Abstract
Clinical practice guidelines recommend performing follow-up cultures for patients with candidemia in order to determine the time when Candida is cleared from the bloodstream. Since this requires culturing blood samples from patients undergoing antifungal treatment, we evaluated two blood culture bottles (the Bactec Mycosis IC/F [MICF], specifically adapted to the growth of fungi, and the Bactec Plus Aerobic/F [PAF], containing resins to inactivate anti-infective agents) for their effectiveness in detecting Candida albicans and Candida glabrata when seeded in concentrations of 1 CFU/ml and 10 CFU/ml, respectively, together with human whole blood and various antifungal agents in therapeutic peak serum concentrations (Cmax). Significant differences between the MICF and PAF vials for the detection of Candida spp. were found when inoculated with caspofungin (0/12 versus 8/12) (P < 0.001) or amphotericin B (3/12 versus 12/12) (P < 0.001). Inoculation of fluconazole or voriconazole did not influence the effectiveness of detection in the MICF and PAF bottles (P = 1.0). Neither the MICF nor the PAF bottles detected Candida spp. reliably when seeded together with anidulafungin (1/12 versus 1/12) (P = 1.0) or micafungin (0/12 versus 1/12) (P = 1.0). The times to positivity of both bottles were significantly prolonged when antifungal agents were added compared to those of controls without antimycotic drugs (P < 0.001). Overall, the results of this in vitro study indicate that the PAF bottles detected Candida spp. more reliably than the MICF bottles when supplemented with certain antifungal agents. Consequently, clinical studies should evaluate whether this holds true when blood cultures from patients undergoing antifungal treatment are performed.
INTRODUCTION
A retrospective assessment among 14,414 patients in 76 countries demonstrated that Candida bloodstream infections affect 6.9 of 1,000 patients in intensive care units (ICUs) and that candidemia is associated with a high mortality rate (42%) and prolonged ICU lengths of stay (1). While Candida albicans is still the predominant species in invasive infections, the prevalence and clinical impact of non-C. albicans species, which are associated with increasing resistance to antifungal agents (2), are increasing (3).
Hence, the management of patients with candidemia and the use of appropriate microbiological diagnostic tests are of utmost importance. Therefore, some studies have evaluated the commercial Bactec system for its ability to detect Candida bloodstream infections. With this system, Bactec Mycosis IC/F (MICF) blood culture bottles, which are specifically adapted to the growth of Candida spp., were demonstrated to have a significantly shorter time to positivity (TTP) and a higher Candida detection rate than conventional (an)aerobic media (such as the Bactec Plus Aerobic F [PAF] and Bactec anaerobic bottles) (4–8).
The Infectious Diseases Society of America (IDSA) and a panel of European microbiologists and infectious disease specialists (within the European Society of Clinical Microbiology and Infectious Diseases [ESCMID]) recommend that the duration of antifungal therapy for patients with candidemia be prolonged for 2 weeks after the clearance of Candida from the bloodstream (and resolution of symptoms attributable to candidemia) (9, 10). This recommendation implies that after confirmation of candidemia and the start of antimycotic therapy, daily follow-up blood cultures should be performed in order to determine the time of bloodstream clearance. However, neither the IDSA nor the ESCMID specifies the diagnostic medium which should be used in these cases, and up to now the diagnostic applicability of the Candida bloodstream infection detection systems with respect to their ability to detect Candida during the course of antifungal therapy has not been systematically evaluated.
The aim of this study was to analyze which Candida-spiked blood culture bottle detects candidemia with the highest sensitivity and the shortest TTP and also in the presence of defined amounts of antifungal agents. For comparing the Bactec MICF and PAF bottles, in vitro experiments were performed using (i) defined quantities of Candida albicans and the emerging species Candida glabrata, (ii) antifungal agents added in concentrations corresponding to their respective therapeutic peak serum concentrations, and (iii) volumes of human blood as recommended by the manufacturer.
Overall, the results of this study can be used to guide clinical diagnostic tests for patients with proven candidemia and to guide specific recommendations by the IDSA and ESCMID regarding this issue.
MATERIALS AND METHODS
Preparation of Candida suspensions.
Candida albicans (ATCC 90028) and Candida glabrata (ATCC 90030) were cultured on Kimmig agar plates (bioMérieux, Marcy l'Etoile, France) for 48 h at 37°C. From these cultures, colonies were suspended in 0.85% sodium chloride (3 ml in vitro diagnostic medium; bioMérieux), vortexed, and diluted to a McFarland standard of 0.5 density. From this suspension, decimal dilution series were prepared with concentrations of 1 CFU/ml and 10 CFU/ml. The final inoculum size of each suspension was checked by plating and incubating the suspension on a Kimmig agar plate for 48 h at 37°C.
Susceptibility testing.
The MICs of both ATCC strains for all tested antifungal agents were determined using the gradient diffusion method (Etest; bioMérieux, Marcy l'Etoile, France) according to the manufacturer's instructions. Briefly, several colonies from an overnight agar plate were emulsified in 0.85% saline solution and adjusted to a 0.5 McFarland standard. A plate rotator was used to distribute the inoculum evenly over an RPMI 1640 agar plate (Sigma-Aldrich, St. Louis, MO, USA). The Etest strips were positioned on agar plates and dried for approximately 15 min, and the inoculated plates were incubated at 37°C. The results were read according to the manufacturer's instructions. This procedure was performed three times for all antifungal agents and each ATCC strain. Each final MIC reported was defined as the median of the three MIC values read at 24 h.
Blood culture systems used.
The blood culture bottles used in this study were Bactec Mycosis IC/F (MICF) bottles (medium comprising 1.0% brain heart infusion broth, 0.5% soybean-casein digest broth, 0.035% yeast extract, 0.6% sucrose, 0.1% dextrose, 0.05% m-inositol, 0.0001% ferric ammonium citrate, 0.05% sodium polyanethol sulfonate [SPS], 0.024% saponin, 0.0037% chloramphenicol, 0.001% tobramycin, 0.01% antifoaming agent; recommended for inoculation of 8 to 10 ml blood) and Bactec Plus Aerobic/F (PAF) bottles (medium comprising 3.0% soybean-casein digest broth, 0.25% yeast extract, animal tissue digest, 0.05% amino acids, sugar, sodium citrate, 0.005% SPS, 0.025% vitamins, 0.005% antioxidants/reductants, 16.0% nonionic adsorbing resin, 1.0% cationic exchange resin; recommended for inoculation of 8 to 10 ml blood).
Preparation of blood samples.
For inoculation of the blood culture bottles according to the manufacturer's recommendation, venous whole-blood samples obtained for quality control purposes from healthy human volunteer donors not receiving systemic medication were retrieved. Donors provided written informed consent. Samples were retrieved in commercial sterile bags (Leukotrap WB T&B; Pall Medical, Port Washington, NY) and inoculated into the blood culture bottles within 4 h after retrieval from the donor.
Preparation of antifungal agents.
For inoculation into the blood culture bottles, we reconstituted the antifungal agents listed below according to the recommendations of the manufacturers. A stock solution containing the maximum plasma concentration (Cmax) for adults (when using a standard dosing regimen) was prepared for amphotericin B deoxycholate (Fungizone; Bristol-Myers Squibb) (Cmax, 2.9 μg/ml) (11), anidulafungin (Ecalta; Pfizer) (Cmax, 8.6 μg/ml) (12), caspofungin (Cancidas; Merck Sharp & Dohme) (Cmax, 12.1 μg/ml) (13), micafungin (Mycamine; Astellas) (Cmax, 10.1 μg/ml) (12), fluconazole (Diflucan IV; Pfizer) (Cmax, 4.68 μg/ml) (14), and voriconazole (Vfend; Pfizer) (Cmax, 5.402 μg/ml) (15).
Inoculation of the blood culture bottles in the first test series.
Each test series set of blood culture bottles consisted of 24 MICF and 24 PAF bottles, which were inoculated using sterile precautions with a Candida suspension (100 μl C. albicans suspension or 200 μl C. glabrata suspension, each at final concentrations of 1 CFU/ml and 10 CFU/ml in the bottle), 10 ml blood, and 100 μl of each of the six antifungal agents (at the concentrations mentioned above). In addition, for each CFU concentration, one culture vial with MICF medium and one with PAF medium were inoculated with blood and a Candida suspension without antifungal agents as controls. This inoculation protocol was repeated three times, yielding a total of 168 culture sets in this test series.
Incubation and data analysis.
All blood culture bottles were incubated at 37°C in a BD Bactec 9240 system for 14 days. After 14 days, 100-μl samples from all negative bottles were streaked onto Kimmig agar plates and incubated for 48 h at 37°C. For all bottles detected as positive within 14 days, the TTPs were recorded. Statistical analyses were performed using the chi-square test, Fisher's exact test, or the Kruskal-Wallis test (SPSS statistical software, version 21).
Additional test series for echinocandins.
After finishing the first test series as described above, which demonstrated significant differences between the MICF and PAF bottles, we repeated the same tests for caspofungin, anidulafungin, and micafungin using identical procedures for the preparation of the Candida suspensions and the human blood samples and inoculation of the bottles. The protocol for preparing the antifungal agents was changed by using stock solutions containing 50%, 25%, 12.5%, and 6.25% of the Cmax for each antifungal agent when inoculating the blood culture bottles. All remaining procedures for inoculation and incubation were the same as those described above. For each of the four concentrations of the antifungal agents and each of the three echinocandins, the experiments were again performed three times for C. albicans and C. glabrata with inoculum suspensions of both 1 CFU/ml and 10 CFU/ml. This resulted in a second test series of 288 culture bottles.
RESULTS
A total of 168 seeded blood culture bottles were incubated in the first test series. For all controls containing Candida spp. without antifungal agents, growth was detected by the blood culture system. The median TTPs for the controls in MICF and PAF bottles were 21.1 h and 24.4 h (P = 0.015) for C. albicans and 22.0 h and 43.2 h (P = 0.002) for C. glabrata, respectively. For the controls without antifungal agents, the median TTPs for different inoculum sizes (1 CFU/ml and 10 CFU/ml) of C. albicans were 24.9 h and 21.4 h (P = 0.07) and for C. glabrata were 33.5 h and 31.3 h (P = 0.24), respectively.
Testing the susceptibility of the reference strains used for inoculation yielded median MICs for C. albicans of 0.19 mg/liter for amphotericin B, 1.0 mg/liter for fluconazole, 0.023 mg/liter for voriconazole, 0.19 mg/liter for caspofungin, 0.004 mg/liter for anidulafungin, and 0.016 mg/liter for micafungin. For C. glabrata, the MICs were 0.125 mg/liter for amphotericin B, 24 mg/liter for fluconazole, 0.5 mg/liter for voriconazole, 0.25 mg/liter for caspofungin, 0.006 mg/liter for anidulafungin, and 0.012 mg/liter for micafungin.
For the following calculations, the controls were excluded from the analysis, and only the culture bottles spiked with antifungal agents were included. Overall, when Candida spp. were inoculated in a concentration of 1 CFU/ml, the BD Bactec 9240 system yielded positive results in 16/36 (44%) cases for both C. albicans and C. glabrata (P = 1.0). When 10 CFU/ml inocula were used, 20/36 (56%) C. albicans and 22/36 (61%) C. glabrata cultures were detected (P = 0.13). Overall, growth was detected more frequently in the PAF bottles (46/72 [64%]) than in the MICF bottles (28/72 [39%]) (P = 0.004). The TTPs did not vary between the MICF and PAF bottles when we compared only the positive culture bottles (P = 0.548). The test results for the various antifungal agents are shown in Table 1. The growth of Candida spp. was more sensitively detected in MICF and PAF bottles in the presence of peak concentrations (Cmax) of fluconazole and voriconazole. When Candida spp. were inoculated with anidulafungin and micafungin in MICF and PAF bottles, the BD Bactec 9240 system mostly did not detect growth (P = 1.0). For amphotericin B (P < 0.001) and caspofungin (P = 0.001), we observed that the level of detection of Candida spp. was significantly less in MICF than in PAF bottles. There were no significant differences regarding the growth of C. albicans versus that of C. glabrata.
Table 1.
Comparison of Bactec Mycosis IC/F and Bactec Plus Aerobic/F blood culture bottles for detection of Candida spp. in the presence of therapeutic peak serum concentrations of different antifungal agents
| Antifungal agent |
Candida spp. detected separately (no. of culture sets with growth detected/no. of culture sets tested [%] and P value) bya: |
Total Candida spp. detected (no. of culture sets with growth detected/no. of culture sets tested [%] and P value) by: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Bactec Mycosis IC/F bottles |
Bactec Plus Aerobic/F bottles |
Bactec Mycosis IC/F bottles | Bactec Plus Aerobic/F bottles | P | |||||
| C. albicans | C. glabrata | P | C. albicans | C. glabrata | P | ||||
| Amphotericin B | 3/6 (50) | 0/6 (0) | 0.18 | 6/6 (100) | 6/6 (100) | 1.0 | 3/12 (25) | 12/12 (100) | <0.001 |
| Fluconazole | 6/6 (100) | 6/6 (100) | 1.0 | 6/6 (100) | 6/6 (100) | 1.0 | 12/12 (100) | 12/12 (100) | 1.0 |
| Voriconazole | 6/6 (100) | 6/6 (100) | 1.0 | 6/6 (100) | 6/6 (100) | 1.0 | 12/12 (100) | 12/12 (100) | 1.0 |
| Anidulafungin | 0/6 (0) | 1/6 (17) | 1.0 | 0/6 (0) | 1/6 (17)a | 1.0 | 1/12 (8) | 1/12 (8)# | 1.0 |
| Caspofungin | 0/6 (0) | 0/6 (0) | 1.0 | 4/6 (67) | 4/6 (67) | 1.0 | 0/12 (0) | 8/12 (67) | 0.001 |
| Micafungin | 0/6 (0) | 0/6 (0) | 1.0 | 1/6 (17) | 0/6 (0) | 1.0 | 0/12 (0) | 1/12 (8) | 1.0 |
In one case, growth was undetected by the automated blood culture system, but C. glabrata grew on solid medium when samples from negative blood culture bottles were plated after 14 days of incubation.
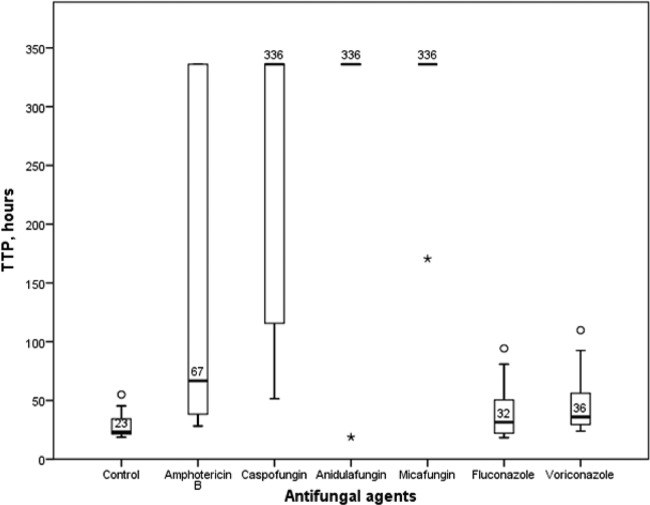
The TTPs of blood culture bottles without antifungal agents (i.e., controls) and those supplemented with antifungals were significantly different (P < 0.001) (Fig. 1). The median TTPs were 336 h (i.e., the maximum incubation time) for all three echinocandins tested, 67 h for amphotericin B, 36 h for voriconazole, 32 h for fluconazole, and 23 h for controls without antifungal agents (Fig. 1).
Fig 1.
Times to positivity (TTPs) in hours for blood culture bottles without antifungal agents (controls) and supplemented with different types of antifungal agents (maximal incubation period, 336 h). Box plots indicate the medians (thick black bars) and 25th and 75th percentiles. Whiskers indicate the smallest and the highest values not classified as outliers. Outliers are indicated by circles and extreme outliers by asterisks.
Compared to detection with inoculation of the Cmax of caspofungin in MICF bottles (as shown in Table 1), C. glabrata was first detected at 0.125× the Cmax (Table 2); in contrast, in PAF bottles, the growth of C. albicans and C. glabrata was more sensitively detected at all concentrations of caspofungin. For micafungin, Candida spp. were detected better in PAF bottles at 0.5× the Cmax, while neither C. albicans nor C. glabrata was detected in the MICF bottles with concentrations higher than 0.125× the Cmax. For anidulafungin, C. glabrata was detected more reliably from 0.5× the Cmax and below in PAF bottles but not before 0.25× the Cmax in MICF bottles. C. albicans was detected in the presence of anidulafungin from ≥0.125× the Cmax in PAF bottles and 0.0625× the Cmax in MICF bottles.
Table 2.
Comparison of Bactec Mycosis IC/F and Bactec Plus Aerobic/F blood culture bottles for detection of Candida spp. in the presence of different concentrations of echinocandins
| Cmax of antifungal agents used |
Candida spp. detected separately (no. of culture sets with growth detected/no. of culture sets tested [%] and P value) bya: |
Total Candida spp. detected (no. of culture sets with growth detected/no. of culture sets tested [%] and P value) by: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Bactec Mycosis IC/F bottles |
Bactec Plus Aerobic/F bottles |
Bactec Mycosis IC/F bottles | Bactec Plus Aerobic/F bottles | P | |||||
| C. albicans | C. glabrata | P | C. albicans | C. glabrata | P | ||||
| 0.5× Cmax | |||||||||
| Anidulafungin | 0/6 (0) | 0/6 (0) | 1.0 | 0/6 (0) | 6/6 (100) | 0.002 | 0/12 (0) | 6/12 (50) | 0.014 |
| Caspofungin | 0/6 (0) | 0/6 (0) | 1.0 | 5/6 (83) | 4/6 (67) | 1.0 | 0/12 (0) | 9/12 (75) | <0.001 |
| Micafungin | 0/6 (0) | 0/6 (0) | 1.0 | 3/6 (50) | 2/6 (33) | 1.0 | 0/12 (0) | 5/12 (42) | 0.037 |
| 0.25× Cmax | |||||||||
| Anidulafungin | 0/6 (0) | 6/6 (100) | 0.002 | 0/6 (0) | 6/6 (100) | 0.002 | 6/12 (50) | 6/12 (50) | 1.0 |
| Caspofungin | 0/6 (0) | 0/6 (0) | 1.0 | 5/6 (83) | 6/6 (100) | 1.0 | 0/12 (0) | 11/12 (92) | <0.001 |
| Micafungin | 0/6 (0) | 0/6 (0) | 1.0 | 6/6 (100) | 5/6 (83) | 1.0 | 0/12 (0) | 11/12 (92) | <0.001 |
| 0.125× Cmax | |||||||||
| Anidulafungin | 0/6 (0) | 4/6 (67) | 0.061 | 5/6 (83) | 4/6 (67) | 1.0 | 4/12 (33) | 9/12 (75) | 0.10 |
| Caspofungin | 0/6 (0) | 0/6 (0) | 1.0 | 6/6 (100) | 5/6 (83) | 1.0 | 0/12 (0) | 11/12 (92) | <0.001 |
| Micafungin | 6/6 (100) | 6/6 (100) | 1.0 | 6/6 (100) | 5/6 (83) | 1.0 | 12/12 (100) | 11/12 (92) | 1.0 |
| 0.0625× Cmax | |||||||||
| Anidulafungin | 3/6 (50) | 6/6 (100) | 0.182 | 6/6 (100) | 6/6 (100) | 1.0 | 9/12 (75) | 12/12 (100) | 0.217 |
| Caspofungin | 0/6 (0) | 2/6 (33) | 0.455 | 5/6 (83) | 6/6 (100) | 1.0 | 2/12 (17) | 11/12 (92) | <0.001 |
| Micafungin | 6/6 (100) | 6/6 (100) | 1.0 | 6/6 (100) | 6/6 (100) | 1.0 | 12/12 (100) | 12/12 (100) | 1.0 |
DISCUSSION
The aim of this study was to investigate to what extent Candida spp. in quantities typically found in the blood of patients with candidemia (16) are detected in two different Bactec blood culture bottles when inoculated together with therapeutic concentrations of various antifungal agents. The study question arose from the IDSA's and ESCMID's recommendations to perform follow-up cultures for patients undergoing antifungal treatment to guide the length of therapy (9, 10). The evidence for this recommendation is based on several prospective clinical studies, where this procedure was associated with therapeutic success (17–19). However, none of these studies described the method of microbiological determination, which may limit the quality of evidence supporting the recommendation.
The rationale for choosing Bactec MICF bottles for these experiments was that in experiments evaluating the general applicability of different commercial blood culture systems for detecting Candida spp., Bactec MICF bottles, which include supplements specifically adapted for the growth of fungi, were shown to be more sensitive and to detect Candida spp. faster than most other conventional blood culture media (4–8). This finding was confirmed by the results of our study, since for controls inoculated without antifungal agents, we found reduced median TTPs in MICF bottles compared to those in PAF bottles (21.1 h versus 24.4 h for C. albicans and 22.0 h versus 43.2 h for C. glabrata, respectively). Bactec PAF bottles were chosen for comparison with MICF bottles, because they contain a standard medium for the detection of bacterial and fungal pathogens not specifically adapted to fungi and include resins, which can bind and inactivate antifungal agents and other drugs. Several investigations demonstrated that Bactec PAF bottles yielded better results for detecting bacteremia in patients undergoing pharmacotherapy, indicating that the resins have an effect on the inactivation of potential inhibitors of microbiological pathogen growth (20, 21).
In this study, we have shown that the presence of fluconazole and voriconazole had no effects on Candida species detection in MICF and PAF bottles, i.e., Candida spp. grew reliably in both bottles. For fluconazole, this finding might be due to the fact that the C. albicans and C. glabrata strains used in this study had high MICs (1.0 mg/liter and 24 mg/liter, respectively) and low Cmax/MIC quotients for this agent compared with those for the other antifungals tested. Another possible explanation for why the two azoles tested did not have major effects on the detection of Candida spp. in the spiked bottles is that compared with echinocandins, which are fungicidal against Candida, azoles are fungistatic (22, 23).
However, for most of the other drugs tested, we found that their presence in therapeutic serum peak concentrations had a significant impact on the detection of Candida spp. For the inoculation of amphotericin B and the three echinocandins, we found that more sensitive detection in MICF than in PAF bottles (as demonstrated for the controls without antifungal agents in this study) did not hold true. Theoretically, one might assume that in these cases, the ability of the resins included in the Bactec PAF medium to bind and inactivate antimycotics led to significantly better detection in the PAF bottles. However, we have shown that when the PAF and MICF bottles were spiked with anidulafungin and micafungin, Candida spp. mostly remained undetected in both bottles. Since echinocandins are considered the therapy of choice for candidemia in the majority of cases (9), for these antifungal agents, we evaluated in more detail how the quantity of the drug inoculated affected the growth of Candida spp. Therefore, we supplemented the MICF and PAF bottles with concentrations of 6.25% to 50% of the Cmax values for these drugs. Overall, we found that the recovery rate was improved (Table 2), but Candida spp. (especially C. glabrata) were mostly not detected in the MICF bottles before the concentrations were reduced to 6.25% to 12.5% of the Cmax. Since the minimum serum concentrations with the standard echinocandin therapy are usually within a range of 1 to 6 mg/liter (13, 24, 25), i.e., about 10 to 50% of the Cmax of the agent, detection is not reliable even when the cultures are collected shortly before serum trough levels of the echinocandins are expected.
A major limitation of this study is that it was performed completely in vitro using Candida ATCC reference strains (rather than clinical Candida isolates) and whole-blood samples from volunteers not taking any systemic medication. This does not reflect the actual situation and underlines the need for performing studies in a clinical setting to confirm our findings.
In summary, in this in vitro study, we have shown significant differences between the abilities of two blood culture bottles (Bactec Aerobic/F and Mycosis IC/F) used in a commercial blood culture detection system to detect putative C. albicans and C. glabrata bloodstream infections when the bottles were spiked with therapeutic concentrations of antifungal agents. These results clearly indicate that it is not sufficient to use MICF bottles when cultures from patients undergoing antifungal therapy are performed in order to determine the time of Candida clearance from the bloodstream. However, clinical studies should be performed to confirm these findings. Moreover, our observations prompt the necessity for performing clinical studies to document more accurately the preferred microbiological candidemia diagnostic tests to increase the evidence for recommendations suggesting that blood cultures be performed while the patient is being treated with antifungals.
ACKNOWLEDGMENTS
We are very grateful to Barbara Grünastel for expert technical assistance.
BD Diagnostics (Heidelberg, Germany) provided the blood culture bottles used in this study.
Footnotes
Published ahead of print 28 August 2013
REFERENCES
- 1. Kett DH, Azoulay E, Echeverria PM, Vincent JL. 2011. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit. Care Med. 39:665–670 [DOI] [PubMed] [Google Scholar]
- 2. Schmalreck AF, Willinger B, Haase G, Blum G, Lass-Florl C, Fegeler W, Becker K. 2012. Species and susceptibility distribution of 1062 clinical yeast isolates to azoles, echinocandins, flucytosine and amphotericin B from a multi-centre study. Mycoses 55:e124–e137. 10.1111/j.1439-0507.2011.02165.x [DOI] [PubMed] [Google Scholar]
- 3. Pfaller MA, Diekema DJ. 2004. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin. Microbiol. Infect. 10:11–23 [DOI] [PubMed] [Google Scholar]
- 4. Chiarini A, Palmeri A, Amato T, Immordino R, Distefano S, Giammanco A. 2008. Detection of bacterial and yeast species with the Bactec 9120 automated system with routine use of aerobic, anaerobic, and fungal media. J. Clin. Microbiol. 46:4029–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fricker-Hidalgo H, Chazot F, Lebeau B, Pelloux H, Ambroise-Thomas P, Grillot R. 1998. Use of simulated blood cultures to compare a specific fungal medium with a standard microorganism medium for yeast detection. Eur. J. Clin. Microbiol. Infect. Dis. 17:113–116 [DOI] [PubMed] [Google Scholar]
- 6. Meyer MH, Letscher-Bru V, Jaulhac B, Waller J, Candolfi E. 2004. Comparison of Mycosis IC/F and plus Aerobic/F media for diagnosis of fungemia by the Bactec 9240 system. J. Clin. Microbiol. 42:773–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ericson EL, Klingspor L, Ullberg M, Ozenci V. 2012. Clinical comparison of the Bactec Mycosis IC/F, BacT/Alert FA, and BacT/Alert FN blood culture vials for the detection of candidemia. Diagn. Microbiol. Infect. Dis. 73:153–156 [DOI] [PubMed] [Google Scholar]
- 8. Klingspor L, Muhammed SA, Ozenci V. 2012. Comparison of the two blood culture systems, Bactec 9240 and BacT/Alert 3D, in the detection of Candida spp. and bacteria with polymicrobial sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 31:2983–2987 [DOI] [PubMed] [Google Scholar]
- 9. Pappas PG, Kauffman CA, Andes D, Benjamin DK, Calandra TF, Edwards JE, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kullberg BJ, Verweij PE, Akova M, Arendrup MC, Bille J, Calandra T, Cuenca-Estrella M, Herbrecht R, Jacobs F, Kalin M, Kibbler CC, Lortholary O, Martino P, Meis JF, Munoz P, Odds FC, De Pauw BE, Rex JH, Roilides E, Rogers TR, Ruhnke M, Ullmann AJ, Uzun O, Vandewoude K, Vincent JL, Donnelly JP. 2011. European expert opinion on the management of invasive candidiasis in adults. Clin. Microbiol. Infect. 17(Suppl 5):1–12 [DOI] [PubMed] [Google Scholar]
- 11. Benson JM, Nahata MC. 1989. Pharmacokinetics of amphotericin B in children. Antimicrob. Agents Chemother. 33:1989–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grayson M. (ed). 2010. Kucers' the use of antibiotics, 6th ed, vol 1 Edward Arnold Publishers Ltd, London, England [Google Scholar]
- 13. Stone JA, Holland SD, Wickersham PJ, Sterrett A, Schwartz M, Bonfiglio C, Hesney M, Winchell GA, Deutsch PJ, Greenberg H, Hunt TL, Waldman SA. 2002. Single- and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob. Agents Chemother. 46:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foulds G, Wajszczuk C, Weidler DJ, Garg DJ, Gibson P. 1988. Steady state parenteral kinetics of fluconazole in man. Ann. N. Y. Acad. Sci. 544:427–430 [DOI] [PubMed] [Google Scholar]
- 15. Purkins L, Wood N, Ghahramani P, Greenhalgh K, Allen MJ, Kleinermans D. 2002. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46:2546–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfeiffer CD, Samsa GP, Schell WA, Reller LB, Perfect JR, Alexander BD. 2011. Quantitation of Candida CFU in initial positive blood cultures. J. Clin. Microbiol. 49:2879–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pappas PG, Rotstein CM, Betts RF, Nucci M, Talwar D, De Waele JJ, Vazquez JA, Dupont BF, Horn DL, Ostrosky-Zeichner L, Reboli AC, Suh B, Digumarti R, Wu C, Kovanda LL, Arnold LJ, Buell DN. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45:883–893 [DOI] [PubMed] [Google Scholar]
- 18. Rex JH, Bennett JE, Sugar AM, Pappas PG, van der Horst CM, Edwards JE, Washburn RG, Scheld WM, Karchmer AW, Dine AP, Candidemia Study Group and the National Institute 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 331:1325–1330 [DOI] [PubMed] [Google Scholar]
- 19. Rex JH, Pappas PG, Karchmer AW, Sobel J, Edwards JE, Hadley S, Brass C, Vazquez JA, Chapman SW, Horowitz HW, Zervos M, McKinsey D, Lee J, Babinchak T, Bradsher RW, Cleary JD, Cohen DM, Danziger L, Goldman M, Goodman J, Hilton E, Hyslop NE, Kett DH, Lutz J, Rubin RH, Scheld WM, Schuster M, Simmons B, Stein DK, Washburn RG, Mautner L, Chu TC, Panzer H, Rosenstein RB, Booth J. 2003. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin. Infect. Dis. 36:1221–1228 [DOI] [PubMed] [Google Scholar]
- 20. Spaargaren J, van Boven CP, Voorn GP. 1998. Effectiveness of resins in neutralizing antibiotic activities in Bactec plus Aerobic/F culture medium. J. Clin. Microbiol. 36:3731–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lelièvre H, Gimenez M, Vandenesch F, Reinhardt A, Lenhardt D, Just HM, Pau M, Ausina V, Etienne J. 1997. Multicenter clinical comparison of resin-containing bottles with standard aerobic and anaerobic bottles for culture of microorganisms from blood. Eur. J. Clin. Microbiol. Infect. Dis. 16:669–674 [DOI] [PubMed] [Google Scholar]
- 22. Chen SC, Slavin MA, Sorrell TC. 2011. Echinocandin antifungal drugs in fungal infections: a comparison. Drugs 71:11–41 [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Nguyen MH, Cheng S, Schmidt S, Zhong L, Derendorf H, Clancy CJ. 2008. A pharmacokinetic/pharmacodynamic mathematical model accurately describes the activity of voriconazole against Candida spp. in vitro. Int. J. Antimicrob. Agents 31:369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu P, Ruhnke M, Meersseman W, Paiva JA, Kantecki M, Damle B. 2013. Pharmacokinetics of anidulafungin in critically ill patients with candidemia/invasive candidiasis. Antimicrob. Agents Chemother. 57:1672–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oshima K, Kanda Y, Kako S, Ohno K, Kishino S, Kurokawa M. 2013. Pharmacokinetics of micafungin in patients undergoing allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 15:323–327 [DOI] [PubMed] [Google Scholar]