Abstract
High-throughput, sensitive, and cost-effective HIV drug resistance (HIVDR) detection assays are needed for large-scale monitoring of the emergence and transmission of HIVDR in resource-limited settings. Using suspension array technology, we have developed a multiplex allele-specific (MAS) assay that can simultaneously detect major HIVDR mutations at 20 loci. Forty-five allele-specific primers tagged with unique 24-base oligonucleotides at the 5′ end were designed to detect wild-type and mutant alleles at the 20 loci of HIV-1 subtype C. The MAS assay was first established and optimized with three plasmid templates (C-wt, C-mut1, and C-mut2) and then evaluated using 148 plasma specimens from HIV-1 subtype C-infected individuals. All the wild-type and mutant alleles were unequivocally distinguished with plasmid templates, and the limits of detection were 1.56% for K219Q and K219E, 3.13% for L76V, 6.25% for K65R, K70R, L74V, L100I, K103N, K103R, Q151M, Y181C, and I47V, and 12.5% for M41L, K101P, K101E, V106A, V106M, Y115F, M184V, Y188L, G190A, V32I, I47A, I84V, and L90M. Analyses of 148 plasma specimens revealed that the MAS assay gave 100% concordance with conventional sequencing at eight loci and >95% (range, 95.21% to 99.32%) concordance at the remaining 12 loci. The differences observed were caused mainly by 24 additional low-abundance alleles detected by the MAS assay. Ultradeep sequencing analysis confirmed 15 of the 16 low-abundance alleles. This multiplex, sensitive, and straightforward result-reporting assay represents a new efficient genotyping tool for HIVDR surveillance and monitoring.
INTRODUCTION
It is estimated that >8.2 million people were receiving antiretroviral therapy (ART) in low- and middle-income countries at the end of 2011, a dramatic 26-fold increase from 2003 (1). As access to ART continues to expand worldwide and without adequate virological monitoring of patients on ART, the emergence and transmission of HIV drug resistance (HIVDR) are valid concerns. In resource-limited settings, studies have revealed that transmitted drug resistance (DR) in recently HIV-infected populations is rising in some countries where access to ART had previously been expanded (2–6), while acquired DR had been detected in a majority of the patients failing to respond to ART (3, 7, 8).
To preserve the efficacy of the limited first-line antiretroviral (ARV) drugs and to prevent the emergence and transmission of HIVDR, it is imperative to conduct HIVDR surveillance and monitoring in resource-limited settings. Data from DR surveillance and monitoring will allow for making evidence-based decisions on the need for ART guideline changes and assisting in the promotion of best practices in the provision of ART services that lead to higher rates of viral suppression and prevention of both acquired and transmitted HIVDR (9, 10).
In the past few decades, two types of genotypic DR detection assays, sequencing-based and allele-specific assays, have been developed for detecting HIVDR mutations. Conventional sequencing-based assays, such as the FDA-approved and commercially available genotyping assays, ViroSeq (11) and TruGene (12), and many in-house assays (13–15), have been widely used for HIVDR detection analyses in clinical settings and surveillance purposes. These assays can provide detailed sequence information and detect all possible mutations that are present in an amount above the detection limit, but they are generally labor-intensive and not sensitive enough to detect low-abundance mutations. Ultradeep sequencing and single-genome sequencing have also been utilized in HIVDR detection analyses in recent years and are highly sensitive in detecting low-abundance mutations; however, these assays require highly technical skills and are expensive, and they have not been used in routine surveillance in resource-limited settings (16–18).
The other type of genotypic assay is allele-specific assays. Several allele-specific assays have been developed, including oligonucleotide ligation assay (OLA), parallel allele-specific sequencing (PASS) (19, 20), allele-specific PCR (AS-PCR) (21–29) and LigAmp (21, 30). These assays offer substantial improvements in their detection sensitivity over conventional sequencing-based assays and are less expensive, and the results are easy to interpret. However, a major limitation of existing allele-specific assays is that they can only detect one or a few mutations at a time. This has limited their use in routine HIVDR surveillance and monitoring. Here, we report the development of a multiplex allele-specific (MAS) DR detection assay based on suspension array technology (31, 32) for high-throughput and sensitive detection of HIVDR mutations, which may be a feasible genotyping tool for large-scale HIVDR surveillance and monitoring in resource-limited settings.
(Data from this study were presented in part at the International Workshop on HIV and Hepatitis Virus Drug Resistance and Curative, 5 to 9 June 2012, Sitges, Spain.)
MATERIALS AND METHODS
Design of allele-specific primer extension primers.
Based on the current WHO recommendations for first- and second-line ARVs in resource-limited settings, we designed allele-specific primer extension (ASPE) primers targeting the drug resistance mutations (DRMs) at 20 loci of HIV-1 subtype C that are associated with resistance to commonly used ARVs in resource-limited settings. These include eight mutations associated with resistance to nucleoside reverse transcriptase inhibitors (NRTIs) (M41L, K65R, K70R, L74V, Y115F, Q151M, M184V, and K219Q/E), seven mutations associated with resistance to nonnucleoside reverse transcriptase inhibitors (NNRTIs) (L100I, K101P/E, K103N/R, V106A/M, Y181C, Y188L, and G190A), and five mutations associated with resistance to protease inhibitors (PIs) (V32I, I47A/V, L76V, I84V, and L90M). For each of the 20 mutation loci, one ASPE primer was designed for the wild-type (WT) and one or two primers were designed for the mutant (Mut) allele(s) based on the HIV-1 subtype C consensus sequence obtained from the HIV sequence database (see http://www.hiv.lanl.gov/content/sequence/NEWALIGN/align.html). Since five loci have two types of Mut alleles, 20 WT alleles and 25 Mut alleles were included in the assay (i.e., a total of 45 ASPE primers were designed). The specificity of each primer was confirmed by NCBI BLAST program analyses (http://www.ncbi.nlm.nih.gov/BLAST/). The ASPE primers contained an allele-specific nucleotide at the 3′ end for each of the WT and Mut alleles, and at the 5′ end, they contained a unique 24-base Tag (Luminex Corp., Austin, TX) that is complementary to the anti-Tag on each of the MagPlex-TAG microspheres (Luminex Corp.) for distinguishing each of the alleles by the suspension array system. We incorporated degenerate bases into the primers to accommodate polymorphisms adjacent to the targeted alleles. The lengths of primers were between 24 and 39 nucleotide bases with melting temperatures (Tm) of around 60°C. Table 1 shows the 45 ASPE primer sequences.
Table 1.
ASPE primers for HIV-1 group M subtype C
| Primer no. | Typea | Sequence (5′ to 3′) | Tag IDb | Positionc |
|---|---|---|---|---|
| 1 | M41 | Tag-AAG ARA AAA TAA AAG CAT TAA YAG MAA TTT GTG AWG ARA | 45 | 2632→2670 |
| 2 | 41L | Tag-AAG ARA AAA TAA AAG CAT TAA YAG MAA TTT GTG AWG ARC | 38 | 2632→2670 |
| 3 | K65 | Tag-AAA TCC ATA TAA CAC TCC ART ATT TGC YAT AAA RAA | 12 | 2708→2743 |
| 4 | 65R | Tag-AAA TCC ATA TAA CAC TCC ART ATT TGC YAT AAA RAG | 13 | 2708→2743 |
| 5 | K70 | Tag-CCA GTA TTT GCC ATA AAG ARG AAR GAY AGT ACT AA | 18 | 2724→2758 |
| 6 | 70R | Tag-CCA GTA TTT GCC ATA AAG ARG AAR GAY AGT ACT AG | 21 | 2724→2758 |
| 7 | L74 | Tag-CAT AAA AAA GAA RGA CAG TAC HAR RTG GAG AAA AT | 67 | 2735→2769 |
| 8 | 74V | Tag-CAT AAA AAA GAA RGA CAG TAC HAR RTG GAG AAA AG | 90 | 2735→2769 |
| 9 | Y115 | Tag-AGT RCT RGA YGT GGG RGA TGC ATA | 73 | 2870→2893 |
| 10 | 115F | Tag-AGT RCT RGA YGT GGG RGA TGC ATT | 62 | 2870→2893 |
| 11 | Q151 | Tag-GGA TTA GRT ATC AAT ATA ATG TRY TNC CAC | 29 | 2971→3000 |
| 12 | 151M | Tag-GGA TTA GRT ATC AAT ATA ATG TRY TNC CAA | 36 | 2971→3000 |
| 13 | M184 | Tag-AGR GCA AAA AAT CCA GAM RTR GTY ATC TRY CAA TAY A | 37 | 3063→3099 |
| 14 | 184V | Tag-AGR GCA AAA AAT CCA GAM RTR GTY ATC TRY CAA TAY G | 22 | 3063→3099 |
| 15 | K219 | Tag-AAR TGG GGR TTT ACY ACA CCA GAC A | 53 | 3180→3204 |
| 16 | 219Q | Tag-AAR TGG GGR TTT ACY ACA CCA GAC C | 44 | 3180→3204 |
| 17 | 219E | Tag-AAR TGG GGR TTT ACY ACA CCA GAK G | 96 | 3180→3204 |
| 18 | L100 | Tag-CAA TTA GGR ATA CCA CAC CCA KCA GGR T | 42 | 2820→2847 |
| 19 | 100I | Tag-CAA TTA GGR ATA CCA CAC CCA KCA GGR A | 58 | 2820→2847 |
| 20 | K101 | Tag-AAT TAG GRA TAC CAC ACC CAK CAG GRW TRA | 55 | 2821→2850 |
| 21 | 101P | Tag-AAT TAG GRA TAC CAC ACC CAK CAG GRW TRC C | 89 | 2821→2851 |
| 22 | 101E | Tag-AAT TAG GRA TAC CAC ACC CAK CAG GRW TRG | 65 | 2821→2850 |
| 23 | K103 | Tag-GAA TAC CAC ACC CAK CAG GGT TRA ARA AGA AA | 19 | 2827→2858 |
| 24 | 103N | Tag-GAA TAC CAC ACC CAK CAG GGT TRA ARA AGA AY | 66 | 2827→2858 |
| 25 | 103R | Tag-GAA TAC CAC ACC CAK CAG GGT TRA ARA AGA GA | 63 | 2827→2858 |
| 26 | V106 | Tag-ACA CCC AKC AGG GTT AAA RAA GAA HAA RTC WGT | 20 | 2834→2866 |
| 27 | 106A | Tag-ACA CCC AKC AGG GTT AAA RAA GAA HAA RTC WGC | 39 | 2834→2866 |
| 28 | 106M | Tag-CAC ACC CAK CAG GGT TAA ARA AGA AHA ART CWA | 75 | 2833→2865 |
| 29 | Y181 | Tag-GCC CTT TAG RRC AMA AAA TCC AGA MVT RGT YAT CTA | 9 | 3056→3091 |
| 30 | 181C | Tag-GCC CTT TAG RRC AMA AAA TCC AGA MVT RGT YAT CTG | 82 | 3056→3091 |
| 31 | Y188 | Tag-CAG AAA TRG TYA TCT RTC AAT AYR TRG ATG AYT TRT A | 83 | 3076→3112 |
| 32 | 188L | Tag-CAG AAA TRG TYA TCT RTC AAT AYR TRG ATG AYT TRC T | 93 | 3076→3112 |
| 33 | G190 | Tag-ATA GTY ATC TRT CAA TAT RTG GAT GAC TTR TAT GTR GG | 97 | 3081→3118 |
| 34 | 190A | Tag-ATA GTY ATC TRT CAA TAT RTG GAT GAC TTR TAT GTR GC | 76 | 3081→3118 |
| 35 | V32 | Tag-CTC TYT TAG AYA CAG GAG CAG ATG AYA CAG | 14 | 2317→2346 |
| 36 | 32I | Tag-CTC TYT TAG AYA CAG GAG CAG ATG AYA CAA | 48 | 2317→2346 |
| 37 | I47 | Tag-TGC CAG GRA RAT GGA AAC CAA RAA TRA | 30 | 2365→2391 |
| 38 | 47V | Tag-GCC AGG RAR ATG GAA ACC AAR AAT RGT | 43 | 2366→2392 |
| 39 | 47A | Tag-GCC AGG RAR ATG GAA ACC AAR AAT RGC | 78 | 2366→2392 |
| 40 | L76 | Tag-AAA TTT GTG GRA AAA ARG CTR TAG GTA CAG TRT | 28 | 2446→2478 |
| 41 | 76V | Tag-AAA TTT GTG GRA AAA ARG CTR TAG GTA CAG TRG | 70 | 2446→2478 |
| 42 | I84 | Tag-AGT ATT ART RGG RCC TAC ACC TGT CAA YA | 35 | 2474→2502 |
| 43 | 84V | Tag-AGT ATT ART RGG RCC TAC ACC TGT CAA YG | 77 | 2474→2502 |
| 44 | L90 | Tag-CCT ACA CCT GTC AAC ATA ATT GGR AGR AAY HTR T | 95 | 2487→2520 |
| 45 | 90 M | Tag-CCT ACA CCT GTC AAC ATA ATT GGR AGR AAY HTR A | 57 | 2487→2520 |
One primer was designed for each wild-type and mutant allele at 20 drug resistance mutation loci, and five loci have two types of mutant allele, so a total of 45 ASPE primers were designed.
Each Tag matches with one of the MagPlex-TAG microspheres (Luminex Corp., Austin, TX, USA). ID, identification.
Positions based on HXB2 sequence positions from 5′ to 3′.
Construction of HIV-1 subtype C wild-type and mutant plasmids.
In the initial assay development, three 990-bp DNA fragments representing WT and Mut types based on the subtype C reference sequence (GenBank accession no. AF457054) (33) were designed. The DNA fragments were synthesized and individually cloned into pUCminusMCS plasmids by Blue Heron Biotechnology (Bothell, WA). Plasmid C-wt contains the wild-type HIV-1 subtype C partial pol gene between nucleotides 2294 and 3283, according to the HIV strain HXB2 reference sequence (34). Plasmid C-mut1 carries the DRMs V32I, I47V, L76V, I84V, and L90M in the protease region, and M41L, K65R, K70R, L74V, L100I, K101P, K103N, V106A, Y115F, Q151M, Y181C, M184V, Y188L, G190A, and K219Q in the reverse transcriptase (RT) region. Plasmid C-mut2 carries the DRM I47A in the protease region and K101E, K103R, V106M, and K219E in the RT region. These three plasmids were used in the initial assay development, optimization, and sensitivity determination.
Development of the multiplex allele-specific HIVDR assay for HIV-1 subtype C.
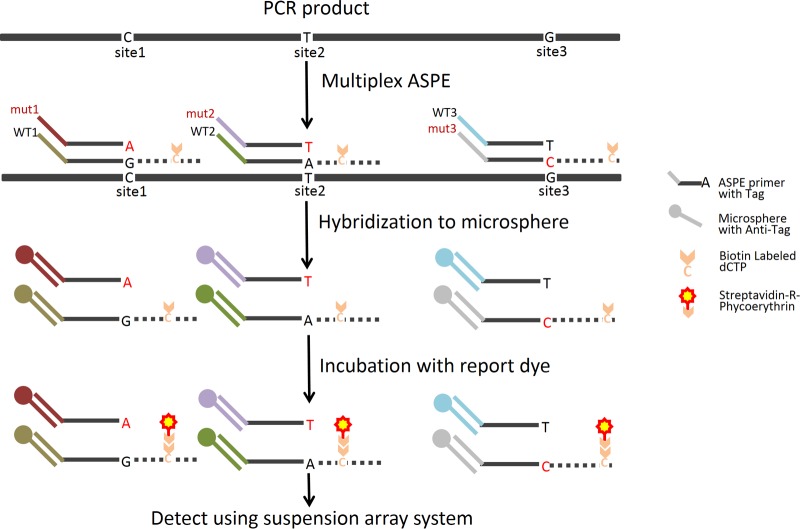
To test the validity of each of the ASPE primers, we amplified the plasmid DNAs individually by PCR. One microliter of 10 ng/μl plasmid DNAs (equivalent to 1,618 viral DNA copies) was added to 100 μl of the PCR mixture, containing 0.2 μM primers M13F (5′-CCCAG TCACG ACGTT GTAAA ACG-3′) and M13R (5′-AGCGG ATAAC AATTT CACAC AGG-3′), 1× High Fidelity PCR buffer, 2.0 mM MgSO4, and 2.0 U of Platinum Taq High Fidelity (Life Technologies, Bethesda, MD). PCR was conducted as follows: 2 min at 94°C, 30 cycles of 20 s at 94°C, 20 s at 55°C, and 90 s at 68°C, and 5 min at 68°C. The PCR products were purified using the ExoSAP-IT PCR cleanup kit (USB Co., Cleveland, OH). The purified PCR products of plasmids C-wt and C-mut1, a mixture of the two at a 1:1 ratio (wt/wt), and a no-target PCR negative control (NC) were used to validate each of the WT and Mut ASPE primers. A multiplex ASPE assay was optimized for several factors affecting the specificity and signal output. These included Mg2+ concentration, polymerase concentration, cycling parameters, annealing temperature, ASPE primer length, ASPE primer concentrations, and the amount of PCR product used in the ASPE reaction mixture. Once the specificities of the individual primers were confirmed and the assay conditions were optimal, we combined all 45 ASPE primers and performed the multiplex ASPE (mASPE) assay in a single tube using Tsp DNA polymerase (Invitrogen, Carlsbad, CA) and reaction buffer that allows for a relatively wide range of annealing temperatures. Briefly, for each 20-μl mASPE reaction mixture, it contained 5 μl purified PCR product, 1.5 U Tsp DNA polymerase, 9.5 × 1011 copies of each ASPE primer (Table 1), 10.0 μM (each) dATP, dTTP, dGTP, and biotin-dCTP (Invitrogen, Carlsbad, CA), 20.0 mM Tris-HCl (pH 8.4), 50.0 mM KCl, and 1.88 mM MgCl2. The reaction mixtures were subjected to the following PCR conditions: initial denaturation at 96°C for 2 min followed by 30 cycles of 94°C for 30 s, 55°C for 1 min, and 74°C for 2 min. Next, 10 μl of mASPE products was added to a 40-μl microsphere hybridization mixture containing 2.2 × 103 of each microsphere set and 2× Tm hybridization buffer (0.2 M Tris-HCl [pH 8.0], 0.4 M NaCl, 0.16% Triton X-100). The ASPE-bead mixtures were subjected to denaturation at 96°C for 90 s and annealing at 37°C for 30 min, and the reaction mixtures were placed on a magnetic separator for 60 s. After discarding the supernatant, the microspheres were resuspended in 100 μl 1× Tm hybridization buffer containing 4 μg/ml streptavidin R-phycoerythrin (Molecular Probes, Eugene, OR) and incubated at 37°C for 15 min. Finally, the 100-μl reaction mixture was analyzed with the Bio-Plex 3D suspension array system to determine the median fluorescence intensity (MFI) value for at least 100 microspheres for each of the 45 sets of microspheres. The MFI value for each bead set was corrected by subtracting the value of the no-target PCR negative control, and the resulting net MFI values were used for the calculation of the allelic ratio (AR). The AR is equal to the net MFI for an allele divided by the sum of the net MFIs for all alleles tested for a given mutation site, which represents the fraction of the total net MFI signal for the mutation site attributed to the presence of a particular allele. Threshold values were determined according to procedures described in previous studies (35, 36); an AR of ≥0.10 indicated the presence of a particular allele, and if more than one allele had an AR of ≥0.10, a mixture was called. Figure 1 is a schematic illustration of the mASPE and suspension array analysis processes.
Fig 1.
Schematic illustration of the MAS assay. The MAS assay starts with an allele-specific primer extension (ASPE) with all allele-specific primers mixed together in one reaction tube containing the reaction reagent mixture and a template. For the primer with a matching 3′-terminal nucleotide, primer extension occurs and biotinylated dCTPs are incorporated into the extended product. During the hybridization step, ASPE products are uniquely annealed to microspheres through the specificity of Tag/anti-Tag recognition. Finally, the hybridization products are read with the suspension array system, which identifies each microsphere set by its internal dye and records the associated reporter dye intensity as the mean fluorescence intensity (MFI).
Determination of the assay sensitivity.
To determine the sensitivity of the MAS assay for each of the Mut alleles, we diluted the two Mut plasmid DNAs with the WT plasmid DNA. Ten nanograms per microliter of C-mut1 or C-mut2 plasmid DNA was mixed with the WT plasmid DNA, resulting in 2-fold serial dilutions with Mut template at levels of 100%, 50%, 25%, 12.5%, 6.25%, 3.12%, 1.56%, and 0%. One microliter of each serial dilution was used for PCR amplification, and each of the 5-μl amplicons was used to perform a multiplex ASPE assay with all 45 ASPE primers in a single tube reaction, and 10 μl of the mASPE products was analyzed following the procedure described above. The sensitivity of the assay for each allele was determined based on the results from three independent experiments. The lowest concentration at which all three replicates were positive was defined as the sensitivity of the assay for a given allele.
Application of the MAS assay with clinical specimens.
Plasma specimens collected from 148 individuals infected with HIV-1 subtype C from Zambia and Malawi (37, 38) with known viral loads (VLs) ranging from 2.43 to 6.50 log10 copies/ml were analyzed. They were collected from 71 ART-experienced patients and 77 ART-naive patients who were eligible for ART at the time of specimen collection. All the specimens were collected under institutional review board-approved protocols. The tests conducted for the MAS assay using deidentified specimens were determined to be non-human subject research by the associate director for Science at the Center for Global Health at CDC. The nested PCR products obtained previously by a broadly sensitive genotyping assay (13, 14) were analyzed using the MAS assay.
Verification of low-abundance alleles detected by the MAS assay.
To verify the additional low-abundance alleles detected by the MAS assay, 16 of the 24 specimens with low-abundance alleles were amplified using primers tagged with multiplex identifiers (39) and then were pooled and pyrosequenced using the Roche 454 GS-FLX Titanium sequencing kit XLR70 (Roche Applied Science, Indianapolis, IN, USA) following the manufacturer's protocol. Six overlapping primer sets (three each forward and reverse) were used for bidirectional coverage of the protease (amino acids 6 to 99) and RT (amino acids 1 to 251) regions (Z. Zhou, K. Tang, G. Zhang, N. Wadonda-Kabondo, L. A. Rowe, J. R. DeVos, N. Wagar, J. Nkengasong, M. Frace, S. Sammons, and C. Yang, unpublished data).
Statistical analysis.
The means, standard deviations (SD), and coefficients of variation (CVs) were calculated for net MFI in the repeat runs of plasmid DNA templates. The agreement between the MAS and in-house sequencing-based assays was assessed by calculating concordance with the 95% confidence interval (CI). The chi-square test was applied for a comparison of the categorical data. Statistical calculations were performed with the SPSS 20.0 (SPSS, Inc., Chicago, IL) and Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA) software packages.
Nucleotide sequence accession numbers.
The nucleotide sequences of the three plasmids C-wt, C-mut1, and C-mut2 were submitted to GenBank under accession no. KF019640 to KF019642.
RESULTS
Development of the MAS assay using specially constructed plasmid DNAs.
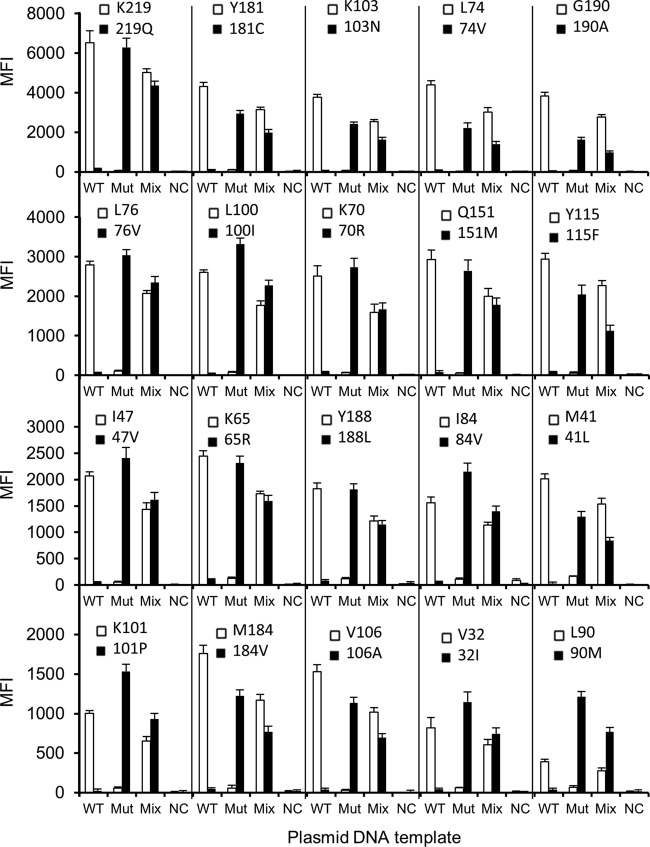
For the initial development of the MAS assay, two plasmids (C-wt and C-mut1) and a mixture of them at a 1:1 ratio (wt/wt) were analyzed. Figure 2 shows the results of the two plasmid templates (C-wt and C-mut1) for all 20 codons from five independent test runs after testing conditions had been optimized. The mean background MFI for each allele ranged from 12.56 to 127.94. The mean ± SD of the highest background value was 127.94 ± 19.41, and the 99% upper confidence limit, which corresponded to the mean plus 2.58 SD, was 178.02. Based on this calculation, the minimal valid allele-calling MFI value was set at 180. If these criteria were met, the allele was determined using the AR. With these criteria, each of the WT and Mut alleles in the plasmid templates was correctly detected. As shown in Fig. 2, the assay had good reproducibility, as evidenced by the low SD between testing runs. The CVs for the positive signals of each WT allele were 2.43% to 15.42%, and the CVs for the positive signals of each Mut allele were 3.41% to 11.68%.
Fig 2.
Detection of drug resistance mutations using plasmid DNA templates. After PCR amplification of each template individually, a mixture of the wild-type template and the mutant template (Mut) at a ratio of 1:1 (wt/wt), amplicons of each individual template, and a no-target negative control (NC) were analyzed with the 45 ASPE primers. The data shown are means plus standard deviations (SD) (error bars) (n = 5).
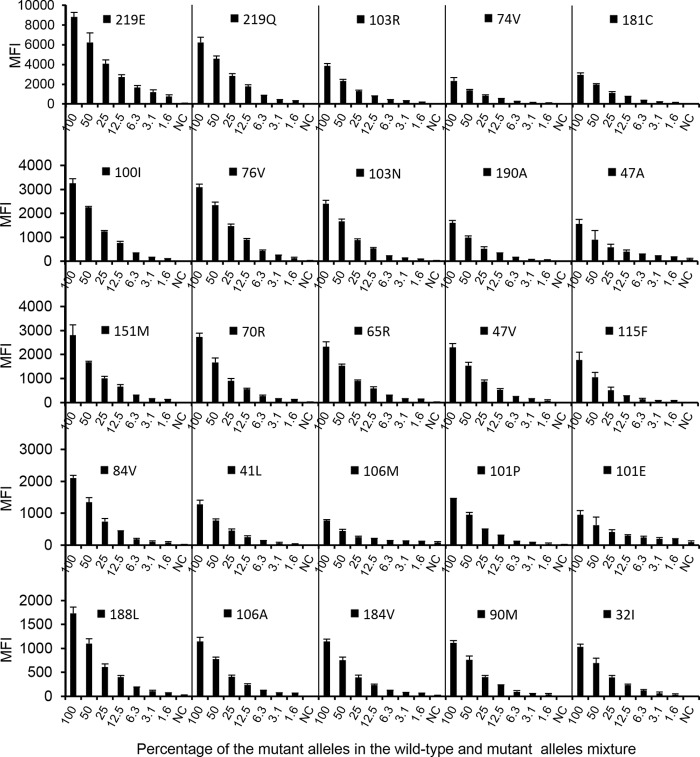
Sensitivity of the MAS assay.
The results from three independent testing runs of serial dilutions of Mut templates (C-mut1 and C-mut2) are shown in Fig. 3. Although the MFI values varied between different Mut alleles, a significant concentration-dependent relationship was observed for each Mut allele. At a cutoff value of 180 and a AR of ≥0.1, the MAS assay detected 1.56% of the minority mutant populations of K219Q and K219E, 3.13% of L76V, 6.25% of K65R, K70R, L74V, L100I, K103N, K103R, Q151M, Y181C, and I47V, and 12.5% of M41L, K101P, K101E, V106A, V106M, Y115F, M184V, Y188L, G190A, V32I, I47A, I84V, and L90M.
Fig 3.
Sensitivity for detection of drug resistance mutations at 20 mutation sites. Twofold serial dilutions of the Mut template against the WT template were tested with the MAS assay. The data shown are the means plus SD (error bars) (n = 3).
Analysis of plasma specimens from ART-experienced and ART-naive patients.
The purified nested PCR products from 148 patient specimens were analyzed using the MAS assay. In all, 2,960 codons (20 codons per specimen, 148 specimens) were analyzed, of which 2,887 (97.53% [95% CI, 96.91 to 98.06]) were genotyped; the remaining 73 codons had indeterminate results due to negative reactions for both the WT and Mut alleles. The mean MFI for the positive signals in patient specimens was 2,823 (95 percentile interval, 525.0 to 6,720.2), and the mean MFI for negative signals in patient specimens was 59.80 (95 percentile interval, 8.48 to 140.0), which are comparable to those obtained from plasmid templates. Analyses of the DRMs at 20 loci showed that eight mutation sites had identical genotyping results, while the remaining 12 sites were 95.21% to 99.32% concordant between the MAS assay and conventional sequencing (Table 2).
Table 2.
Concordance between population sequencing and the MAS assay in DR mutation genotyping of patient specimens (n = 148) at each resistance mutation site
| pol region | Position | No. (%) of alleles detected | No. (%) of specimens with concordant resultsa | No. of discrepancies | No. of additional mixturesb |
|---|---|---|---|---|---|
| rt | 41 | 145 (97.97) | 144 (99.31) | 1 | 0 |
| 65 | 148 (100.0) | 145 (97.97) | 3 | 3 | |
| 70 | 142 (95.95) | 141 (99.30) | 1 | 1 | |
| 74 | 144 (97.30) | 144 (100.0) | 0 | 0 | |
| 115 | 145 (97.97) | 145 (100.0) | 0 | 0 | |
| 151 | 143 (96.62) | 143 (100.0) | 0 | 0 | |
| 184 | 137 (92.57) | 134 (97.81) | 3 | 2 | |
| 219 | 142 (95.95) | 141 (99.30) | 1 | 0 | |
| 100 | 143 (96.62) | 143 (100.0) | 0 | 0 | |
| 101c | 147 (99.32) | 143 (97.28) | 4 | 2 | |
| 103 | 146 (98.65) | 139 (95.21) | 7 | 5 | |
| 106 | 148 (100.0) | 146 (98.65) | 2 | 2 | |
| 181c | 137 (92.57) | 135 (98.54) | 2 | 1 | |
| 188c | 143 (96.62) | 141 (98.60) | 2 | 2 | |
| 190c | 145 (97.97) | 140 (96.55) | 5 | 5 | |
| prt | 32 | 148 (100.0) | 148 (100.0) | 0 | 0 |
| 47 | 148 (100.0) | 148 (100.0) | 0 | 0 | |
| 76 | 140 (94.59) | 140 (100.0) | 0 | 0 | |
| 84 | 148 (100.0) | 148 (100.0) | 0 | 0 | |
| 90 | 148 (100.0) | 147 (99.32) | 1 | 1 | |
| Total | 2,887 (97.53) | 2,855 (98.89) | 32 | 24 |
The concordance rate equals the number of concordant specimens divided by the number of alleles detected.
Number of additional mixtures detected by the MAS assay.
Six untargeted alleles at four loci could not be detected because no ASPE primers were designed for them; these include one K101Q, Y181I, Y188H, G190S, and two Y188C.
Overall, among the 2,887 codons detected by both MAS and sequencing-base assays, 2,855 (98.89% [95% CI, 98.44 to 99.21]) were identical and 32 were discordant between the two assays. The concordance rates between the two methods in ART-experienced and ART-naive patients were 98.54% (1,353/1,373) (95% CI, 97.76% to 99.05%) and 99.21% (1,502/1,514) (95% CI, 98.62% to 99.55%), respectively, which were not significantly different between the two groups of patients (χ2 = 2.90, P = 0.09). In total, there were 32 discordant allele calls, and among them, 24 were identified as mixtures by the MAS assay, while they were identified as nonmixtures by sequencing-based assay (15 WTs and 9 Muts). To the contrary, seven codons identified as mixtures by the sequencing-based assay were interpreted as WTs (n = 5) and Muts (n = 2) by the MAS assay, and the one remaining specimen, MW4644 from Malawi, was a Mut at 181C using the sequencing assay and was a wild-type Y181 using the MAS assay. Sequencing results showed that the MAS assay missed these alleles due to mismatches between the ASPE primers and viral sequences.
Of the 73 indeterminate results from the MAS assay, sequencing results indicated that 58 were WTs, seven were Muts, two were a mixture of WTs and Muts, and the remaining six were untargeted alleles, including one K101Q, Y181I, Y188H, and G190S, and two Y188Cs. These indeterminate results were caused by the failure of the ASPE primers to either hybridize or extend during primer extension. Analysis of the sequences generated by conventional sequencing revealed three reasons for the indeterminate results: (i) an unexpected mutation within close proximity to the polymorphism site (at the 3′ end of ASPE primer), (ii) multiple mutations within the span of the ASPE primers, or (iii) an untargeted Mut allele that was not included in the assay.
Results of 454 ultradeep pyrosequencing.
Sixteen of the 24 low-abundance alleles detected using the MAS assay were further analyzed by Roche 454 ultradeep sequencing. All but one low-abundance allele (K65R in MW5549 collected from Malawi) were verified by ultradeep sequencing. The ultradeep sequencing tagged with multiplex identifiers yielded an average of 2,258 reads per base, with a range of 332 to 5,591 reads per base, and the results showed that the proportions of low-abundance Mut alleles missed by Sanger sequencing were 3.61% to 27.29%. Since several steps of amplification were involved prior to pyrosequencing, the accuracy of these proportions is unverifiable. Nevertheless, the ultradeep sequencing results confirmed the higher sensitivity of the MAS assay than that of conventional sequencing in detecting low-abundance alleles.
DISCUSSION
We describe the development and validation of a multiplex allele-specific HIV-1 subtype C DR detection assay using suspension array technology that can simultaneously detect a set of genetic mutations associated with the resistance of HIV-1 to the commonly used NNRTIs, NRTIs, and PIs. This novel assay can simultaneously detect 45 alleles in a single reaction tube and has good concordance with a well-validated in-house sequencing-based method (13). Specimens from both ART-experienced and ART-naive patients were analyzed successfully by the MAS assay, indicating the potential utility of this assay to detect both acquired and transmitted DRMs.
The results of 2-fold serial dilutions of the Mut templates in a WT background demonstrated that the MAS assay reliably detected Mut alleles at low levels of 1.56% to 12.5%, while direct sequence analysis has less sensitivity for polymorphism detection, approximately 15 to 20% (40). More low-abundance alleles in patient specimens were detected by the MAS assay as verified by 454 ultradeep sequencing, further confirming the increased sensitivity of the MAS assay. This increased sensitivity will reduce the chance of missing low-abundance DRMs, which may have clinical significance for treatment outcomes (17, 41). However, like any allele-specific DR detection assay, the higher sensitivity for detecting low-abundance variants is input copy number dependent; thus, low-abundance variants may still be missed when the patient specimens used for the analysis have low viral load levels.
Traditional sequencing-based DR assays require six to eight sequencing reaction tubes/wells per sample to cover the minimal regions for which sequence data need to be collected in the pol region. This increases the time spent to complete testing for each sample and the analysis time for generating a consensus sequence from the 6 to 8 contigs to a week or more. The use of the 45-plex suspension array assay reduces the number of tubes/wells used for each sample testing to one, and the time to results is substantially reduced. After initial reverse transcription-PCR (RT-PCR) and nested PCR amplification and purification, it takes <8 h to perform the entire procedure, including the mASPE reaction (4.0 h), hybridization to microspheres (1.0 h), incubation with reporter dye (0.5 h), instrument readout (1.0 h), and data analysis (0.5 h). As only one tube/reaction well is required per sample, this allows for the genotyping of 96 or 384 samples (including controls) to occur in one plate, which can be accomplished within one work day. Therefore, this method is especially suitable for large-scale ongoing surveillance of HIVDR.
The high-throughput nature of the MAS assay substantially reduces its cost. To estimate the reagent cost, we included the cost associated with RNA extraction, primer synthesis, RT-PCR and nested PCR amplification, PCR purification, mASPE, hybridization, and suspension array detection. We calculated a cost of $40.90 (U.S. market price) per sample for the MAS assay, which is comparable to the inexpensive in-house sequencing assay ($40.00 per sample) and considerably lower than that for commercially available genotyping assays, such as ViroSeq ($213.20 per sample) and TruGene ($172.86 per sample) (14). Moreover, the instrumentation for the MAS assay is more affordable. The latest compact suspension array system, Magpix (Luminex Corp., Austin, TX), costs only one-tenth that of an ABI 3730 sequencer (Applied Biosystems, Foster City, CA).
Another advantage of the MAS assay over sequencing is that this assay is simpler to perform and the resulting data are easier to interpret. Once an assay run is completed, the genotyping results are immediately available and the data can be reported.
Due to the flexibility of the suspension array technology, which currently permits multiplexed analysis of up to 500 individual analytes per tube or well, additional mutations of biological significance can be easily incorporated into the current assay. As such, the MAS assay can be readily adapted for different surveillance purposes, as well as for clinical use, by adding or removing ASPE primers and the corresponding microspheres to the assay.
As with any DR genotyping assays, the MAS assay has its limitations. First, it can only be used to analyze known DR mutations and it does not provide sequence data. This issue is common to any other point mutation assays, such as the AS-PCR, OLA, and PASS. Second, due to significant genetic variation between different HIV-1 subtypes, ASPE primers designed based on one subtype sequence may not work well for other subtypes. Currently, we are modifying the primers and validating the assay for HIV-1 non-C subtypes. Third, the assay is a qualitative assay and cannot be used for the quantification of DR mutations.
In conclusion, we have developed and validated a multiplex allele-specific subtype C DR detection assay. This assay not only saves time and resources for high-throughput detection of DR mutations but also has the flexibility to add or delete DR mutations based on specific needs. Therefore, the MAS assay may represent an efficient and flexible approach for the surveillance and monitoring of HIVDR in countries where subtype C viruses predominate.
ACKNOWLEDGMENTS
This project was supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Centers for Disease Control and Prevention, grants from the U.S. National Institutes of Health (no. RO1 GM065057), and the appointment of Guoqing Zhang to the International Emerging Infectious Diseases (EID) fellowship program sponsored by the Association of Public Health Laboratories (APHL) and the Centers for Disease Control and Prevention.
We thank all the local staff in Zambia and Malawi who participated in the study, and we are grateful to Kathi L. Kellar and R. Suzanne Beard for critically reading the manuscript.
Chunfu Yang, Feng Gao, Guoqing Zhang, and Fangping Cai are the inventors in U.S. patent application no. 61/655,216. This does not alter the authors' adherence to journal policies on sharing data and materials.
The use of trade names is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, the Public Health Service, or the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 28 August 2013
REFERENCES
- 1.WHO 2012. WHO HIV drug resistance report 2012. World Health Organization, Geneva, Switzerland: http://www.who.int/hiv/pub/drugresistance/report2012/en/ [Google Scholar]
- 2. Wheeler WH, Ziebell RA, Zabina H, Pieniazek D, Prejean J, Bodnar UR, Mahle KC, Heneine W, Johnson JA, Hall HI, Variant, Atypical, and Resistant HIV Surveillance Group 2010. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U.S.-2006. AIDS 24:1203–1212 [DOI] [PubMed] [Google Scholar]
- 3. Stadeli KM, Richman DD. 2013. Rates of emergence of HIV drug resistance in resource-limited settings: a systematic review. Antivir. Ther. 18:115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamers RL, Wallis CL, Kityo C, Siwale M, Mandaliya K, Conradie F, Botes ME, Wellington M, Osibogun A, Sigaloff KC, Nankya I, Schuurman R, Wit FW, Stevens WS, van Vugt M, de Wit TF, PharmAccess African Studies to Evaluate Resistance (PASER) 2011. HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect. Dis. 11:750–759 [DOI] [PubMed] [Google Scholar]
- 5. Price MA, Wallis CL, Lakhi S, Karita E, Kamali A, Anzala O, Sanders EJ, Bekker LG, Twesigye R, Hunter E, Kaleebu P, Kayitenkore K, Allen S, Ruzagira E, Mwangome M, Mutua G, Amornkul PN, Stevens G, Pond SL, Schaefer M, Papathanasopoulos MA, Stevens W, Gilmour J, IAVI Early Infection Cohort Study Group 2011. Transmitted HIV type 1 drug resistance among individuals with recent HIV infection in east and southern Africa. AIDS Res. Hum. Retroviruses 27:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jain V, Liegler T, Vittinghoff E, Hartogensis W, Bacchetti P, Poole L, Loeb L, Pilcher CD, Grant RM, Deeks SG, Hecht FM. 2010. Transmitted drug resistance in persons with acute/early HIV-1 in San Francisco, 2002–2009. PLoS One 5:e15510. 10.1371/journal.pone.0015510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wood E, Montaner JS. 2011. Time to get serious about HIV antiretroviral resistance. Lancet Infect. Dis. 11:723–724 [DOI] [PubMed] [Google Scholar]
- 8. Sigaloff KC, Calis JC, Geelen SP, van Vugt M, de Wit TF. 2011. HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: a systematic review. Lancet Infect. Dis. 11:769–779 [DOI] [PubMed] [Google Scholar]
- 9. Jordan MR, Bennett DE, Wainberg MA, Havlir D, Hammer S, Yang C, Morris L, Peeters M, Wensing AM, Parkin N, Nachega JB, Phillips A, De Luca A, Geng E, Calmy A, Raizes E, Sandstrom P, Archibald CP, Perriëns J, McClure CM, Hong SY, McMahon JH, Dedes N, Sutherland D, Bertagnolio S. 2012. Update on World Health Organization HIV drug resistance prevention and assessment strategy: 2004–2011. Clin. Infect. Dis. 54(Suppl 4):S245–S249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO 2012. World Health Organization global strategy for the surveillance and monitoring of HIV drug resistance 2012. World Health Organization, Geneva, Switzerland: http://www.who.int/hiv/pub/drugresistance/drug_resistance_strategy/en/index.html [Google Scholar]
- 11. Eshleman SH, Crutcher G, Petrauskene O, Kunstman K, Cunningham SP, Trevino C, Davis C, Kennedy J, Fairman J, Foley B, Kop J. 2005. Sensitivity and specificity of the ViroSeq human immunodeficiency virus type 1 (HIV-1) genotyping system for detection of HIV-1 drug resistance mutations by use of an ABI Prism 3100 genetic analyzer. J. Clin. Microbiol. 43:813–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grant RM, Kuritzkes DR, Johnson VA, Mellors JW, Sullivan JL, Swanstrom R, D'Aquila RT, Van Gorder M, Holodniy M, Lloyd RM, Jr, Reid C, Morgan GF, Winslow DL. 2003. Accuracy of the TRUGENE HIV-1 genotyping kit. J. Clin. Microbiol. 41:1586–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang C, McNulty A, Diallo K, Zhang J, Titanji B, Kassim S, Wadonda-Kabondo N, Aberle-Grasse J, Kibuka T, Ndumbe PM, Vedapuri S, Zhou Z, Chilima B, Nkengasong JN. 2010. Development and application of a broadly sensitive dried-blood-spot-based genotyping assay for global surveillance of HIV-1 drug resistance. J. Clin. Microbiol. 48:3158–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou Z, Wagar N, DeVos JR, Rottinghaus E, Diallo K, Nguyen DB, Bassey O, Ugbena R, Wadonda-Kabondo N, McConnell MS, Zulu I, Chilima B, Nkengasong J, Yang C. 2011. Optimization of a low cost and broadly sensitive genotyping assay for HIV-1 drug resistance surveillance and monitoring in resource-limited settings. PLoS One 6:e28184. 10.1371/journal.pone.0028184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buckton AJ, Bissett SL, Myers RE, Beddows S, Edwards S, Cane PA, Pillay D. 2008. Development and optimization of an internally controlled dried blood spot assay for surveillance of human immunodeficiency virus type-1 drug resistance. J. Antimicrob. Chemother. 62:1191–1198 [DOI] [PubMed] [Google Scholar]
- 16. Dudley DM, Chin EN, Bimber BN, Sanabani SS, Tarosso LF, Costa PR, Sauer MM, Kallas EG, O'Connor DH. 2012. Low-cost ultra-wide genotyping using Roche/454 pyrosequencing for surveillance of HIV drug resistance. PLoS One 7:e36494. 10.1371/journal.pone.0036494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simen BB, Simons JF, Hullsiek KH, Novak RM, Macarthur RD, Baxter JD, Huang C, Lubeski C, Turenchalk GS, Braverman MS, Desany B, Rothberg JM, Egholm M, Kozal MJ, Terry Beirn Community Programs for Clinical Research on AIDS 2009. Low-abundance drug-resistant viral variants in chronically HIV-infected, antiretroviral treatment-naive patients significantly impact treatment outcomes. J. Infect. Dis. 199:693–701 [DOI] [PubMed] [Google Scholar]
- 18. Wang C, Mitsuya Y, Gharizadeh B, Ronaghi M, Shafer RW. 2007. Characterization of mutation spectra with ultradeep pyrosequencing: application to HIV-1 drug resistance. Genome Res. 17:1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai F, Chen H, Hicks CB, Bartlett JA, Zhu J, Gao F. 2007. Detection of minor drug-resistant populations by parallel allele-specific sequencing. Nat. Methods 4:123–125 [DOI] [PubMed] [Google Scholar]
- 20. Liu J, Miller MD, Danovich RM, Vandergrift N, Cai F, Hicks CB, Hazuda DJ, Gao F. 2011. Analysis of low-frequency mutations associated with drug resistance to raltegravir before antiretroviral treatment. Antimicrob. Agents Chemother. 55:1114–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Church JD, Towler WI, Hoover DR, Hudelson SE, Kumwenda N, Taha TE, Eshleman JR, Eshleman SH. 2008. Comparison of LigAmp and an ASPCR assay for detection and quantification of K103N-containing HIV variants. AIDS Res. Hum. Retroviruses 24:595–605 [DOI] [PubMed] [Google Scholar]
- 22. Johnson JA, Li JF, Wei X, Lipscomb J, Bennett D, Brant A, Cong ME, Spira T, Shafer RW, Heneine W. 2007. Simple PCR assays improve the sensitivity of HIV-1 subtype B drug resistance testing and allow linking of resistance mutations. PLoS One 2:e638. 10.1371/journal.pone.0000638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson JA, Li JF, Wei X, Lipscomb J, Irlbeck D, Craig C, Smith A, Bennett DE, Monsour M, Sandstrom P, Lanier ER, Heneine W. 2008. Minority HIV-1 drug resistance mutations are present in antiretroviral treatment-naive populations and associate with reduced treatment efficacy. PLoS Med. 5:e158. 10.1371/journal.pmed.0050158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Metzner KJ, Rauch P, Walter H, Boesecke C, Zöllner B, Jessen H, Schewe K, Fenske S, Gellermann H, Stellbrink HJ. 2005. Detection of minor populations of drug-resistant HIV-1 in acute seroconverters. AIDS 19:1819–1825 [DOI] [PubMed] [Google Scholar]
- 25. Metzner KJ, Giulieri SG, Knoepfel SA, Rauch P, Burgisser P, Yerly S, Gunthard HF, Cavassini M. 2009. Minority quasispecies of drug-resistant HIV-1 that lead to early therapy failure in treatment-naive and -adherent patients. Clin. Infect. Dis. 48:239–247 [DOI] [PubMed] [Google Scholar]
- 26. Bansal V, Metzner KJ, Niederöst B, Leemann C, Böni J, Günthard HF, Fehr JS. 2011. Minority K65R variants and early failure of antiretroviral therapy in HIV-1-infected Eritrean immigrant. Emerg. Infect. Dis. 17:1966–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flys T, Nissley DV, Claasen CW, Jones D, Shi C, Guay LA, Musoke P, Mmiro F, Strathern JN, Jackson JB, Eshleman JR, Eshleman SH. 2005. Sensitive drug-resistance assays reveal long-term persistence of HIV-1 variants with the K103N nevirapine (NVP) resistance mutation in some women and infants after the administration of single-dose NVP: HIVNET 012. J. Infect. Dis. 192:24–29 [DOI] [PubMed] [Google Scholar]
- 28. Boltz VF, Maldarelli F, Martinson N, Morris L, McIntyre JA, Gray G, Hopley MJ, Kimura T, Mayers DL, Robinson P, Mellors JW, Coffin JM, Palmer SE. 2010. Optimization of allele-specific PCR using patient-specific HIV consensus sequences for primer design. J. Virol. Methods 164:122–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boltz VF, Ambrose Z, Kearney MF, Shao W, KewalRamani VN, Maldarelli F, Mellors JW, Coffin JM. 2012. Ultrasensitive allele-specific PCR reveals rare preexisting drug resistant variants and a large replicating virus population in macaques infected with a simian immunodeficiency virus containing human immunodeficiency virus reverse transcriptase. J. Virol. 86:12525–12530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi C, Eshleman SH, Jones D, Fukushima N, Hua L, Parker AR, Yeo CJ, Hruban RH, Goggins MG, Eshleman JR. 2004. LigAmp for sensitive detection of single-nucleotide differences. Nat. Methods 1:141–147 [DOI] [PubMed] [Google Scholar]
- 31. Dunbar SA. 2006. Applications of Luminex xMAP technology for rapid, high-throughput multiplexed nucleic acid detection. Clin. Chim. Acta 363:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Houser B. 2012. Bio-Rad's Bio-Plex suspension array system, xMAP technology overview. Arch. Physiol. Biochem. 118:192–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dowling WE, Kim B, Mason CJ, Wasunna KM, Alam U, Elson L, Birx DL, Robb ML, McCutchan FE, Carr JK. 2002. Forty-one near full-length HIV-1 sequences from Kenya reveal an epidemic of subtype A and A-containing recombinants. AIDS 16:1809–1820 [DOI] [PubMed] [Google Scholar]
- 34. Ratner L, Haseltine W, Patarca R, Livak KJ, Starcich B, Josephs SF, Doran ER, Rafalski JA, Whitehorn EA, Baumeister K, Ivanoff L, Petteway SR, Pearson ML, Lautenberger JA, Papas TS, Ghrayeb J, Chang NT, Gallo RC, Wong-Staal F. 1985. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature 313:277–284 [DOI] [PubMed] [Google Scholar]
- 35. Bortolin S, Black M, Modi H, Boszko I, Kobler D, Fieldhouse D, Lopes E, Lacroix JM, Grimwood R, Wells P, Janeczko R, Zastawny R. 2004. Analytical validation of the tag-it high-throughput microsphere-based universal array genotyping platform: application to the multiplex detection of a panel of thrombophilia-associated single-nucleotide polymorphisms. Clin. Chem. 50:2028–2036 [DOI] [PubMed] [Google Scholar]
- 36. Li G, Luo X, He J, Zhu Z, Yu G, Qin H, Zeng T, Liu Z, Wu S, Xu J, Ren-Heidenreich L. 2011. A novel liquidchip platform for simultaneous detection of 70 alleles of DNA somatic mutations on EGFR, KRAS, BRAF and PIK3CA from formalin-fixed and paraffin-embedded slides containing tumor tissue. Clin. Chem. Lab. Med. 49:191–195 [DOI] [PubMed] [Google Scholar]
- 37. Wadonda-Kabondo N, Bennett D, van Oosterhout JJ, Moyo K, Hosseinipour M, DeVos J, Zhou Z, Aberle-Grasse J, Warne TR, Mtika C, Chilima B, Banda R, Pasulani O, Porter C, Phiri S, Jahn A, Kamwendo D, Jordan MR, Kabuluzi S, Chimbwandira F, Kagoli M, Matatiyo B, Demby A, Yang C. 2012. Prevalence of HIV drug resistance before and 1 year after treatment initiation in 4 sites in the Malawi antiretroviral treatment program. Clin. Infect. Dis. 54:S362–S368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stringer JS, McConnell MS, Kiarie J, Bolu O, Anekthananon T, Jariyasethpong T, Potter D, Mutsotso W, Borkowf CB, Mbori-Ngacha D, Muiruri P, Ong'ech JO, Zulu I, Njobvu L, Jetsawang B, Pathak S, Bulterys M, Shaffer N, Weidle PJ. 2010. Effectiveness of non-nucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in women previously exposed to a single intrapartum dose of nevirapine: a multi-country, prospective cohort study. PLoS Med. 7:e1000233. 10.1371/journal.pmed.1000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoffmann C, Minkah N, Leipzig J, Wang G, Arens MQ, Tebas P, Bushman FD. 2007. DNA bar coding and pyrosequencing to identify rare HIV drug resistance mutations. Nucleic Acids Res. 35:e91. 10.1093/nar/gkm435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tsiatis AC, Norris-Kirby A, Rich RG, Hafez MJ, Gocke CD, Eshleman JR, Murphy KM. 2010. Comparison of Sanger sequencing, pyrosequencing, and melting curve analysis for the detection of KRAS mutations: diagnostic and clinical implications. J. Mol. Diagn. 12:425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nicot F, Saliou A, Raymond S, Sauné K, Dubois M, Massip P, Marchou B, Delobel P, Izopet J. 2012. Minority variants associated with resistance to HIV-1 nonnucleoside reverse transcriptase inhibitors during primary infection. J. Clin. Virol. 55:107–113 [DOI] [PubMed] [Google Scholar]