Abstract
Cutaneous infections due to Listeria monocytogenes are rare. Typically, infections manifest as nonpainful, nonpruritic, self-limited, localized, papulopustular or vesiculopustular eruptions in healthy persons. Most cases follow direct inoculation of the skin in veterinarians or farmers who have exposure to animal products of conception. Less commonly, skin lesions may arise from hematogenous dissemination in compromised hosts with invasive disease. Here, we report the first case in a gardener that occurred following exposure to soil and vegetation.
INTRODUCTION
Human listeriosis most frequently is recognized as a food-borne invasive illness leading to bacteremia or central nervous system infection (1). In utero transmission from mother to fetus may result in disseminated neonatal infection that sometimes is associated with diffuse skin lesions (1, 2), but cutaneous listeriosis outside the neonatal period is distinctly rare. We report here a case of primary skin infection due to Listeria monocytogenes and review the available literature concerning cutaneous listeriosis.
CASE REPORT
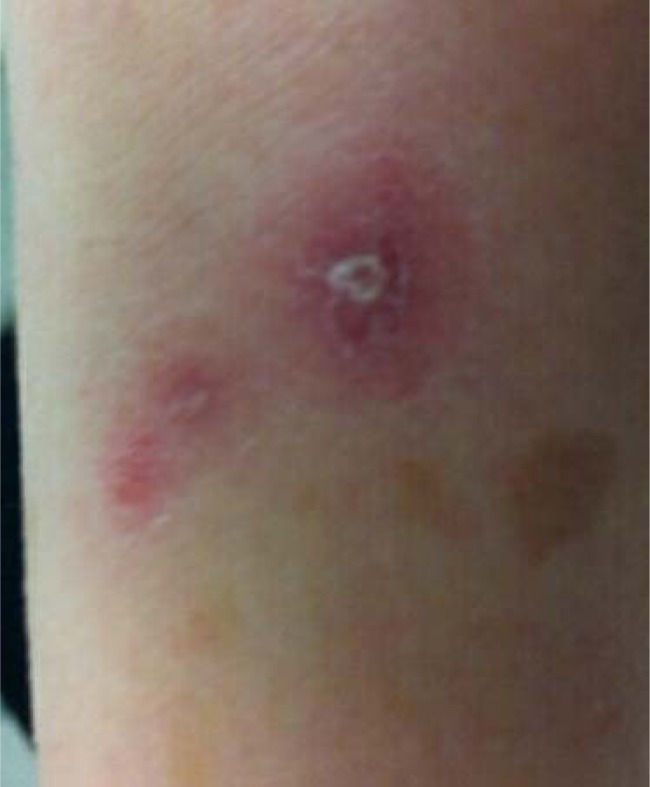
A 66-year-old woman was referred to an infectious diseases clinic for a rash on her right wrist. Two weeks earlier, the patient spent time digging out plants and bushes in her California garden. She has a large estate and described the area in which she worked as “more wild” than her usual gardening locations. She did not recall specific trauma to her skin. The day after gardening, she experienced generalized achiness and noticed she was sleeping more than usual. She had no fever, chills, nausea, vomiting, diarrhea, headache, or neck stiffness. One day later, she developed a rash on her right wrist (Fig. 1) without associated joint pain. The rash was neither painful nor pruritic. Three days after gardening, she saw her primary care physician, who unroofed and swabbed a skin lesion for culture. Blood cultures were not obtained. Listeria monocytogenes susceptible to ampicillin and penicillin was isolated from the swab culture; a single colony of coagulase-negative Staphylococcus was also isolated.
Fig 1.
Rash on volar aspect of wrist 24 h after onset demonstrating two clusters of vesiculopustular lesions with surrounding erythema. Culture from an unroofed lesion grew Listeria monocytogenes. Although the patient reported no trauma, an interrupted, linear, healing scratch can be seen lateral to and extending into one of the lesions (arrows).
When the culture result became known, 7 days after the onset of the rash, the patient was brought back to her primary care physician's office. At that time, the rash was thought to appear more prominent, but because the patient was well, she was not given any antibiotic therapy and was referred to an infectious disease specialist.
When evaluated at the infectious diseases clinic, 2 weeks after the onset of her rash, she felt well; her energy level was back to normal. She reported a diagnosis of osteoporosis and a history of herpes labialis for which she occasionally took acyclovir. She had had nonpainful zoster of the abdomen in the 1990s and had not received the zoster vaccine. She was taking no medications; ciprofloxacin caused a rash.
The patient reported that she lives with her husband on the San Francisco peninsula in California. She volunteers at a Ronald McDonald House and at a local hospital. Her last travel was to Europe in 2010. She occasionally eats artisanal cheese but does not eat queso fresco. She ate cantaloupe and deli-style pastrami prior to the rash. She had no animal exposure. She had no family history of unusual or recurrent infections.
In the infectious diseases clinic, her vital signs and examination were normal except for a resolving rash on the volar surface of her wrist (Fig. 2). There were no remaining pustules or vesicles from which to obtain a culture, and no other skin lesions were present. The white blood cell count was 10.2 × 109/liter with 55.5% neutrophils, 34% lymphocytes, 6% monocytes, and 3.5% eosinophils. A swab from the surface of the resolving skin lesions and two blood cultures yielded no growth. No treatment was given.
Fig 2.
Resolving rash on day seven. The vesiculopustular lesions are gone, and desquamation is evident. No antimicrobial therapy had been given.
She was seen again several weeks later, at which time she was asymptomatic and the rash had resolved completely.
MATERIALS AND METHODS
We performed an English language literature search for cases of cutaneous listeriosis employing the PubMed and Ovid databases and using the search terms “listeriosis” and “Listeria monocytogenes” combined with the terms “cutaneous,” “rash,” or “skin.” We selected for review all nonneonatal cases of skin lesions attributable to Listeria monocytogenes whether they were primary (limited to the skin) or secondary to systemic infection. References cited in the articles found through these searches were evaluated for potential additional cases. Cases from references not located by a traditional literature search, along with those originally reported in languages other than English, were included if they were summarized in previous English language reviews. Although several cases were detail deficient in terms of patient age and sex, type of skin lesion, occurrence of systemic symptoms, need for treatment, and outcome, we included them in order to have as complete a review as possible.
RESULTS
Cases of nonneonatal listeriosis with cutaneous manifestations are summarized in Table 1. Twenty-three instances of cutaneous listeriosis occurring after the neonatal period were reported in the literature between 1957 and 2009 (3–14); our report represents the 24th case. Patients ranged in age from 26 to 66 years. Reflecting occupational exposure as veterinarians and farmers, most cases were in men, with only 3 cases in women. Contact with an aborted bovine fetus was the most common exposure. The time from exposure to development of a rash ranged from 6 h to 7 days with a median of 2 days. Seventeen of the 24 cases occurred as the result of direct skin inoculation. In three instances, the skin was secondarily involved in patients with invasive disease through hematogenous spread, and in four, the mechanism of cutaneous infection was unknown. Most episodes of cutaneous listeriosis manifested as papules or pustules; there was one instance of cellulitis with abscess formation. Again reflecting occupational exposure, skin eruptions most often occurred on the arms and/or hands, with only 3 cases of skin lesions noted on the lower extremities. Most patients experienced systemic symptoms, with fever being the most common, occurring in 17 of 24 cases. Three patients had regional adenopathy, and one of these three had lymphangitis. Three individuals showed no evidence of illness other than the rash. Bacteremia was documented in only one case, a person with hairy cell leukemia and cerebritis. All three patients with invasive disease and hematogenous dissemination to the skin had a serious underlying disease (leukemia, HIV, or bone marrow transplant); all those whose skin lesions developed after direct inoculation were in good health. Antibiotic use was reported for only 5 of the 21 patients without invasive disease. Although one patient with direct inoculation cutaneous listeriosis after exposure to a bovine abortion died (the age and presence of any underlying are disease unknown), all others with direct inoculation infection for whom the outcome was recorded recovered without incident, including two patients who were clearly documented as not having received antimicrobial treatment.
Table 1.
Features of cutaneous listeriosis cases
| Yr of report (reference or first author) | Patient age (yr)/sexc | Exposure scenario | Incubation period | Authors' description of skin lesions | Location(s) of skin lesions | Direct inoculation or hematogenous spread | Systemic symptom(s) | Other site(s) of infection | Underlying illness(es)d | Treatmente | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1957 (Novaka) | NA/F | Laboratory technician | NA | Papules, vesicles | Face and neck | NA | Headache, fever, swollen lymph nodes, vomiting | NA | NA | NA | Full recovery |
| 1959 (Dijkstrab) | NA | Bovine abortion | 2 days | Papular/pustular | Arms or hands | Direct inoculation | NA | NA | NA | NA | NA |
| 1960 (4) | NA/M | Bovine abortion | 2–3 days | Papular/pustular | Right arm, left wrist | Direct inoculation | Fever, malaise, headache, dizziness | None | None | Sulfonamide, unknown duration | Full recovery |
| 1960 (Kalkoffb) | NA/M | Bovine abortion | 1 day | Nodules, pustules, surrounding erythema | Forearms and upper arms | Direct inoculation | Chills, fever | NA | NA | NA | Full recovery |
| 1961 (Seeligerb) | NA | Bovine abortion | 1–2 days | Papular/pustular | Arms or hands | Direct inoculation | Fever, lymphangitis, adenitis | NA | NA | NA | Died |
| 1966 (Moutonb) | NA | Bovine abortion | 3 days | Papular/pustular | Arms or hands | Direct inoculation | Fever | NA | NA | NA | NA |
| 1966 (Moutonb) | NA | NA | 2 days | Papular/pustular | Arms or hands | NA | Fever | NA | NA | NA | NA |
| 1966 (Moutonb) | NA | NA | 3 days | Papular/pustular | Arms or hands | NA | Fever | NA | NA | NA | NA |
| 1986 (5) | 64/M | Bovine abortion | 1–2 days | Red, vesicular/pustular | Both arms and hands | Direct inoculation | Fever, chills, aches | None | None | Erythromycin, unknown duration | Full recovery |
| 1986 (6) | 53/M | NA | NA | Nonerythematous papules | Upper extremities then lower extremities | Hematogenous spread | Fever, headache, night sweats, fatigue, weakness | Bacteremia, cerebritis | Hairy cell leukemia | Parenteral ampicillin and gentamicin for 6 wk, then oral ampicillin for 6 wk | Partial recovery |
| 1990 (7) | NA | Handled meat carcasses | NA | Papular/pustular | Arms or hands | Direct inoculation | NA | NA | NA | NA | NA |
| 1992 (8) | NA/M | Bovine abortion | 2 days | Small pustules | Bilateral forearms | Direct inoculation | None | None | None | None | Full recovery |
| 1994 (9) | 29/M | NA | NA | Localized abscess in area of cellulitis | Pretibial | Hematogenous spread | Fever, malaise | None | HIV | Cloxacillin, then parenteral ampicillin and gentamicin for 10 days, then oral amoxicillin for 12 days | Full recovery |
| 1994 (10) | NA | Bovine abortion | 3–4 days | Papular/pustular | Arms or hands | Direct inoculation | None | NA | NA | NA | NA |
| 1994 (10) | NA | Rectal examination of heifer | 1 day | Papular/pustular | Arms or hands | Direct inoculation | NA | NA | NA | NA | NA |
| 1994 (10) | NA | NA | NA | Papular/pustular | Arms or hands | NA | Fever | NA | NA | NA | NA |
| 1994 (10) | NA | Bovine abortion | NA | Papular/pustular | Arms or hands | Direct inoculation | NA | NA | NA | NA | NA |
| 1994 (10) | NA | Delivery of calf | NA | Papular/pustular | Arms or hands | Direct inoculation | Fever | NA | NA | NA | NA |
| 1994 (10) | NA | Bovine abortion | NA | Papular/pustular | Arms or hands | Direct inoculation | Fever | NA | NA | NA | NA |
| 2004 (11) | 36/M | NA | NA | Single purple papule | Thigh | Hematogenous spread | Fever | Cerebritis, pneumonia | NHL, BMT | Ampicillin and TMP-SMX for 4 days, then ampicillin and gentamicin for 15 days, then amoxicillin for 7 days | Full recovery |
| 2005 (12) | 26/F | Bovine abortion | 7 days | Pustular rash | Both arms | Direct inoculation | Fever, headache, myalgia | None | None | Amoxicillin-clavulanate for 10 days | Full recovery |
| 2008 (13) | 55/M | Bovine abortion | 1–2 days | Pustular rash | Both hands and arms | Direct inoculation | Fever, headache, myalgia, axillary nodes | None | None | Amoxicillin-clavulanate for 10 days | Full recovery |
| 2009 (14) | 38/M | Bovine abortion | 6 hr | Large pustules | Both hands and wrists, spreading to forearms | Direct inoculation | Fever, rigors, myalgia | None | None | Parenteral penicillin and gentamicin for 2 days, then amoxicillin-clavulanate for 10 days | Full recovery |
| Present report | 66/F | Soil and plant vegetation | 1 day | Vesiculopustular | Right wrist | Direct inoculation | Achiness, malaise, no fever | None | None | None | Full recovery |
DISCUSSION
Human listeriosis typically follows food-borne transmission and manifests as bacteremia and/or central nervous system infection in persons at risk due to impaired cell-mediated immunity from underlying disease or medical therapy, pregnancy, or advanced age (1). Less often, focal infections of joints, liver, spleen, pericardium, and other body sites may follow hematogenous dissemination. When fetal infection occurs, the newborn may exhibit skin lesions as part of widely disseminated disease (granulomatosis infantiseptica). Cutaneous listeriosis outside the neonatal period is quite rare. Our literature review yielded just 23 cases.
Cutaneous listeriosis after the neonatal period is mostly an occupational infection, with the majority of episodes representing primary cutaneous involvement in veterinarians or farmers who were exposed to bovine products of conception. In these cases, L. monocytogenes infection caused the intrauterine demise of the bovine fetus and was then transmitted via direct inoculation to the person who assisted at the delivery. In many of these cases, the farmer or veterinarian did not wear birthing gloves. Unlike most cases of human listeriosis, primary cutaneous infection happens in otherwise healthy individuals or those who are presumed to be healthy. Although infection appears confined to the skin, fever and other systemic symptoms are common.
The papulopustular or papulovesicular rash that occurs is most often self-limited, and full recovery without antibiotic treatment is usual.
Our present case occurred in a gardener with no underlying illness. We believe that this is the first published case of primary cutaneous listeriosis in a gardener. She developed papulovesicular skin lesions on her wrist 2 days after digging out plants and bushes in an overgrown area of her property. Listeria monocytogenes is prevalent in soil and on vegetation (15). As illustrated in Fig. 1, her lesions cropped up adjacent to a scratch that may have been the inoculation site. Her lesions were neither pruritic nor tender, were well healed in less than 2 weeks, and resolved completely in about 1 month. Given the widespread presence of L. monocytogenes in soil and on plants, and the common occurrence of skin trauma during gardening, it is somewhat surprising that there have been no previous reports of cutaneous infection in gardeners. It is possible that some cases may have been misdiagnosed as folliculitis, contact dermatitis, or localized herpetic infection, but a more likely explanation is that it takes a large inoculum of Listeria to produce infection. The occurrence of cutaneous listeriosis in veterinarians and farmers having contact with bovine products of conception may be related to the very high concentrations of bacteria found in infected amniotic fluid (estimated to be 108 CFU/ml [10]). In this regard, cutaneous listeriosis is reminiscent of febrile gastroenteritis due to L. monocytogenes. It also occurs in healthy persons but requires a very large inoculum to produce illness (16).
Skin involvement after hematogenous dissemination of L. monocytogenes infection has been documented in three instances. Each patient had an underlying condition that severely impaired cell-mediated immunity (hairy cell leukemia, AIDS, and bone marrow transplant for non-Hodgkin lymphoma) along with evidence of severe systemic illness. In these three cases, L. monocytogenes was grown from culture of skin lesions. In two cases, the skin lesions were solitary, with one of the case patients having an abscess within an area of cellulitis. In the third case, there was a widespread eruption of papules similar to the cutaneous lesions seen in neonatal cases of listeriosis.
Cutaneous listeriosis is rare, and its frequency is hard to determine. McLauchlin and Low (10) reported the incidence of skin involvement in human listeriosis in Great Britain to be between 0.1 and 1.1%. There were 1,651 cases of listeriosis in the United States from 2009 through 2011 (17); we are unaware of any reports of cutaneous infection during that time period.
Direct inoculation listeriosis should be considered whenever a veterinarian or farmer presents with a rash within days of assisting at the delivery of a calf. Such persons should wear protective gloves when attending deliveries. Primary cutaneous listeriosis also should be considered when a gardener presents with a papulopustular or papulovesicular rash within several days of being exposed to soil and/or vegetation.
Primary cutaneous listeriosis appears to be self-limited in almost all instances, and the role for antibiotics is unclear. Our patient was not treated with antibiotics, because at the time she was seen by the infectious diseases consultant she was totally well, and the rash was almost gone. However, since most patients experience systemic symptoms, including fever, we believe it would be prudent to treat those having documented infection with a brief (5- to 7-day) course of oral amoxicillin or trimethoprim-sulfamethoxazole.
ACKNOWLEDGMENTS
This study was unfunded.
The authors declare no conflict of interest.
Footnotes
Published ahead of print 21 August 2013
REFERENCES
- 1.Lorber B. 2012. Listeria monocytogenes, p 762–767 In Long SS, Pickering LK, Prober CG. (ed), Principles and practice of pediatric infectious diseases, 4th ed. Elsevier, London, United Kingdom [Google Scholar]
- 2.Smith KJ, Skelton HG, III, Angritt P, James WD, Yeager J, Wagner K. 1991. Cutaneous lesions of listeriosis in a newborn. J. Cutan. Pathol. 18:474–476 [DOI] [PubMed] [Google Scholar]
- 3.Dincsoy MY, Booker CR, Scott RB. 1965. Skin manifestation in Listeria infection. A case report and review of the literature. J. Natl. Med. Assoc. 57:290–296 [PMC free article] [PubMed] [Google Scholar]
- 4.Owen CR, Meis A, Jackson JW, Stoenner HG. 1960. A case of primary cutaneous listeriosis. N. Engl. J. Med. 262:1026–1028 [DOI] [PubMed] [Google Scholar]
- 5.Cain DB, McCann VL. 1986. An unusual case of cutaneous listeriosis. J. Clin. Microbiol. 23:976–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salata RA, King RE, Gose F, Pearson RD. 1986. Listeria monocytogenes cerebritis, bacteremia, and cutaneous lesions complicating hairy cell leukemia. Am. J. Med. 81:1068–1072 [DOI] [PubMed] [Google Scholar]
- 7.McLauchlin J. 1990. Human listeriosis in Britain, 1967–1985, a summary of 722 cases. 2. Listeriosis in non-pregnant individuals, a changing pattern of infection and seasonal incidence. Epidemiol. Infect. 104:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allcock JG. 1992. Cutaneous listeriosis. Vet. Rec. 130:18–19 [DOI] [PubMed] [Google Scholar]
- 9.Vásquez A, Ramos JM, Pacho E, Rodríguez-Pérez A, Cuenca-Estrella M, Esteban J. 1994. Cutaneous listeriosis in a patient infected with the immunodeficiency virus. Clin. Infect. Dis. 19:988–989 [DOI] [PubMed] [Google Scholar]
- 10.McLauchlin J, Low JC. 1994. Primary cutaneous listeriosis in adults: an occupational disease of veterinarians and farmers. Vet. Rec. 135:615–617 [PubMed] [Google Scholar]
- 11.Lambotte O, Fihman V, Poyart C, Buzyn A, Berche P, Soumelis V. 2005. Listeria monocytogenes skin infection with cerebritis and haemophagocytosis syndrome in a bone marrow transplant recipient. J. Infect. 50:356–358 [DOI] [PubMed] [Google Scholar]
- 12.Regan EJ, Harrison GA, Butler S, McLauchlin J, Thomas M, Mitchell S. 2005. Primary cutaneous listeriosis in a veterinarian. Vet. Rec. 157:207. [DOI] [PubMed] [Google Scholar]
- 13.Laureyns J, Moyaert H, Werbrouck H, Catry B, de Kruif A, Pasmans F. 2008. Pustular dermatitis by Listeria monocytogenes after the assisted delivery of a dead calf. Vlaams Diergeneeskundig Tijdschrift. 77:29–34 [Google Scholar]
- 14.Gilchrist M. 2009. Cutaneous Listeria infection. Br. J. Hosp. Med. (Lond.) 70:659. [DOI] [PubMed] [Google Scholar]
- 15.Sauders BD, Wiedmann M. 2007. Listeria species and L. monocytogenes in the natural environment, p 21–53 In Ryser ET, Marth EH. (ed), Listeria, listeriosis and food Safety, 3rd ed. CRC Press, Boca Raton, FL [Google Scholar]
- 16.Ooi ST, Lorber B. 2005. Gastroenteritis due to Listeria monocytogenes. Clin. Infect. Dis. 40:1327–1332 [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) 2013. Vital signs: Listeria illnesses, deaths, and outbreaks—United States, 2009–2011. MMWR Morb. Mortal. Wkly. Rep. 62:448–452 [PMC free article] [PubMed] [Google Scholar]