Abstract
Sporadic hand, foot, and mouth disease (HFMD) outbreaks and other infectious diseases in recent years have frequently been associated with certain human enterovirus (HEV) serotypes. This study explored the prevalences and genetic characteristics of non-HEV71 and non-coxsackievirus A16 (CV-A16) human enterovirus-associated HFMD infections in Shenzhen, China. A total of 2,411 clinical stool specimens were collected from hospital-based surveillance for HFMD from 2008 to 2012. The detection of HEV was performed by real-time reverse transcription-PCR (RT-PCR) and RT-seminested PCR, and spatiotemporal phylogenetic analysis was performed based on the VP1 genes. A total of 1,803 (74.8%) strains comprising 28 different serotypes were detected. In the past 5 years, the predominant serotypes were HEV71 (60.0%), followed by CV-A16 (21.2%) and two uncommon serotypes, CV-A6 (13.0%) and CV-A10 (3.3%). However, CV-A6 replaced CV-A16 as the second most common serotype between 2010 and 2012. As an emerging pathogen, CV-A6 became as common a causative agent of HFMD as HEV71 in Shenzhen in 2012. Phylogenetic analysis revealed that little variation occurred in the Chinese HEV71 and CV-A16 strains. The genetic characteristics of the Chinese CV-A6 and CV-A10 strains displayed geographic differences. The CV-A6 and CV-A10 strains circulating in Shenzhen likely originated in Europe. It was found that human enteroviruses have a high mutation rate due to evolutionary pressure and frequent recombination (3.2 × 10−3 to 6.4 ×10−3 substitutions per site per year for HEV71, CV-A6, CV-A16, and CV-A10). Since certain serotypes are potential threats to the public health, this study provides further insights into the significance of the epidemiological surveillance of HFMD.
INTRODUCTION
Coxsackievirus A6 (CV-A6) and coxsackievirus A10 (CV-A10) are naked positive single-stranded RNA viruses which belong to the human enteroviruses (HEVs). HEVs, including poliovirus (PV), coxsackievirus A and B (CV-A and CV-B), echovirus (E), and new human enterovirus (HEV), are among the most common human infectious viruses and mainly infect neonates and young children (1). Based on their molecular characterizations, HEVs include the species A to D.
Although most HEV infections are asymptomatic, they can cause a wide range of clinical manifestations ranging from mild symptoms to fatal disease, such as hand, foot, and mouth disease (HFMD), herpangina, onychomadesis, acute hemorrhagic conjunctivitis, acute respiratory tract infection, aseptic meningitis, encephalitis, myocarditis, and acute flaccid paralysis (1, 2). HFMD infection in children younger than 5 years old typically presents as a brief, generally mild, febrile illness with a papulovesicular rash on the palms and soles and multiple oral ulcers (3).
As a major causative agent of HFMD, epidemic waves of human enterovirus 71 (HEV71) have swept through countries in the Asia-Pacific region since 1997 (4). In mainland China, the HEV71 strain was first isolated in 1987 from an HFMD patient without neurological symptoms (5). Since a large outbreak of HFMD with 405 severe infections and 78 deaths in Taiwan occurred in 1998, HEV71 has become the dominant cause of HFMD, which is prevalent in mainland China. Three large HEV71 outbreaks resulted in 14, 23, and 126 deaths, respectively, in Linyi, Shandong province, in 2007, Fuyang, Anhui province, in 2008, and Taiwan in 2008 (6–8). Severe HFMD with neurological system illness (acute flaccid paralysis, brainstem encephalitis associated with cardiopulmonary edema) has been mainly caused by HEV71, according to a few large outbreaks of HFMD in the world. In contrast, worldwide epidemiological studies of HFMD showed that CV-A16 and a number of other HEV-A serotypes usually cause mild self-limiting infections (9).
In the past few years, HEV-B has been relatively more prevalent than HEV-A in certain regions. Recently, CV-A6 and CV-A10 of the HEV-A serotype have been increasingly associated with infectious disease, such as HFMD, herpangina, and onychomadesis (10–16). The prevalences of other HEV-A infections were underestimated for many years because more attention has been paid to HEV71 and CV-A16 in China. Shenzhen, as a special economic zone in China, is located on the southern coast. Its high population density, high population mobility, and subtropical environment make Shenzhen an HFMD-prone area. From 2008 to 2012, the detection ratio of non-HEV71 and non-CV-A16 enteroviruses indicated an upward trend by real-time reverse transcription-PCR (RT-PCR) from the sentinel surveillance systems for HFMD (Shenzhen Center for Disease Control and Prevention [CDC]). Therefore, a prospective observational study on the causative agents of HFMD was performed to clarify the roles of other human enterovirus types, with an emphasis on exploring the prevalences and genetic characteristics of CV-A6 and CV-A10.
MATERIALS AND METHODS
Specimen collection and study protocol.
From 2008 to 2012, sentinel pediatricians were requested to collect clinical specimens from patients presenting with HFMD. Each specimen along with a standardized report form was sent to the virology laboratory at the Shenzhen CDC for the detection of HEVs. The report form recorded information on patient demographics and clinical findings. According to the diagnostic criteria defined previously by the Ministry of Health, children were clinically diagnosed as having HFMD if they had a fever and onset of at least one of the following features: maculopapular or vesicular rash on the palms and/or soles and vesicles or ulcers in the mouth. Children with serious complications, including encephalitis, meningitis, acute flaccid paralysis, cardiorespiratory failure, or death, were considered to have severe HFMD. Children diagnosed with HFMD but without the abovementioned serious complications were classified as having mild HFMD.
A total of 2,411 clinical stool specimens were collected and archived by the Department of Microbiology at the Shenzhen CDC between 2008 and 2012 (590 in 2008, 299 in 2009, 458 in 2010, 628 in 2011, and 436 in 2012). HEV RNA was detected by real-time RT-PCR and reverse transcription seminested PCR (RT-snPCR) (17, 18). The study was performed according to the Declaration of Helsinki II and was approved by the ethics committees of Shenzhen Children's Hospital, Long Gang Center Hospital, and Ping Shan People's Hospital. Written informed consent was obtained from all patients or their caretakers.
Viral RNA extraction and real-time RT-PCR.
Specimen processing was performed as previously described (18). Viral RNA was extracted from 200 μl supernatant using the High Pure viral RNA kit (Roche, Germany) and was subsequently used for HEV71 and CV-A16 detection using a real-time RT-PCR kit (Shenzhen Taitai Genomics, Inc., China). A known-negative stool specimen was included in each extraction run, and the extract was tested by real-time RT-PCR along with the clinical specimens to monitor for cross-contamination. The runs that occasionally yielded false-positive results occasionally were excluded from the analysis.
RT-PCR, RT-snPCR amplification, and sequencing.
Samples that were positive for HEV71 or CV-A16 by real-time RT-PCR were randomly selected to amplify the entire VP1 gene for phylogenetic analysis using RT-PCR with specific primers (6). For specimens that were negative for both HEV71 and CV-A16, the typing of HEVs based on the VP1 gene was performed by three rounds of independent reverse transcription-seminested PCR (RT-snPCR). First, cDNA was synthesized in a 10-μl volume reaction mixture using the PrimeScript II 1st Strand cDNA synthesis kit (TaKaRa, Japan).
Next, a 3-μl cDNA solution was used as a template in each round of RT-snPCR. The first round of RT-snPCR based on the complete VP1 gene was performed to detect HEV-A using the primers HEVAS1495, HEVAR2C, and HEVAR2807 (19). The second round of RT-snPCR based on the complete VP1 gene was performed to detect HEV-B using the primers HEVBS1695, EV2C, and HEVBR132 (20). The third round of RT-snPCR based on the partial VP1 gene was performed to detect other HEVs using two pairs of CODEHOP primers, 224/222 and AN89/AN88 (18). Amplified DNA was purified using a commercial kit (catalog no. D823A, MiniBEST agarose gel DNA extraction kit version 3.0; TaKaRa, Japan) and sequenced by the ABI PrismTM 3730xl DNA analyzer using the BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems). A contig was assembled with forward and reverse nucleotide sequences.
Identification of HEVs serotypes and phylogenetic analyses.
Assembled sequences were used to identify the serotypes of HEV strains using the online Enterovirus Genotyping Tool (http://www.rivm.nl/mpf/enterovirus/typingtool) or a BLAST search. The nucleotide sequences of HEV strains assigned to serotypes HEV71, CV-A6, CV-A16, and CV-A10 were compared with homologous sequences available in GenBank to identify variants and analyze the phylogenetic relationships with strains that are circulating globally. Multiple sequence alignments were performed by ClustalX 2.0.12, which is available in the European Bioinformatics Institute. All reference sequences were derived from GenBank. The neighbor-joining (NJ), maximum-likelihood (ML), and Bayesian Markov chain Monte Carlo (BMCMC) methods were used to comparatively analyze the phylogenesis of the partial VP1 genes of CV-A6 and CV-A10 using MEGA 5.05 (21) and BEAST 1.7.40 (22). The spatiotemporal evolution of these four pathogens was inferred by BMCMC analysis. Bayesian analyses were performed using a relaxed molecular clock model (the uncorrelated lognormal distributed model [UCLD]) (23). The analyses were independently performed using the Hasegawa-Kishino-Yano (HKY) (24) and general time-reversible (GTR) (25) nucleotide substitution models, with a gamma-distributed among-site rate variation with four rate categories. BMCMC analyses were repeated using the constant size and exponential growth models in order to investigate the degree to which the dating estimates were affected by the demographic model chosen.
Statistical analyses.
Data were analyzed using the statistical software SPSS version 18.0. The statistical differences of the male/female and severe/mild ratios between different HEVs serotypes were tested by chi-square test. An analysis of variance was used to compare the means of ages. A P value of <0.05 was regarded as statistically significant.
Nucleotide sequence accession numbers.
The sequences determined in this study (n = 779) were submitted to GenBank/EMBL/DDBJ under the accession numbers KC866623 to KC867102, JX154899 to JX155009, and JX473292 to JX473479.
RESULTS
The distribution of HEV infection.
Among the 2,411 total HFMD cases, 1,803 (74.8%) were positive for HEVs. A total of 28 different serotypes were identified by sequencing between 2008 and 2012 (Table 1; see also Table S1 in the supplemental material), in which 9 serotypes belonged to HEV-A species, 18 serotypes belonged to HEV-B species, one serotype belonged to HEV-C species, and no HEV-D species were detected. HEV-A species were the most prevalent (96.7% [1,744/1,803]), followed by HEV-B species (3.2% [57/1,803]).
Table 1.
Distributions of HEV infections from HFMD patients between 2008 and 2012
| Yr | No. (%) of positive cases | Prevalence of serotypes by ranka: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
|||||||
| Serotype | No. (%) of cases | Serotype | No. (%) of cases | Serotype | No. (%) of cases | Serotype | No. (%) of cases | Serotype | No. (%) of cases | ||
| 2008 | 407 (69.0) | HEV71 | 240 (59.0) | CV-A16 | 147 (36.1) | CV-B3 | 5 (1.2) | CV-A10 | 3 (0.7) | CV-A4 | 3 (0.7) |
| 2009 | 244 (81.6) | HEV71 | 131 (53.7) | CV-A16 | 71 (29.1) | CV-A6 | 20 (8.2) | CV-A10 | 15 (6.1) | CV-A5 | 2 (0.8) |
| 2010 | 330 (72.1) | HEV71 | 233 (70.6) | CV-A6 | 37 (11.2) | CV-A16 | 20 (6.1) | CV-A10 | 13 (3.9) | CV-A4 | 4 (1.2) |
| 2011 | 472 (75.2) | HEV71 | 302 (64.0) | CV-A6 | 79 (16.7) | CV-A16 | 57 (12.1) | CV-A10 | 19 (4.0) | CV-B2 | 3 (0.6) |
| 2012 | 350 (80.3) | HEV71 | 121 (34.6) | CV-A6 | 96 (27.4) | CV-A16 | 87 (24.9) | CV-A2 | 12 (3.4) | CV-A10 | 10 (2.9) |
The other ranks are listed in Table S1 in the supplemental material.
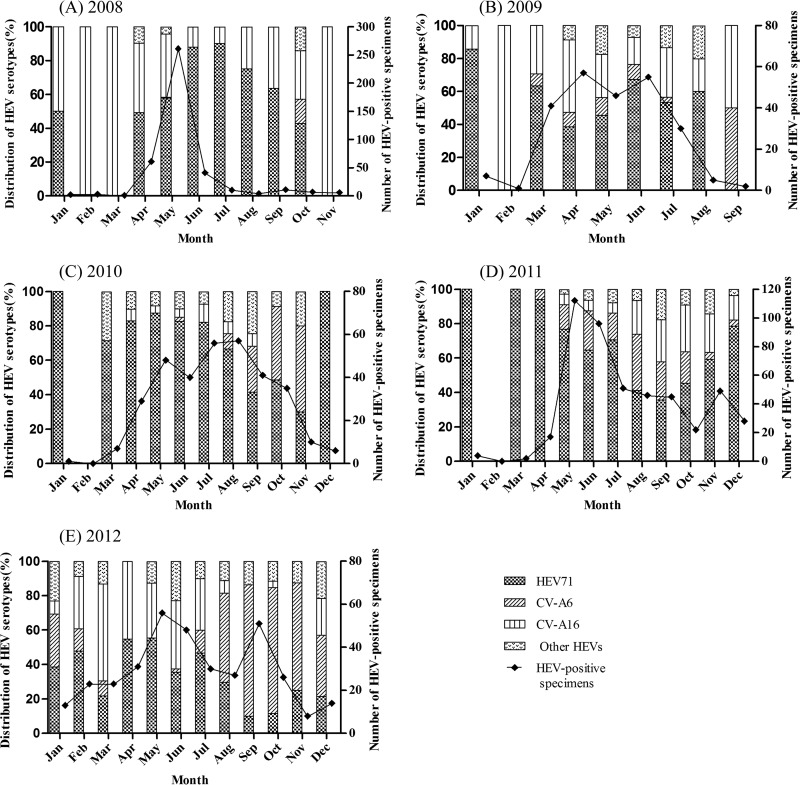
During 2008 and 2009, the most prevalent serotype was HEV71, followed by CV-A16. Although HEV71 remained the most common serotype between 2010 and 2012, CV-A6 replaced CV-A16 as the second most common HEV. Within 5 years, only CV-A6 showed a trend of increasing prevalence (from 0.5% to 27.4% between 2008 and 2012), while CV-A10 strains circulated at a relatively low prevalence (<7%). CV-A6 has recently emerged as a serotype circulating with increasing frequency in Shenzhen, and in 2012, it turned into as common a causative agent of HFMD as HEV71 and CV-A16.
Epidemiological data.
The HEV infections mainly occurred between April and September during the years 2008 and 2012 (Fig. 1A to E). HEV71 infections generally occurred throughout the year between 2008 and 2012 but occurred mainly during the warm seasons. The proportion of HEV71 infections (among all HEVs) significantly dropped in 2012 relative to the previous 4 years. CV-A16 infections occurred throughout the year during 2008 and 2009, but the proportion of CV-A16 infections obviously declined in 2010 and then increased gradually from 2011 to 2012. Contrary to HEV71, CV-A6 infections mainly occurred during the autumn and winter.
Fig 1.
Distribution of the number of human enterovirus-positive specimens and prevalent serotypes by month between 2008 and 2012 in Shenzhen, China. (A) 2008; (B) 2009; (C) 2010; (D) 2011; (E) 2012. CV-A, coxsackievirus A; HEV, human enterovirus.
Statistical analysis indicated that the mean age of CV-A6- or CV-A10-infected patients was lower than the mean age of those infected with HEV71 in 2009, 2010, and 2012 (P < 0.05) (Table 2). The male/female ratio of CV-A16-infected patients was higher than that of patients infected with other HEVs in 2010 (P = 0.025), and the male/female ratio of CV-A6-infected patients was higher than that of patients infected with other HEVs in 2012 (P < 0.001). The majority of HEV infection cases (78.5% [1,416/1,803]) presented with mild symptoms. However, the severe/mild ratio was higher in HEV71-infected patients than in those who tested positive for CV-A6, CV-A16, or CV-A10 (P < 0.001) during 2009 and 2012. Severe symptoms included accelerated breathing, severe vomiting, muscle twitches, abnormal eye movements, brainstem encephalitis, and aseptic meningitis. In patients with severe symptoms with HEV71, CV-A6, CV-A16, and CV-A10 infections, the common symptoms included accelerated breathing and muscle twitches (data not shown).
Table 2.
Demographics associated with the different HEVs from 2008 to 2012
| Demographic and years of infection | Results by HEV serotype |
P | |||
|---|---|---|---|---|---|
| EV71 | CV-A6 | CV-A16 | CV-A10 | ||
| Mean age ± SD (yr) | |||||
| 2008 | 3.0 ± 1.6 | 1.0 ± 0 | 2.9 ± 1.4 | 1.6 ± 0.9 | 0.089 |
| 2009 | 2.0 ± 1.3 | 1.6 ± 1.1 | 2.2 ± 1.5 | 1.4 ± 0.5 | 0.024 |
| 2010 | 2.1 ± 1.4 | 1.8 ± 0.9 | 2.1 ± 1.5 | 1.1 ± 0.5 | 0.014 |
| 2011 | 2.2 ± 1.3 | 2.1 ± 1.5 | 2.1 ± 1.8 | 1.9 ± 1.4 | 0.589 |
| 2012 | 2.3 ± 1.4 | 1.8 ± 1.0 | 2.3 ± 1.5 | 1.8 ± 0.9 | 0.014 |
| Male/female ratio | |||||
| 2008 | 2.24 | NAa | 1.83 | 2.00 | 0.489 |
| 2009 | 2.05 | 2.33 | 3.18 | 2.00 | 0.602 |
| 2010 | 1.33 | 2.08 | 5.67 | 0.63 | 0.025 |
| 2011 | 1.54 | 1.39 | 1.48 | 2.80 | 0.666 |
| 2012 | 1.88 | 2.69 | 1.72 | 1.5 | <0.001 |
| Severe/mild ratiob | |||||
| 2008 | 0.01 | 0 | 0 | 0 | 0.398 |
| 2009 | 0.72 | 0.33 | 0.16 | 0.07 | <0.001 |
| 2010 | 0.73 | 0.06 | 0.11 | 0 | <0.001 |
| 2011 | 0.75 | 0.10 | 0.14 | 0.19 | <0.001 |
| 2012 | 0.51 | 0.08 | 0.09 | 0 | <0.001 |
NA, not available.
Patients presenting with hand, foot, and mouth disease with serious complications, including encephalitis, meningitis, acute flaccid paralysis, cardiorespiratory failure, or death, were considered to have severe cases. Patients without the abovementioned serious complications were classified as having mild cases.
Spatiotemporal phylogenetic analysis.
Phylogenetic analysis of complete VP1 sequences showed that all HEV71 strains (n = 192) identified in this study belonged to subgenogroup C4. A high degree of similarity (93.4% to 100%) was observed between the sequences. Since 1998, the subgenogroup C4 has been responsible for almost all HEV71 infections in mainland China. Maximum-likelihood phylogenetic analysis of the Chinese HEV71 sequences indicated that there were 2 stages of HEV71 circulation in mainland China between 1998 and 2012 (see Fig. S1 in the supplemental material). The HEV71 strains isolated from Shenzhen showed a shift from evolutionary branch C4b to C4a between 2003 and 2004 (see Fig. S1 in the supplemental material). We estimated that the common ancestor HEV71 likely emerged at the beginning of the 20th century, and its evolutionary rate was estimated to be 3.2 ×10−3 to 3.3 ×10−3 substitutions per site per year (see Table S2 in the supplemental material). Comparing the estimated time of origin with the dates of first detection of each subgenotype, we found that divergent B and C subgenotypes circulated recessively for approximately 1 to 7 years before causing large HFMD outbreaks (see Table S2 in the supplemental material). Phylogenetic analysis based on complete VP1 sequences indicated that CV-A16 strains (n = 102) in this study were grouped with B1a and B1b with 86.9 to 100% identity (see Fig. S2 in the supplemental material). The prevalent subgenotypes B1a and B1b mainly cocirculated in the Asia-Pacific regions. CV-A16 strains isolated from Shenzhen revealed the cocirculation of B1a and B1b from 2005 to 2012. The common ancestor CV-A16 likely emerged between 1804 and 1806 (exponential growth model), and its evolutionary rate was estimated to be 4.0 ×10−3 to 4.1 ×10−3 substitutions per site per year (see Table S3 in the supplemental material). Genotype B2 and divergent B1 subgenotypes were estimated to circulate recessively for 3 to 4 years before causing large HFMD outbreaks.
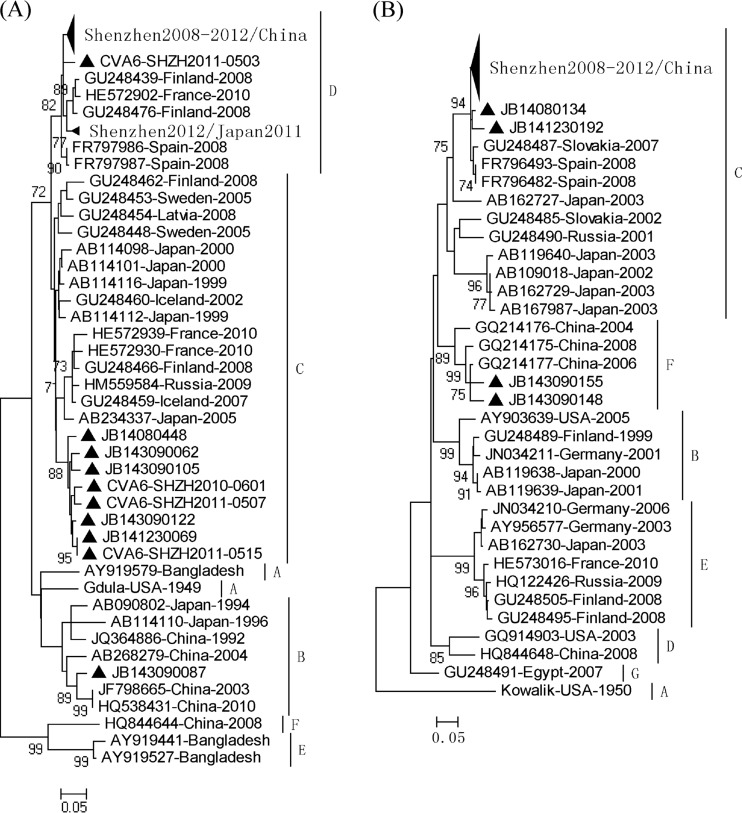
The NJ, ML, and BMCMC phylogenetic trees of CV-A6 based on partial VP1 sequences (269 nt) showed the same topology (Fig. 2; see also Fig. S3 and S4 in the supplemental material). Genotyping for CV-A6 strains was performed in a similar fashion as that which was described for HEV71 (26). A difference of ≥15% in the VP1 genes was used to distinguish between the different genotypes. Accordingly, the CV-A6 strains were classified into six major clusters, denoted A, B, C, D, E, and F (Fig. 2). The complete VP1 genes of 142 CV-A6 strains in this study showed 73.2% to 99.9% similarity. All CV-A6 strains (n = 234) determined in this study between 2008 and 2012 were categorized into three genogroups, the majority of which clustered with genogroup D, 8 strains (isolated from 2008 to 2012) clustered with genogroup C, and only one variant strain, JB143090087 (isolated in 2009), grouped with genogroup B. A majority of the CV-A6 strains isolated from Shenzhen between 2008 and 2012 and from Japan in 2011 displayed a close genetic relationship with 2008 Finnish strains associated with an HFMD outbreak, 2008 Spanish strains associated with onychomadesis after HFMD, and 2010 French strains associated with an HFMD/herpangina outbreak (10, 12, 15, 16). The genetic characteristics of the CV-A6 strains isolated in China displayed geographical differences: the predominant genotype of CV-A6 was genogroup D between 2008 and 2012 in Shenzhen, whereas the prevalent genotype was B in other regions in China. Therefore, we inferred that the Chinese CV-A6 strains had different geographical origins, and CV-A6 strains from Shenzhen likely originated in Europe. The most common ancestor of CV-A6 emerged between 1857 and 1860 (exponential growth model), and its evolutionary rate was estimated to be 4.5 ×10−3 to 4.6 ×10−3 substitutions per site per year (Table 3). The prevalent genotypes C and D were estimated to circulate recessively for 5 to 6 years before causing infectious diseases.
Fig 2.
The ML phylogenetic trees based on partial VP1 sequences of global CV-A6 (269 nt) (A) and CV-A10 isolates (264 nt) (B). The nucleotide substitution model used was the general time-reversible method. The sequences of this study are labeled with ▲ (indicating a single sequence) or ⊇ (indicating multiple sequences). The trees were midpoint rooted, and bootstrap support values of <70% (1,000 bootstrap replicates) were not indicated. The reference sequences are labeled as GenBank accession no.-location-year.
Table 3.
Evolutionary characteristics of CV-A6, CV-A10, and their genogroups
| tMRCA by serotype and other characteristicsa | Data by BMCMC growth model (mean [95% CI])b: |
|||
|---|---|---|---|---|
| Constant size |
Exponential growth |
|||
| HKY + γ4 | GTR + γ4 | HKY + γ4 | GTR + γ4 | |
| tMRCA CV-A6 (1949) | 1867 (1780–1949) | 1866 (1780–1949) | 1857 (1780–1949) | 1860 (1760–1949) |
| Evolutionary ratec | 4.5 (2.8–6.5) | 4.5 (2.8–6.7) | 4.5 (2.8–6.9) | 4.4 (2.7–6.5) |
| Coefficient of variation | 0.41 (0–0.74) | 0.40 (0–0.76) | 0.42 (0–0.78) | 0.39 (0–0.73) |
| tMRCA B (1992) | 1981 (1972–1988) | 1981 (1971–1988) | 1981 (1972–1989) | 1980 (1971–1988) |
| tMRCA C (1999) | 1994 (1990–1997) | 1994 (1990–1997) | 1994 (1990–1997) | 1994 (1990–1997) |
| tMRCA D (2008) | 2002 (1998–2005) | 2003 (1998–2005) | 2003 (1998–2005) | 2003 (1998–2005) |
| tMRCA CV-A10 (1950) | 1907 (1866–1949) | 1906 (1861–1946) | 1905 (1858–1945) | 1903 (1857–1944) |
| Evolutionary ratec | 6.2 (4.0–8.6) | 6.4 (4.0–8.9) | 6.3 (4.2–8.8) | 6.2 (4.2–8.6) |
| Coefficient of variation | 0.37 (0.02–0.69) | 0.39 (0–0.68) | 0.36 (0–0.67) | 0.35 (0–0.66) |
| tMRCA B (1999) | 1994 (1990–1998) | 1994 (1990–1998) | 1994 (1990–1998) | 1994 (1990–1998) |
| tMRCA C (2001) | 1990 (1983–1996) | 1990 (1984–1996) | 1990 (1983–1996) | 1990 (1984–1996) |
| tMRCA D (2003) | 1997 (1991–2001) | 1997 (1991–2002) | 1997 (1991–2002) | 1996 (1990–2002) |
| tMRCA E (2003) | 2001 (1999–2003) | 2001 (1999–2003) | 2001 (2000–2003) | 2001 (1999–2003) |
| tMRCA F (2004) | 1999 (1995–2003) | 1999 (1995–2003) | 1999 (1995–2003) | 1999 (1995–2003) |
The year in parentheses is the the earliest time of identification of the serotype or genogroup. tMRCA, time to the most common ancestor.
Values are estimated years of emergence of the most common ancestor unless otherwise indicated. BMCMC, Bayesian Markov chain Monte Carlo method; HKY, Hasegawa-Kishino-Yano method; GTR, general time-reversible method; CI, confidence interval; γ4, a gamma-distributed among-sites rate variation with four rate categories that allows rate variation between sites in the associated alignment.
Evolutionary rates are expressed as ×10−3 substitutions per site per year.
The NJ, ML, and BMCMC phylogenetic trees of CV-A10 based on partial VP1 sequences (264 nt) also displayed the same topology (Fig. 2; see also Fig. S5 and S6 in the supplemental material). CV-A10 strains were classified into seven major clusters based on the criteria described above, designated A, B, C, D, E, F, and G (Fig. 2). The complete VP1 sequences of 55 CV-A10 strains in this study showed 81.3% to 100% similarity. All CV-A10 strains (n = 60) determined in this study between 2008 and 2012 were included in genogroup C except for two variant strains, JB143090148 and JB143090155 (isolated in 2009), which were clustered in genogroup F. The majority of CV-A10 strains isolated from Shenzhen between 2008 and 2012 had a close genetic relationship with the 2007 Slovakian strains and the 2008 Spanish strains associated with onychomadesis after HFMD (15); however, they displayed a distant genetic relationship with the 2008 Finnish strains associated with an HFMD outbreak and the 2010 French strains associated with an HFMD/herpangina outbreak (10, 12). CV-A10 strains circulated in China with a low prevalence, and the genetic characteristics of the CV-A10 strains isolated from Shenzhen were different from those isolated from other regions in China. The most common ancestor of CV-A10 emerged almost at the same time as HEV71, and its evolutionary rate was estimated to be 6.2 ×10−3 to 6.4 ×10−3 substitutions per site per year (Table 3). Genotypes B, C, D, E, and F were estimated to circulate recessively for 2 to 11 years before causing infectious diseases.
DISCUSSION
As a common infectious disease, HFMD is a serious threat to public health. Numerous large epidemics of HFMD have occurred mainly in eastern and southeastern Asian countries and regions in the past decades, with HEV71 being the most commonly responsible causative agent. In recent years, however, some uncommon HEVs emerged as being occasionally prevalent. CV-A6 and CV-A10 especially began to cocirculate with increasingly frequency in some European countries, which resulted in these serotypes becoming as common causes of HFMD as were HEV71 and CV-A16 in certain regions (10–16). In mainland China, the surveillance of HFMD has been focused mainly on HEV71 and CV-A16. Therefore, little is known about the pathogenic roles of other HEVs, their geographic distributions, and epidemiological data. Our prospective study is the first to provide a 5-year surveillance of HFMD over the past 5 years in China. The results demonstrated that CV-A6 and CV-A10 emerged and cocirculated with a variety of other HEVs in Shenzhen, although HEV71 remained the major pathogen. As an emerging pathogen, CV-A6 increasingly became as common a causative agent of HFMD in Shenzhen as was HEV71. Although HEV71 and CV-A6 have been the main pathogens of HFMD in Shenzhen in recent years, HEV71 and CV-A16 remained the major causes of HFMD in other regions in mainland China.
HEV-A species were the most common HEV types (94.3% to 98.8% between 2008 and 2012) among the pathogens causing HFMD in Shenzhen, followed by HEV-B and HEV-C species, while no HEV-D species were detected. However, HEV-B species were the most frequent HEV types in healthy children (<5 years) and in an aquatic environment between 2010 and 2011 in Shenzhen (27, 35). These results suggest that the prevalences of HEVs in Shenzhen have been different in different environments.
Previous studies demonstrated that HEV71 was more likely to cause serious complications than other HEVs and often led to acute flaccid paralysis, brainstem encephalitis associated with cardiopulmonary edema, and death. However, in our study, in patients with severe HEV71 or CV-A16 infections, the shared common symptoms were accelerated breathing and muscle twitches. Phylogenetic analysis showed that little variation appeared in the Chinese HEV71 and CV-A16 strains; Chinese HEV71 strains have been the C4 genotype since 1998, and Chinese CV-A16 strains have had subgenotypes B1a and B1b since 1999. Further studies should be intensified to clarify the relationship between pathogenic features and the genetic characteristics of the pathogens.
In consideration of further evolutionary dynamics analysis of the pathogens, three RT-snPCR methods were chosen to identify the causative agents of HFMD in this study, which not only ensured the high sensitivity of detection but also amplified complete VP1 genes. Spatiotemporal phylogenetic analysis of CV-A6 and CV-A10 strains revealed genetic diversity. There was great genetic variation among the CV-A6 and CV-A10 strains from different regions in mainland China. We inferred that CV-A6 strains circulating in Shenzhen likely originated in Europe. The prevalent genotypes C and D were estimated to circulate for 5 to 6 years before causing infectious diseases. Therefore, we must be on the alert for the possibility that CV-A6 will cause an HFMD outbreak in the next few years in China. Although the genotyping of CV-A6 and CV-A10 was performed by three statistical methods, a robust spatiotemporal phylogenetic analysis of CV-A6 and CV-A10 based on complete VP1 sequences is needed.
The molecular evolutionary rates of several human enterovirus serotypes have been estimated to be 3 × 10−3 to 9 × 10−3 substitutions/site/year (28–31). Since RNA viruses have an estimated mutation rate of between 10−3 and 10−5 substitutions/site/generation (32), this suggests that the molecular evolutionary rates of HEVs are higher than those of the other RNA viruses. HEVs underwent rapid variation under evolutionary pressure and frequent recombination (8, 28, 33, 34). For this reason, the pathogens of HFMD have evolved into multiple genotypes that have served as a bottleneck for vaccine development against HEVs. In summary, our study indicated that a variety of HEV serotypes have been involved in HFMD infections, according to our 5-year surveillance in China. Identifying the causative agents of HFMD has been challenging. Continuing surveillance is needed to identify other unusual strains.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Natural Science Foundation of China (no. 31170874).
We are grateful to the pediatricians who are from the sentinel surveillance system for HFMD in Shenzhen, China.
Footnotes
Published ahead of print 21 August 2013
Supplemental material may be found for this article at http://dx.doi.org/10.1128/JCM.01231-13.
REFERENCES
- 1.Palacios G, Oberste MS. 2005. Enteroviruses as agents of emerging infectious diseases. J. Neurovirol. 11:424–433 [DOI] [PubMed] [Google Scholar]
- 2.Chang LY, Lin TY, Hsu KH, Huang YC, Lin KL, Hsueh C, Shih SR, Ning HC, Hwang MS, Wang HS, Lee CY. 1999. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 354:1682–1686 [DOI] [PubMed] [Google Scholar]
- 3.Shah VA, Chong CY, Chan KP, Ng W, Ling AE. 2003. Clinical characteristics of an outbreak of hand, foot and mouth disease in Singapore. Ann. Acad. Med. Singapore 32:381–387 [PubMed] [Google Scholar]
- 4.Tee KK, Lam TT, Chan YF, Bible JM, Kamarulzaman A, Tong CY, Takebe Y, Pybus OG. 2010. Evolutionary genetics of human enterovirus 71: origin, population dynamics, natural selection, and seasonal periodicity of the VP1 gene. J. Virol. 84:3339–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng ZM, He PJ, Caueffield D, Neumann M, Specter S, Baker CC, Bankowski MJ. 1995. Enterovirus 71 isolated from China is serologically similar to the prototype E71 BrCr strain but differs in the 5′-noncoding region. J. Med. Virol. 47:161–167 [DOI] [PubMed] [Google Scholar]
- 6.Yang F, Ren L, Xiong Z, Li J, Xiao Y, Zhao R, He Y, Bu G, Zhou S, Wang J, Qi J. 2009. Enterovirus 71 outbreak in the People's Republic of China in 2008. J. Clin. Microbiol. 47:2351–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Tan XJ, Wang HY, Yan DM, Zhu SL, Wang DY, Ji F, Wang XJ, Gao YJ, Chen L, An HQ, Li DX, Wang SW, Xu AQ, Wang ZJ, Xu WB. 2009. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J. Clin. Virol. 44:262–267 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang Y, Mao N, Xu S, Zhu S, Cui A, Zhang Y, Yan D, Li Q, Dong X, Zhang J, Zhao Y, Wan J, Feng Z, Sun J, Wang S, Li D, Xu W. 2010. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol. J. 7:94. 10.1186/1743-422X-7-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Yeo A, Phoon MC, Tan EL, Poh CL, Quak SH, Chow VT. 2010. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int. J. Infect. Dis. 14:e1076–e1081. 10.1016/j.ijid.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 10.Blomqvist S, Klemola P, Kaijalainen S, Paananen A, Simonen ML, Vuorinen T, Roivainen M. 2010. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J. Clin. Virol. 48:49–54 [DOI] [PubMed] [Google Scholar]
- 11.Lo SH, Huang YC, Huang CG, Tsao KC, Li WC, Hsieh YC, Chiu CH, Lin TY. 2011. Clinical and epidemiologic features of coxsackievirus A6 infection in children in northern Taiwan between 2004 and 2009. J. Microbiol. Immunol. Infect. 44:252–257 [DOI] [PubMed] [Google Scholar]
- 12.Mirand A, Henquell C, Archimbaud C, Ughetto S, Antona D, Bailly JL, Peigue-Lafeuille H. 2011. Outbreak of hand, foot and mouth disease/herpangina associated with coxsackievirus A6 and A10 infections in 2010, France: a large citywide, prospective observational study. Clin. Microbiol. Infect. 18:E110–E118. 10.1111/j.1469-0691.2012.03789.x [DOI] [PubMed] [Google Scholar]
- 13.Wei SH, Huang YP, Liu MC, Tsou TP, Lin HC, Lin TL, Tsai CY, Chao YN, Chang LY, Hsu CM. 2011. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect. Dis. 11:346. 10.1186/1471-2334-11-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamashita T, Ito M, Taniguchi A, Sakae K. 2005. Prevalence of coxsackievirus A5, A6, and A10 in patients with herpangina in Aichi Prefecture, 2005. Jpn. J. Infect. Dis. 58:390–391 [PubMed] [Google Scholar]
- 15.Bracho MA, González-Candelas F, Valero A, Córdoba J, Salazar A. 2011. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg. Infect. Dis. 17:2223–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimoto T, Iizuka S, Enomoto M, Abe K, Yamashita K, Hanaoka N, Okabe N, Yoshida H, Yasui Y, Kobayashi M, Fujii Y, Tanaka H, Yamamoto M, Shimizu H. 2012. Hand, foot, and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg. Infect. Dis. 18:337–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberste MS, Peñaranda S, Rogers SL, Henderson E, Nix WA. 2010. Comparative evaluation of Taqman real-time PCR and semi-nested VP1 PCR for detection of enteroviruses in clinical specimens. J. Clin. Virol. 49:73–74 [DOI] [PubMed] [Google Scholar]
- 18.Nix WA, Oberste MS, Pallansch MA. 2006. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 44:2698–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirand A, Schuffenecker I, Henquell C, Billaud G, Jugie G, Falcon D, Mahul A, Archimbaud C, Terletskaia-Ladwig E, Diedrich S, Huemer HP, Enders M, Lina B, Peigue-Lafeuille H, Bailly JL. 2010. Phylogenetic evidence for a recent spread of two populations of human enterovirus 71 in European countries. J. Gen. Virol. 91:2263–2277 [DOI] [PubMed] [Google Scholar]
- 20.Mirand A, Henquell C, Archimbaud C, Chambon M, Charbonne F, Peigue-Lafeuille H, Bailly JL. 2008. Prospective identification of enteroviruses involved in meningitis in 2006 through direct genotyping in cerebrospinal fluid. J. Clin. Microbiol. 46:87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7:214. 10.1186/1471-2148-7-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biol. 4:e88. 10.1371/journal.pbio.0040088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasegawa M, Kishino H, Yano T. 1985. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 22:160–174 [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez F, Oliver JL, Marín A, Medina JR. 1990. The general stochastic model of nucleotide substitution. J. Theor. Biol. 142:485–501 [DOI] [PubMed] [Google Scholar]
- 26.Oberste MS, Maher K, Kilpatrick DR, Flemister MR, Brown BA, Pallansch MA. 1999. Typing of human enteroviruses by partial sequencing of VP1. J. Clin. Microbiol. 37:1288–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Liu Q, Wang D, Chen Y, Feng B, Li G, Yao W, Shu B, He Y. 2013. Surveillance and analysis of enteroviruses in water environments in Shenzhen from 2010 to 2011. Arch. Virol. 158:1343–1347 [DOI] [PubMed] [Google Scholar]
- 28.McWilliam Leitch EC, Cabrerizo M, Cardosa J, Harvala H, Ivanova OE, Kroes AC, Lukashev A, Muir P, Odoom J, Roivainen M, Susi P, Trallero G, Evans DJ, Simmonds P. 2010. Evolutionary dynamics and temporal/geographical correlates of recombination in the human enterovirus echovirus types 9, 11, and 30. J. Virol. 4:9292–9300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu PY, Lu PL, Tsai YL, Hsi E, Yao CY, Chen YH, Hsu LC, Wang SY, Wu HS, Lin YY, Su HJ, Lin KH. 2011. Spatiotemporal phylogenetic analysis and molecular characterization of coxsackievirus A4. Infect. Genet. Evol. 11:1426–1435 [DOI] [PubMed] [Google Scholar]
- 30.Bailly JL, Mirand A, Henquell C, Archimbaud C, Chambon M, Regagnon C, Charbonné F, Peigue-Lafeuille H. 2011. Repeated genomic transfers from echovirus 30 to echovirus 6 lineages indicate co-divergence between co-circulating populations of the two human enterovirus serotypes. Infect. Genet. Evol. 11:276–289 [DOI] [PubMed] [Google Scholar]
- 31.Gullberg M, Tolf C, Jonsson N, Mulders MN, Savolainen-Kopra C, Hovi T, Van Ranst M, Lemey P, Hafenstein S, Lindberg AM. 2010. Characterization of a putative ancestor of coxsackievirus B5. J. Virol. 84:9695–9708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drake JW, Charlesworth B, Charlesworth D, Crow JF. 1998. Rates of spontaneous mutation. Genetics 148:1667–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu YF, Yang F, Du J, Dong J, Zhang T, Wu ZQ, Xue Y, Jin Q. 2011. Complete genome analysis of coxsackievirus A2, A4, A5, and A10 strains isolated from hand, foot, and mouth disease patients in China revealing frequent recombination of human enterovirus A. J. Clin. Microbiol. 49:2426–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oberste MS, Maher K, Pallansch MA. 2004. Evidence for frequent recombination within species human enterovirus B based on complete genomic sequences of all thirty-seven serotypes. J. Virol. 78:855–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu W, Xu WB, Chen L, Chen HL, Liu Q, Wang DL, Chen YJ, Yao W, Li G, Feng B, Shu BH, Zhou YK, He YQ. 2013. Molecular identification and analysis of human enteroviruses isolated from healthy children in Shenzhen, China from 2010 to 2011. PLoS One 8(6):e64889. 10.1371/journal.pone.0064889 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.