline and (ii) to evaluate the use of tetracycline as a surrogate to predict the doxycycline susceptibility of Staphylococcus pseudintermedius isolates. MICs and inhibition zone diameters were determined for 168 canine S. pseudintermedius isolates according to Clinical and Laboratory Standards Institute (CLSI) standards. Tetracycline resistance genes were detected by PCR, and time-kill curves were determined for representative strains. In vitro pharmacodynamic and target animal pharmacokinetic data were analyzed by Monte Carlo simulation (MCS) for the development of MIC interpretive criteria. Optimal zone diameter breakpoints were defined using the standard error rate-bounded method. The two drugs displayed bacteriostatic activity and bimodal MIC distributions. Doxycycline was more active than tetracycline in non-wild-type strains. MCS and target attainment analysis indicated a certainty of ≥90% for attaining an area under the curve (AUC)/MIC ratio of >25 with a standard dosage of doxycycline (5 mg/kg of body weight every 12 h) for strains with MICs of ≤0.125 μg/ml. Tetracycline predicted doxycycline susceptibility, but current tetracycline breakpoints were inappropriate for the interpretation of doxycycline susceptibility results. Accordingly, canine-specific doxycycline MIC breakpoints (susceptible, ≤0.125 μg/ml; intermediate, 0.25 μg/ml; resistant, ≥0.5 μg/ml) and zone diameter breakpoints (susceptible, ≥25 mm; intermediate, 21 to 24 mm; resistant, ≤20 mm) and surrogate tetracycline MIC breakpoints (susceptible, ≤0.25 μg/ml; intermediate, 0.5 μg/ml; resistant, ≥1 μg/ml) and zone diameter breakpoints (susceptible, ≥23 mm; intermediate, 18 to 22 mm; resistant, ≤17 mm) were proposed based on the data generated in this study.
INTRODUCTION
Tetracyclines are broad-spectrum bacteriostatic agents that act by binding to the 30S ribosomal subunit, thereby inhibiting protein synthesis (1). Doxycycline is the most widely used tetracycline in small-animal practice. This tetracycline has enhanced lipophilic properties, compared to older tetracyclines such as tetracycline and oxytetracycline (2).
Two different commercial doxycycline products are available for use in dogs; doxycycline hyclate tablets (Ronaxan; Merial) are available in several European countries, and doxycycline monohydrate paste or tablets (VibraVet; Pfizer) are available in other countries, including Australia, New Zealand, and South Africa. These products are used for treatment of respiratory tract infections and other infections caused by a variety of bacteria, including staphylococci.
Staphylococcus pseudintermedius is the most important staphylococcal pathogen in dogs, where it can be responsible for a variety of infections, primarily skin infections and otitis (3). Due to widespread resistance, doxycycline is a second-choice antibiotic in the therapy of canine staphylococcal infections, and prescription is often guided by susceptibility testing. No veterinary clinical breakpoints are available for doxycycline, however, and human tetracycline breakpoints are currently employed for interpretation of doxycycline susceptibility data for canine clinical isolates. Human clinical breakpoints as currently applied in Clinical and Laboratory Standards Institute (CLSI) veterinary standards may not be appropriate for interpretation of susceptibility data for isolates from dogs because (i) the pharmacokinetic (PK) characteristics and protein binding of doxycycline may be different in dogs and humans and (ii) the susceptibility of bacterial isolates may be different in dogs and humans. The two objectives of the present study were (i) to determine canine-specific interpretive criteria for establishing breakpoints for doxycycline and (ii) to evaluate the use of tetracycline as a surrogate drug for interpretation of the doxycycline susceptibility of canine S. pseudintermedius isolates. A multistage approach was used to pursue these objectives. First, the distributions of doxycycline and tetracycline MICs and zone diameters were determined in a collection of canine S. pseudintermedius strains isolated from different countries, and the strains were characterized with respect to their tetracycline resistance gene contents. Second, the bacteriostatic activities of doxycycline and tetracycline were evaluated by time-kill experiments with selected strains representing the wild-type population. Third, the available in vitro pharmacodynamic (PD), target animal PK, and efficacy data were analyzed by Monte Carlo simulation (MCS) for the development of MIC breakpoints. Finally, correlates were developed for interpretive breakpoints for disk diffusion data.
(This study was presented in part in an abstract at the 6th Antimicrobial Agents in Veterinary Medicine [AAVM] Conference, Washington, DC, 23 to 26 October 2012.)
MATERIALS AND METHODS
Bacterial isolates.
A total of 168 canine S. pseudintermedius isolates from Denmark (n = 93), the United States (n = 30), Italy (n = 26), and Germany (n = 19) were included in this study. The strain collection included isolates from 2004 to 2007 (19.6%) or 2008 to 2012 (80.4%). The isolates were identified to the species level by either species-specific PCR (4) or PCR-restriction fragment length polymorphism (RFLP) analysis of the pta gene followed by MboI digestion (5).
MIC determinations.
MICs were determined by the broth microdilution method, in cation-adjusted Mueller-Hinton broth (CAMHB) (Sigma-Aldrich, Germany), as described by the Clinical and Laboratory Standards Institute (6). The following ranges of concentrations were tested: 0.031 to 64 μg/ml for tetracycline and 0.031 to 32 μg/ml for doxycycline. Drugs were purchased from Sigma-Aldrich (Germany). Fresh antimicrobial stock solutions in sterile water were prepared on each day of testing.
Disk diffusion.
Inhibition zone diameters were determined on Mueller-Hinton agar (Oxoid, United Kingdom) by disk diffusion, according to CLSI standards (6). Doxycycline (30 μg) and tetracycline (30 μg) disks were purchased from Oxoid (United Kingdom).
PCR for tetracycline resistance genes.
All isolates belonging to the tetracycline-resistant subpopulation were screened by PCR for the presence of resistance genes previously reported in this staphylococcal species, i.e., tet(K), tet(M), tet(L), and tet(O) genes. Primers and cycling conditions were the same as reported previously (7, 8).
Time-kill curves.
Time-kill kinetics were determined for two isolates displaying the most common MICs for tetracycline (0.125 μg/ml) and doxycycline (0.063 μg/ml) in the wild-type population. The method was based on that described by Blondeau et al. (9), with minor modifications. Experiments were undertaken in cation-adjusted Mueller-Hinton broth (CAMHB) (Sigma-Aldrich, Germany). Antibiotics were added at concentrations corresponding to 1, 2, 4, 8, and 16 times the MIC of the strain tested. Aliquots of 0.1 ml were collected after 0, 2, 4, 6, 8, and 24 h.
Pharmacokinetics and pharmacodynamics.
The pharmacokinetics of doxycycline in dogs have been assessed in previously published studies on the oral administration of doxycycline (10, 11), the findings of a conference presentation (A. Dominguez, B. Kukanich, and K. Kukanich, presented at the American Academy of Veterinary Pharmacology and Therapeutics 18th Biennial Symposium, Potomac, MD, 20 to 23 May 2013), and one unpublished study performed by one of the veterinary drug sponsors (S. T. B. Rogar, unpublished data). Other studies of nonoral administration in dogs have been published for comparison with studies of oral administration and determination of protein binding (12–17). The oral administration studies used for this analysis used a validated assay and uniform methods. One study was an unpublished study conducted by a drug sponsor. One of the authors (M.G.P.) performed pharmacokinetic analyses, using commercial software (Phoenix WinNonlin), on the reported concentrations from that study. The pharmacokinetic data are shown in Table 1.
Table 1.
Oral doxycycline pharmacokinetics in dogs
| Source | No. of subjects | Dose (mg/kg) | Mean ± SDa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| t1/2 (h) | kel (h−1) | Vd/F (liters/kg) | CL/F (liters/kg/h) | AUC (μg·h/ml) | Cmax (μg/ml) | Absorption t1/2 (h) | Tmax (h) | F (%) | |||
| van Gool et al. (11) | 4 | 10 | 8.34 ± 2.77 | 0.0831 ± 0.0277 | 1.28 ± 0.5 | 0.1 ± 0.02 | NR | 4.03 ± 1.25 | 0.87 ± 0.4 | 3.04 ± 1.15 | 57.3 ± 17.2 |
| Gutierrez et al. (10) | 12 | 10 | 5.59 ± 0.03 | 0.124 ± 0.0007 | 1.49 ± 0.27 | 0.137 ± 0.012 | 72.89 ± 6.3 | 5.58 ± 0.5 | 1.49 ± 0.07 | 3.88 ± 0.4 | 74.88 |
| Dominguez et al. (presented at the American Academy of Veterinary Pharmacology and Therapeutics 18th Biennial Symposium) | 5 | 10 | NR | NR | NR | 0.215 ± 0.065 | NR | NR | NR | NR | NR |
| Rogar (unpublished data) | 6 | 5.023 | 29.496 ± 6.15 | 0.0235 ± 0.0049 | 2.299 ± 1.333 | 0.0676 ± 0.035 | 92.962 ± 53.89 | 3.968 ± 3.904 | NR | 4.125 ± 2.326 | 53.36 ± 28.57 |
| Overall mean ± SD | 14.475 ± 3.066 | 0.077 ± 0.015 | 1.690 ± 0.559 | 0.102 ± 0.012 | 82.926 ± 33.651 | 4.526 ± 1.789 | 1.180 ± 0.233 | 3.682 ± 0.971 | 61.847 ± 8.040 | ||
| Weighted mean ± SD | 27 | 12.61 ± 11.10 | 1.67 ± 0.81 | 0.131 ± 0.061 | |||||||
t1/2, terminal half-life; kel, terminal rate constant; Vd/F, apparent volume of distribution per fraction absorbed; CL/F, systemic clearance per fraction absorbed; AUC, area under the curve; Cmax, maximal (peak) plasma concentration; absorption t1/2, half-life for oral absorption; Tmax, time to peak concentration; F, fraction of oral dose absorbed; NR, not reported in the study. Weighted means and standard deviations (described in more detail in the text) account for weighted differences in the numbers of subjects per study and within-study variations. Weighted means were calculated only for parameters that were necessary for Monte Carlo simulations.
In order to utilize the pharmacokinetic data for Monte Carlo simulations, overall means of the data and standard deviations had to be determined. Because each study had different numbers of subjects and there were both within-study and between-study variations, an analysis that allows pooling of data from a variety of published articles to obtain estimates of the population mean and variance for each parameter of interest was performed. The resulting estimated parameter means and variances provided the input for Monte Carlo simulations (MCSs). Because studies had different sample sizes, the data were weighted according to the sample size so that all observations (all animals) had equal weights. Then, for estimations of parameter values based upon the pooling of several studies, variances were estimated as products of both within-study and between-study sums of squares. These data are included in Table 1 for the parameters used in the MCSs, i.e., clearance, half-life, and volume of distribution.
Monte Carlo simulation.
Monte Carlo simulations were performed in order to estimate the certainty of attaining the PK/PD target for tetracycline antimicrobials. Pharmacokinetic/pharmacodynamic target attainment analysis using Monte Carlo simulations to integrate interpatient variability in drug exposure, drug potency, and in vivo exposure targets predictive of positive therapeutic outcomes can be used to derive clinical breakpoints (18–20).
In this study, the PK/PD target chosen was the ratio of the area under the curve (AUC) of the free (protein unbound) drug concentration in plasma over time to the MIC (AUC/MIC). The AUC/MIC ratio has been established as the preferred target for tetracycline antibiotics (21). Christianson et al. (22) found that the optimal AUC/MIC ratio in a mouse infection model was 25. Andes and Craig (21) concluded that, for doxycycline, the free-drug 24-h AUC/MIC value associated with a net bacteriostatic effect (static dose) was near 25. This exposure index (24-h AUC/MIC) necessary for efficacy is not impacted by drug resistance to β-lactams, macrolides, or tetracyclines (21). In contrast, other authors have cited free-drug AUC/MIC targets of 30 (23) and as low as 12 to 13 (24) in clinical studies. Although the bacteriostatic effect was observed at low ratios of ≤25, a higher ratio of 40 to 50 may be necessary for a 2-log10 CFU reduction in bacteria in a mouse thigh infection model. We felt that these data justified our analysis of free-drug AUC/MIC targets ranging from 40 to 13.
Target attainment (the certainty of attaining the PK/PD target) tables were constructed by performing MCSs using commercial software (Oracle Crystal Ball, fusion edition, release 11.1.2.2.000 [32 bit]). AUC/MIC calculations were performed using the following equation: AUC/MIC = (fu · F · 24 h · dose)/(CL · MIC), where fu is the fraction unbound (protein unbound), F is the fraction absorbed, and CL is the systemic clearance. The value for fu used in the analysis was 0.084, because protein binding levels of 91.75% ± 0.63% (12) and 91.4% (17) have been reported for dogs. Other parameters in the equation have been defined previously. For this simulation, a doxycycline dose for dogs of 5 mg/kg of body weight every 12 h was used. This is the most common clinical dose used for dogs in the United States and is cited as a consensus value in handbooks and reviews (25, 26). The MCS consisted of 1,000 random trials with input for the parameters listed in Table 1, across a MIC range of 0.03 to 4 μg/ml. Target attainment (certainty of meeting target) was simulated for free-drug AUC/MIC values of 40, 25, and 13.
Statistical analysis of susceptibility data.
The MIC distributions for doxycycline and tetracycline were compared by cross-tabulation. Wild-type (epidemiological) cutoff values for tetracycline and doxycycline were estimated by the statistical technique (27) and confirmed by visual inspection.
Disk diffusion zone diameter correlates were estimated using the error rate-bounded method described by CLSI (28) and implemented in the dBETS online software (http://glimmer.rstudio.com/dbets/dBETS). Correlates were determined for both tetracycline and doxycycline 30-μg disks.
RESULTS
MIC distributions and wild-type cutoff values.
The MIC distributions for both drugs were bimodal (Table 2), and visual inspection readily distinguished wild-type from non-wild-type populations. Wild-type cutoff values for tetracycline and doxycycline were 0.25 and 0.125 μg/ml, respectively.
Table 2.
Doxycycline and tetracycline MIC distributions of 168 canine S. pseudintermedius isolates
| Antimicrobial | Range tested (μg/ml) | No. of S. pseudintermedius isolates with an MIC (μg/ml) of: |
MIC50 (μg/ml) | MIC90 (μg/ml) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.031 | 0.063 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ≥64 | ||||
| Doxycycline | 0.031–32 | 12 | 76 | 10 | 0 | 0 | 1 | 1 | 65 | 3 | 0 | 0 | 0 | 0.063 | 4 |
| Tetracycline | 0.031–64 | 0 | 27 | 70 | 1 | 0 | 0 | 0 | 1 | 1 | 13 | 54 | 1 | 0.125 | 32 |
Doxycycline versus tetracycline MICs.
The MICs of doxycycline and tetracycline correlated well for both wild-type strains and strains with acquired resistance genes (Fig. 1). Doxycycline was approximately 2-fold more active than tetracycline in the wild-type strains, which increased to about 8-fold more active against the majority of strains with acquired resistance mechanisms. Tetracycline MIC interpretive criteria of 0.25 μg/ml (susceptible), 0.5 μg/ml (intermediate), and 1 μg/ml (resistant) resulted in no very major, major, or minor errors in predicting doxycycline susceptibility.
Fig 1.
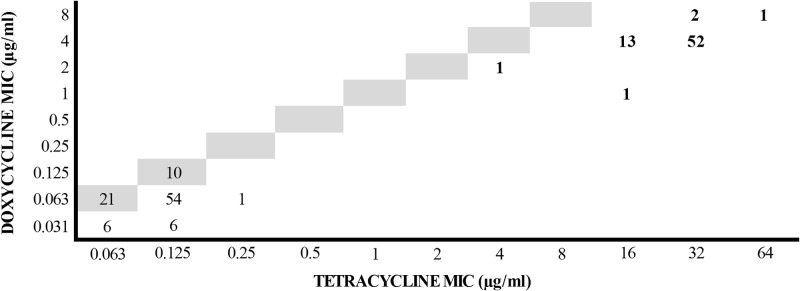
Scattergram of doxycycline versus tetracycline MICs for 168 canine S. pseudintermedius isolates. Numbers represent the numbers of isolates. Highlighted values represent the line of identity. Isolates harboring known resistance genes (n = 70) are shown in bold type.
Resistance genes.
Seventy of the isolates were resistant to tetracycline, of which 66 harbored tet(M) alone. Two isolates harbored tet(K) alone, 1 isolate both tet(K) and tet(M), and 1 isolate tet(O) alone. None of the isolates demonstrated tet(L). All isolates harboring resistance genes displayed higher MICs for both drugs than did the wild-type population. The tet(O)-containing isolate displayed MICs of 2 and 4 μg/ml for doxycycline and tetracycline, respectively.
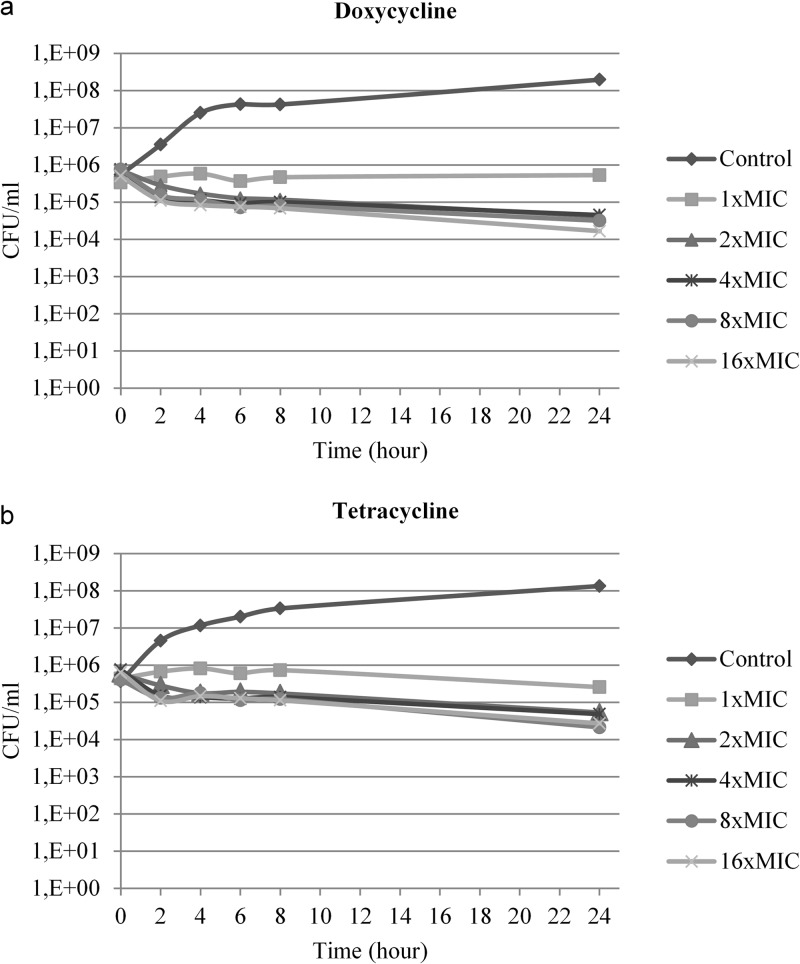
Time-kill curves.
Time-kill kinetics for the two drugs were similar for the two S. pseudintermedius isolates tested (Fig. 2). The rate and extent of in vitro bacterial inhibition were independent of the antimicrobial concentration for both doxycycline and tetracycline.
Fig 2.
Twenty-four-hour time-kill kinetics of one representative canine S. pseudintermedius isolate against doxycycline (MIC, 0.063 μg/ml) (a) and tetracycline (MIC, 0.125 μg/ml) (b).
Pharmacokinetics and pharmacodynamics.
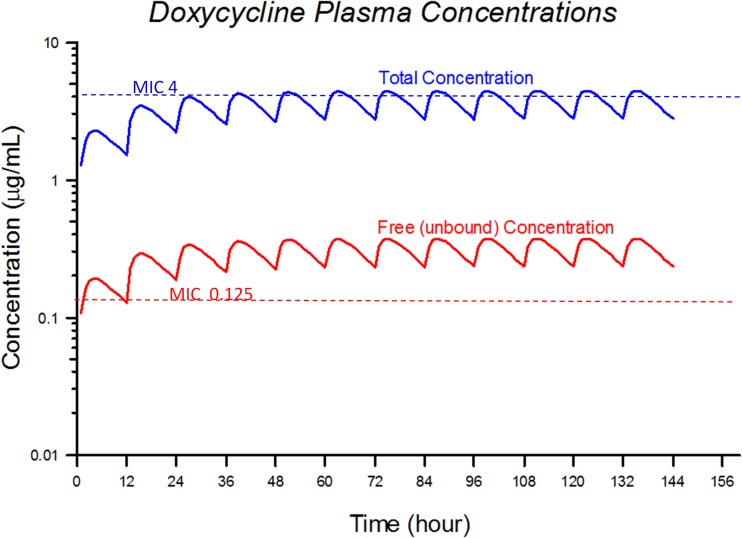
The pharmacokinetic variables are shown in Table 1. These parameters were used in the MCS for development of the target attainment table. The mean values for the pharmacokinetic parameters were used to simulate a doxycycline dose for dogs of 5 mg/kg administered orally every 12 h. This simulation, which represents the predicted mean plasma concentrations for total and free (unbound) drug, is shown in Fig. 3. Also indicated in Fig. 3 are MIC values of 4 μg/ml (the current human breakpoint) and 0.125 μg/ml (the proposed breakpoint in this study). Figure 3 represents simple average simulated concentrations and does not account for the variability in the population. The MCS was performed to account for this variation.
Fig 3.
Average total and unbound doxycycline plasma concentrations simulated from repeated oral administration of 5 mg/kg every 12 h.
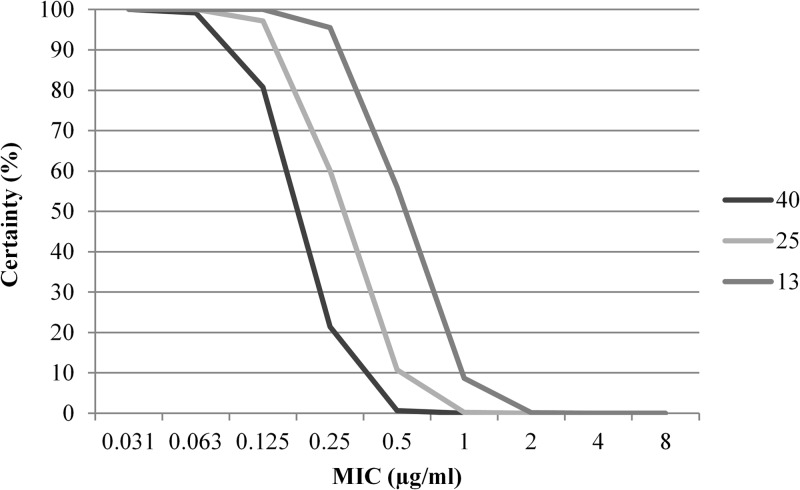
Monte Carlo simulation target attainment analysis.
The target attainment table from the MCS is shown in Table 3 and Fig. 4. Target attainment of ∼100% certainty was reached with MIC values of ≤0.125 μg/ml, using free-drug AUC/MIC ratios of 25 and 13. For MIC values of 0.25 μg/ml, target attainment values were 21.4%, 60.2%, and 95.5% with AUC/MIC ratios of 40, 25, and 13, respectively (Table 3). Figure 4 shows the steep decline in certainty at MIC values of ≥0.25 μg/ml; for higher MIC values, the probability is that essentially 0% of the population will reach the desired PK/PD target (Table 3).
Table 3.
Monte Carlo simulation of target attainment for a simulated canine population (n = 1,000) after oral administration of doxycycline at 5 mg/kg every 12 ha
| AUC/MIC | Certainty (%) with an MIC (μg/ml) of: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | |
| 40 | 100 | 99.2 | 80.72 | 21.44 | 0.62 | 0 | 0 | 0 | 0 |
| 25 | 100 | 100 | 97.17 | 60.15 | 10.75 | 0.24 | 0 | 0 | 0 |
| 13 | 100 | 100 | 100 | 95.54 | 55.97 | 8.65 | 0.12 | 0 | 0 |
Certainty levels (probability of target attainment) are shown for PK/PD targets of free-drug AUC/MIC ratios of 40, 25, and 13.
Fig 4.
Monte Carlo simulation of target attainment for a simulated canine population (n = 1,000) after oral administration of doxycycline at 5 mg/kg every 12 h. Target attainments are shown for PK/PD targets of free-drug AUC/MIC values of 40, 25, and 13. The plot shows MIC values versus percent certainty (probability) of attaining the AUC/MIC target.
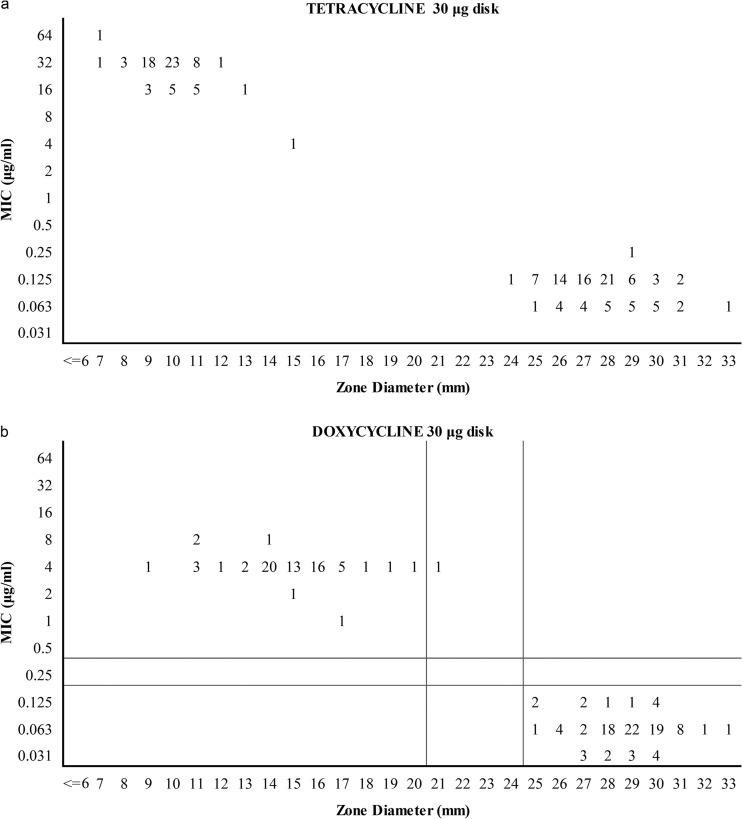
Inhibition zone diameters and correlations with MICs.
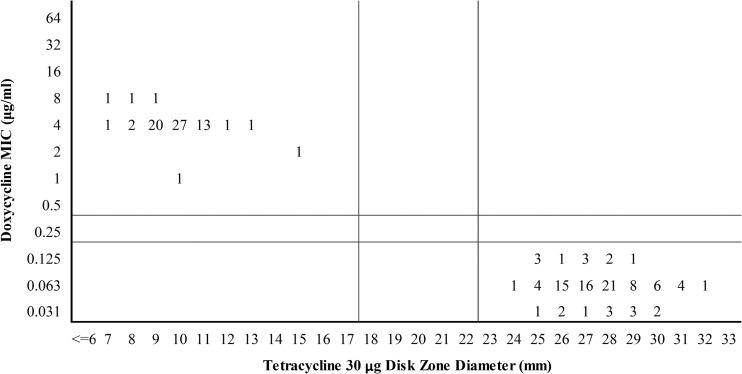
All isolates belonging to the wild-type population displayed zone diameters of ≥25 mm for doxycycline and ≥24 mm for tetracycline (Fig. 5a and b). Isolates belonging to the non-wild-type subpopulation displayed zone diameters of 9 to 21 mm for doxycycline and 7 to 15 mm for tetracycline. All doxycycline-susceptible isolates (MICs of ≤0.125 μg/ml) displayed zone diameters of ≥24 mm with tetracycline disks (Fig. 6). Doxycycline-resistant isolates (MICs of 1 to 8 μg/ml) had tetracycline zone diameters in the range of 7 to 15 mm. Statistical evaluation using proposed doxycycline MIC breakpoints of ≤0.125 μg/ml (susceptible), 0.25 μg/ml (intermediate), and ≥0.5 μg/ml (resistant) revealed corresponding zone diameter breakpoints of ≥23 mm (susceptible), 18 to 22 mm (intermediate), and ≤17 mm (resistant) for 30-μg tetracycline disks (Fig. 6) and ≥25 mm (susceptible), 21 to 24 mm (intermediate), and ≤20 mm (resistant) for 30-μg doxycycline disks (Fig. 5b).
Fig 5.
Scattergrams of tetracycline (a) and doxycycline (b) MICs versus disk diffusion zone diameters for 168 canine S. pseudintermedius isolates. Numbers represent the numbers of isolates. For doxycycline, the horizontal and vertical lines represent MIC and disk diffusion breakpoints, respectively. No breakpoints are proposed for tetracycline.
Fig 6.
Scattergram of doxycycline MICs versus tetracycline disk diffusion zone diameters for 168 canine S. pseudintermedius isolates. Numbers represent the numbers of isolates. Horizontal and vertical lines represent proposed doxycycline MIC and tetracycline surrogate disk diffusion breakpoints, respectively.
DISCUSSION
In order to set clinical breakpoints, several types of data are essential, including MIC distributions and other PD data such as time-kill kinetics, genetic resistance markers, target PK/PD index and ratio values, PK data for the target animal species, and clinical efficacy data (29). Prior to this study, a few studies have been published to describe PK properties of doxycycline in dogs (10–17). One additional study was identified (30), but analysis of the plasma concentrations from that study produced values far out of the range of those listed in Table 1; therefore, those data were not incorporated into this analysis. In addition to PK data, doxycycline MIC distributions from canine S. pseudintermedius isolates have been published (31). In this work, we made an attempt to generate and to analyze the PD and PK data required to set canine-specific clinical breakpoints for doxycycline susceptibility testing of this important veterinary pathogen. Canine-specific breakpoints, rather than human breakpoints, are preferred, and some commercial testing systems (e.g., Sensititre [Trek Diagnostic Systems]) list doxycycline as the only tetracycline on the panel for canine pathogens.
According to the current CLSI standards for susceptibility testing of bacteria of veterinary interest (6), strains susceptible to tetracycline should be regarded as susceptible to doxycycline and minocycline, whereas strains that have intermediate resistance or are resistant to tetracycline may be susceptible to doxycycline, minocycline, or both. Until now, the human breakpoints for tetracycline (which were set many years ago, before modern methods of determining breakpoints had been developed) have been used as class indicator breakpoints. Veterinary-specific breakpoints for doxycycline have not yet been approved by the CLSI. Therefore, interpretation using the human breakpoints considers MIC values of ≤4 μg/ml to indicate susceptibility. The evidence presented in this paper indicates that such a breakpoint for susceptible bacteria is not appropriate for canine isolates of S. pseudintermedius, because it does not match the MIC distribution of isolates and does not meet PK/PD criteria for tetracyclines using a typical dose administered to dogs.
The MIC distributions observed in this study are likely representative of S. pseudintermedius worldwide, as isolates from different countries and continents were included. The doxycycline and tetracycline MIC distributions are similar to those reported in previous studies in France (31) and Canada (32), respectively. Epidemiological cutoff values, defined as the highest MICs present in the wild-type population (33), were ≤0.125 μg/ml for doxycycline and ≤0.25 μg/ml for tetracycline according to our data set.
Resistance to tetracyclines in staphylococci is mediated by four different resistance genes (34, 35); tet(K) and tet(L) encode drug efflux pumps (35), while tet(M) and tet(O) mediate ribosomal protection of the drug target (36). The distribution of resistance genes found in our study is in agreement with previous studies showing that tet(M) is the most prevalent tetracycline resistance gene in S. pseudintermedius (34, 37). Among Gram-positive species, tet(O) is commonly found in streptococci (38) and enterococci (7) but is rare in staphylococci. To the best of our knowledge, this is the second time that tet(O) has been reported for S. pseudintermedius (34). The tetracycline MIC of this isolate (4 μg/ml) is unusually low for a strain carrying a tetracycline resistance gene mediating ribosomal protection and is remarkably lower than the values for the rest of the resistant subpopulation. This may be due to heterologous expression of tet(O) acquired from a distantly related species.
Regardless of the presence of resistance genes, all isolates were recorded as either susceptible (n = 165) or intermediate resistant (n = 3) to doxycycline by applying tetracycline breakpoints. This finding is in agreement with a recent study by Jones et al. (39), investigating doxycycline and tetracycline susceptibility of a large number of Gram-positive isolates of human origin. Their study describes the discordance between interpretative criteria for the tetracyclines published by different organizations, and it is concluded that reassessment of tetracycline breakpoints is necessary also for human Gram-positive species (39). The increased potency of doxycycline over tetracycline is mediated through enhanced lipophilic properties of the former drug (2). Whether the higher level of doxycycline in vitro susceptibility is reflected clinically depends on additional PK/PD drug properties. The in vitro antibacterial activity of both doxycycline and tetracycline against S. pseudintermedius is time dependent, which is in agreement with previous findings for Staphylococcus aureus (40). According to the definition by LaPlante et al. (24), in which a <3-log10 CFU/ml reduction in colony count from that of the initial inoculum defines bacteriostatic activity, while a ≥3-log10 CFU/ml reduction in colony count from that of the initial inoculum defines bactericidal activity, the activities of both drugs against S. pseudintermedius were regarded as bacteriostatic.
There is consensus among experts that PK/PD analysis of antibiotics should use only the unbound drug fraction (41). As only free unbound drug in plasma is able to penetrate tissues and reach extravascular infection sites, protein binding is a major factor limiting the tissue distribution of drugs. Doxycycline is highly protein bound in canine plasma, i.e., ∼91% according to the study by Bidgood and Papich (12). Their study in dogs showed that the high degree of protein binding restricted the distribution of free drug to tissues. As can be seen from the pharmacokinetic simulations (Fig. 3), for an average dog treated with 5 mg/kg drug every 12 h, the unbound level of drug is remarkably lower than the total plasma concentration. With an AUC/MIC ratio of 25, target attainment values were 97.2%, 60.2%, 10.8%, 0.2%, 0%, and 0% for MICs of 0.125, 0.25, 0.5, 1, 2, and 4 μg/ml, respectively.
A >97% probability of attaining a free-drug AUC/MIC ratio of 25 or 13 was achieved at a MIC value of 0.125 μg/ml. Other investigators have recommended that the probability of target attainment should be ≥90% to define the susceptible breakpoint (19, 42). We propose the same probability level to define 0.125 μg/ml as the PD cutoff value in this study. This PD cutoff value is in perfect agreement with the epidemiological cutoff value determined by MIC distributions. We therefore suggest canine-specific doxycycline breakpoints of ≤0.125 μg/ml (susceptible), 0.25 μg/ml (intermediate), and ≥0.5 μg/ml (resistant). Corresponding doxycycline zone diameter breakpoints are ≥25 mm (susceptible), 21 to 24 mm (intermediate), and ≤20 mm (resistant). These MIC breakpoints are 5 log2 units lower than the current tetracycline breakpoints of ≤4 μg/ml (susceptible), 8 μg/ml (intermediate), and ≥16 μg/ml (resistant); therefore, the current use of tetracycline breakpoints for the interpretation of doxycycline susceptibility data for canine S. pseudintermedius isolates cannot be justified. Based on our data, it is possible to predict doxycycline susceptibility using tetracycline as a surrogate for doxycycline in broth microdilution plate assays with a high degree of confidence, using breakpoints of ≤0.25 μg/ml (susceptible), 0.5 μg/ml (intermediate), and ≥1 μg/ml (resistant). The observed agreement of doxycycline MICs with tetracycline MICs and zone diameters validates the use of tetracycline as a surrogate drug for MIC testing and disk diffusion studies.
Like any other study, this study has some limitations. First, although isolates from different countries were included, all results are from a single laboratory. Ideally, a multicenter study is needed to take into account interlaboratory variability in the results of susceptibility testing. With regard to the PK analysis, different dosage regimens are recommended for the different doxycycline products marketed. Calculations were based on a dosage regimen of 5 mg/kg every 12 h. For Ronaxan (Merial), a daily dose of 10 mg/kg is recommended. For VibraVet (Pfizer), a loading dose of 5 mg/kg is followed at 12-h intervals by two doses of 2.5 mg/kg. Maintenance doses of 2.5 mg/kg are given subsequently at 24-h intervals for 5 days. Administration of a loading dose to reach the PK/PD target after the first dose is recommended in order to enhance clinical efficacy (43). Our proposed susceptible breakpoint of 0.125 μg/ml is sufficiently conservative that dosage regimens used in other countries or failure to administer a loading dose also would likely result in clinical success, but we cannot exclude the possibility that differences in dosage regimens may give somewhat different results in the Monte Carlo target attainment analysis. It would be ideal to have additional pharmacokinetic studies, preferably population pharmacokinetic studies in a large population of clinically affected dogs, to derive pharmacokinetic parameters for Monte Carlo simulations. Because such studies are not available, we used the only pharmacokinetic data available to us. Clinical breakpoints based on epidemiological and PD cutoff values should ideally be validated by clinical cutoff values (29). It would be preferable to have a controlled clinical trial to determine the MIC cutoff values for clinical cures versus failures in canine patients treated with doxycycline at the dose used in this study. The only evidence of clinical success with doxycycline in dogs is the licensing of this medication for treatment of routine infections in dogs in several countries outside the United States. The AUC/MIC ratio of 25 for a static effect applied in the target attainment analysis was derived from a study with Streptococcus pneumoniae (22) and not staphylococci. However, LaPlante et al. (24) used methicillin-resistant S. aureus in a murine thigh infection model and an in vitro model. In those models, the bacteriostatic activity values for the doxycycline free-drug AUC/MIC ratio were 12.4 and 20.4, respectively. Finally, doxycycline is known to possess biological properties other than purely antimicrobial effects, including anti-inflammatory effects (44). Whether (and how) these effects contribute to the clinical response in canine pyoderma is unknown. Thus, this aspect could not be taken into consideration in the development of the proposed breakpoints.
This study shows that tetracycline can be used as a surrogate drug to predict the doxycycline susceptibility of canine S. pseudintermedius isolates. However, tetracycline breakpoints are inappropriate for the interpretation of doxycycline susceptibility testing results. For this purpose, we recommend tentative canine-specific doxycycline breakpoints for broth microdilution (susceptible, ≤0.125 μg/ml; intermediate, 0.25 μg/ml; resistant, ≥0.5 μg/ml) and disk diffusion (susceptible, ≥25 mm; intermediate, 21 to 24 mm; resistant, ≤20 mm) findings. Tetracycline 30-μg disks may also be used as surrogates for doxycycline disks using the proposed interpretive criteria (susceptible, ≥23 mm; intermediate, 18 to 22 mm; resistant, ≤17 mm), and tetracycline breakpoints of ≤0.25 μg/ml (susceptible), 0.5 μg/ml (intermediate), and ≥1 μg/ml (resistant) may be used for MIC susceptibility tests.
ACKNOWLEDGMENTS
The study was supported by the Danish Centre for Antimicrobial Research and Development (DANCARD), funded by the Danish Council for Strategic Research.
We thank Tatjana Petrovna Kristensen for excellent laboratory assistance. We are grateful to Stefan Schwarz, Antonio Battisti, and David Bemis for providing bacterial isolates from Germany, Italy, and the United States, respectively.
Footnotes
Published ahead of print 21 August 2013
REFERENCES
- 1.Chopra I, Roberts M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryskier A. 2005. Tetracyclines, p 642–651 In Bryskier A. (ed), Antimicrobial agents. Antibacterials and antifungals. ASM Press, Washington, DC [Google Scholar]
- 3.Bannoehr J, Guardabassi L. 2012. Staphylococcus pseudintermedius in the dog: taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet. Dermatol. 23:253–266 [DOI] [PubMed] [Google Scholar]
- 4.Sasaki T, Tsubakishita S, Tanaka Y, Sakusabe A, Ohtsuka M, Hirotaki S, Kawakami T, Fukata T, Hiramatsu K. 2010. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J. Clin. Microbiol. 48:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannoehr J, Franco A, Iurescia M, Battisti A, Fitzgerald JR. 2009. Molecular diagnostic identification of Staphylococcus pseudintermedius. J. Clin. Microbiol. 47:469–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard—third edition. M31-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7.Aarestrup FM, Agerso Y, Gerner-Smidt P, Madsen M, Jensen LB. 2000. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 37:127–137 [DOI] [PubMed] [Google Scholar]
- 8.Akinbowale OL, Peng H, Barton MD. 2007. Diversity of tetracycline resistance genes in bacteria from aquaculture sources in Australia. J. Appl. Microbiol. 103:2016–2025 [DOI] [PubMed] [Google Scholar]
- 9.Blondeau JM, Borsos S, Blondeau LD, Blondeau BJ. 2012. In vitro killing of Escherichia coli, Staphylococcus pseudintermedius and Pseudomonas aeruginosa by enrofloxacin in combination with its active metabolite ciprofloxacin using clinically relevant drug concentrations in the dog and cat. Vet. Microbiol. 155:284–290 [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez L, Velasco ZH, Vazquez C, Vargas D, Sumano H. 2012. Pharmacokinetics of an injectable long-acting formulation of doxycycline hyclate in dogs. Acta Vet. Scand. 54:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Gool FJPJE, Santoul C, Giuseppin-Huet AM. 1988. Doxycycline: pharmacokinetics and suggested dosage in dogs and cats. Tijdschr. Diergeneeskd. 113:1189–1193 (in Dutch.) [PubMed] [Google Scholar]
- 12.Bidgood TL, Papich MG. 2003. Comparison of plasma and interstitial fluid concentrations of doxycycline and meropenem following constant rate intravenous infusion in dogs. Am. J. Vet. Res. 64:1040–1046 [DOI] [PubMed] [Google Scholar]
- 13.Schach von Wittenau M, Twomey TM. 1971. Disposition of doxycycline by man and dog. Chemotherapy 16:217–228 [DOI] [PubMed] [Google Scholar]
- 14.Wilson BJ, Norris JM, Malik R, Martin PA, Wigney DI, Baral R, Govendir M. 2006. Susceptibility of bacteria from feline and canine urinary tract infections to doxycycline and tetracycline concentrations attained in urine four hours after oral dosage. Aust. Vet. J. 84:8–11 [DOI] [PubMed] [Google Scholar]
- 15.Wilson RC, Kemp DT, Kitzman JV, Goetsch DD. 1988. Pharmacokinetics of doxycycline in dogs. Can. J. Vet. Res. 52:12–14 [PMC free article] [PubMed] [Google Scholar]
- 16.Barza M, Brown RB, Shanks C, Gamble C, Weinstein L. 1975. Relation between lipophilicity and pharmacological behavior of minocycline, doxycycline, tetracycline, and oxytetracycline in dogs. Antimicrob. Agents Chemother. 8:713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riond JL, Vaden SL, Riviere JE. 1990. Comparative pharmacokinetics of doxycycline in cats and dogs. J. Vet. Pharmacol. Ther. 13:415–424 [DOI] [PubMed] [Google Scholar]
- 18.Ambrose PG. 2006. Monte Carlo simulation in the evaluation of susceptibility breakpoints: predicting the future: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 26:129–134 [DOI] [PubMed] [Google Scholar]
- 19.Frei CR, Wiederhold NP, Burgess DS. 2008. Antimicrobial breakpoints for Gram-negative aerobic bacteria based on pharmacokinetic-pharmacodynamic models with Monte Carlo simulation. J. Antimicrob. Chemother. 61:621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts JA, Kirkpatrick CMJ, Lipman J. 2011. Monte Carlo simulations: maximizing antibiotic pharmacokinetic data to optimize clinical practice for critically ill patients. J. Antimicrob. Chemother. 66:227–231 [DOI] [PubMed] [Google Scholar]
- 21.Andes D, Craig W. 2007. Pharmacokinetics and pharmacodynamics of tetracyclines, p 267–278 In Nightingale CH, Ambrose PG, Drusano GL, Murakawa T. (ed), Antimicrobial pharmacodynamics in theory and clinical practice, 2nd ed. Informa Healthcare USA, New York, NY [Google Scholar]
- 22.Christianson J, Andes D, Craig W. 2001. Magnitude of the 24-h AUC/MIC required for efficacy of doxycycline (DOXY) against Streptococcus pneumoniae (SP) in a murine thigh-infection model. Clin. Infect. Dis. 33:1169 [Google Scholar]
- 23.Agwuh KN, MacGowan A. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 58:256–265 [DOI] [PubMed] [Google Scholar]
- 24.LaPlante KL, Leonard SN, Andes DR, Craig WA, Rybak MJ. 2008. Activities of clindamycin, daptomycin, doxycycline, linezolid, trimethoprim-sulfamethoxazole, and vancomycin against community-associated methicillin-resistant Staphylococcus aureus with inducible clindamycin resistance in murine thigh infection and in vitro pharmacodynamic models. Antimicrob. Agents Chemother. 52:2156–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papich MG. 2011. Saunders handbook of veterinary drugs, 3rd ed. Elsevier-Saunders, St; Louis, MO [Google Scholar]
- 26.Papich MG, Riviere JE. 2009. Tetracycline antibiotics, p 1524 In Riviere JE, Papich MG. (ed), Veterinary pharmacology and therapeutics, 9th ed. Wiley-Blackwell Publishing, Ames, IA [Google Scholar]
- 27.Turnidge J, Kahlmeter G, Kronvall G. 2006. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin. Microbiol. Infect. 12:418–425 [DOI] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute 2008. Development of in vitro susceptibility testing criteria and quality control parameters. Approved standard—third edition. M23-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 29.Turnidge J, Paterson DL. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin. Microbiol. Rev. 20:391–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SE, Kim S, Jeong M, Lee Y, Ahn JT, Park YW, Ahn JS, Lee E, Ryu DY, Seo K. 2013. Experimental determination of a subantimicrobial dosage of doxycycline hyclate for treatment of periodontitis in beagles. Am. J. Vet. Res. 74:130–135 [DOI] [PubMed] [Google Scholar]
- 31.Ganiere JP, Medaille C, Mangion C. 2005. Antimicrobial drug susceptibility of Staphylococcus intermedius clinical isolates from canine pyoderma. J. Vet. Med. B Infect. Dis. Vet. Public Health 52:25–31 [DOI] [PubMed] [Google Scholar]
- 32.Weese JS, Sweetman K, Edson H, Rousseau J. 2013. Evaluation of minocycline susceptibility of methicillin-resistant Staphylococcus pseudintermedius. Vet. Microbiol. 162:968–971 [DOI] [PubMed] [Google Scholar]
- 33.Kahlmeter G, Brown DFJ, Goldstein FW, MacGowan AP, Mouton JW, Osterlund A, Rodloff A, Steinbakk M, Urbaskova P, Vatopoulos A. 2003. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J. Antimicrob. Chemother. 52:145–148 [DOI] [PubMed] [Google Scholar]
- 34.Schwarz S, Roberts MC, Werckenthin C, Pang YJ, Lange C. 1998. Tetracycline resistance in Staphylococcus spp. from domestic animals. Vet. Microbiol. 63:217–227 [DOI] [PubMed] [Google Scholar]
- 35.Roberts MC. 1996. Tetracycline resistance determinants: mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol. Rev. 19:1–24 [DOI] [PubMed] [Google Scholar]
- 36.Taylor DE, Chau A. 1996. Tetracycline resistance mediated by ribosomal protection. Antimicrob. Agents Chemother. 40:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norstrom M, Sunde M, Tharaldsen H, Mork T, Bergsjo B, Kruse H. 2009. Antimicrobial resistance in Staphylococcus pseudintermedius in the Norwegian dog population. Microb. Drug Resist. 15:55–59 [DOI] [PubMed] [Google Scholar]
- 38.Schwarz S, Wibawan IWT, Lammler C. 1994. Distribution of genes conferring combined resistance to tetracycline and minocycline among group-B streptococcal isolates from humans and various animals. Zentralbl. Bakteriol. 281:526–533 [DOI] [PubMed] [Google Scholar]
- 39.Jones RN, Stilwell MG, Wilson ML, Mendes RE. 2013. Contemporary tetracycline susceptibility testing: doxycycline MIC methods and interpretive criteria (CLSI and EUCAST) performance when testing Gram-positive pathogens. Diagn. Microbiol. Infect. Dis. 76:69–72 [DOI] [PubMed] [Google Scholar]
- 40.Cunha BA, Domenico P, Cunha CB. 2000. Pharmacodynamics of doxycycline. Clin. Microbiol. Infect. 6:270–273 [DOI] [PubMed] [Google Scholar]
- 41.Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. 2005. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J. Antimicrob. Chemother. 55:601–607 [DOI] [PubMed] [Google Scholar]
- 42.Zelenitsky SA, Ariano RE, Zhanel GG. 2011. Pharmacodynamics of empirical antibiotic monotherapies for an intensive care unit (ICU) population based on Canadian surveillance data. J. Antimicrob. Chemother. 66:343–349 [DOI] [PubMed] [Google Scholar]
- 43.Martinez MN, Papich MG, Drusano GL. 2012. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob. Agents Chemother. 56:2795–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sapadin AN, Fleischmajer R. 2006. Tetracyclines: nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 54:258–265 [DOI] [PubMed] [Google Scholar]