Abstract
Accurate noninvasive tests for diagnosing Helicobacter pylori infection in very young children are strongly required. We investigated the agreement between the [13C]urea breath test ([13C]UBT) and a monoclonal ELISA (HpSA) for detection of H. pylori antigen in stool. From October 2007 to July 2011, we enrolled 414 infants (123 from Brazil and 291 from Peru) of ages 6 to 30 months. Breath and stool samples were obtained at intervals of at least 3 months from Brazilian (n = 415) and Peruvian (n = 908) infants. [13C]UBT and stool test results concurred with each other in 1,255 (94.86%) cases (kappa coefficient = 0.90; 95% confidence interval [CI] = 0.87 to 0.92). In the H. pylori-positive group, delta-over-baseline (DOB) and optical density (OD) values were positively correlated (r = 0.62; P < 0.001). The positivity of the tests was higher (P < 0.001; odds ratio [OR] = 6.01; 95% CI = 4.50 to 8.04) in Peru (546/878; 62.2%) than in Brazil (81/377; 21.5%) and increased with increasing age in Brazil (P = 0.02), whereas in Peru it decreased with increasing age (P < 0.001). The disagreement between the test results was associated with birth in Brazil and female gender but not with age and diarrhea. Our results suggest that both [13C]UBT and the stool monoclonal test are reliable for diagnosing H. pylori infection in very young children, which will facilitate robust epidemiological studies in infants and toddlers.
INTRODUCTION
Helicobacter pylori infection is acquired primarily in early childhood and is predominantly transmitted within families, infected mother and siblings being the most common familial source of the microorganism (1–5). Infants and toddlers most frequently acquire and lose the infection (3, 6), but there are substantial knowledge gaps in respect to the predictors of initial acquisition, as well as the persistence of the infection, in this age group. In addition, in children, H. pylori infection has been associated with iron deficiency anemia, diarrheic disease, and impairment of growth, weight, and cognitive functions (7, 8). Thus, a simple and reliable noninvasive test to detect H. pylori infection in this age group is required, especially in developing countries, where the prevalence of H. pylori infection is very high.
The noninvasive [13C]urea breath test ([13C]UBT) (9–12) and stool antigen test (13–15) are very reliable for the diagnosis of H. pylori infection in children older than 6 years. The stool test based on monoclonal antibodies has proved to be highly accurate in all age groups (13–15), but the specificity of the [13C]UBT varies from 82% to 100% for young children (9–12). However, studies of this subject are scarce and have not included large enough numbers of infants and toddlers to obtain reliable results. Furthermore, different “gold standard” tests have been used to validate the [13C]UBT, and most studies are in developed countries, where the prevalence of infection is very low (11, 12, 16, 17).
To validate noninvasive tests for diagnosis of H. pylori infection, the indicated “gold standard” includes at least two invasive tests, which is a difficult task with young children due to the current rarity of symptomatic H. pylori infection in this period of life. In addition, as stated by Goodman and Correa, validation using invasive tests has some limitations; recent short-term colonization and patchy distribution of the bacterium in the gastric mucosa may decrease the sensitive of biopsy-based tests in very young children (18). Such problems currently restrict epidemiological investigations of the acquisition of H. pylori infection in infants and toddlers in developing-country settings.
The aim of this study was to investigate whether the two independent noninvasive tests for H. pylori infection diagnosis, the [13C]UBT and monoclonal stool antigen test, have good concordance in young children. For that, we evaluated a cohort of infants and toddlers living in impoverished regions of two developing countries in South America. Our hypothesis was that if the [13C]UBT and monoclonal stool antigen test had good agreement in infants and toddlers, either of the two noninvasive tests could be used for the diagnosis of H. pylori in this age group. We also aimed to investigate causes linked to discordant results between the two tests.
MATERIALS AND METHODS
Children and methods.
The study was approved by the Ethics Committees of the participant institutions. The study was also reviewed by the EU (European Union) Ethics Committee. Informed written consent was obtained from the parents or guardians of the included children.
From October 2007 to July 2011, 123 children living in Parque Universitário, a poor urban community neighborhood of Fortaleza, Ceará, in the northeastern region of Brazil, and 291 children from Las Pampas de San Juan and Nuevo Paraiso, an established shanty town on the southern edge of the Lima, Peru, were included in a longitudinal birth cohort study that aimed to evaluate the influence of H. pylori infection on the risk of acute diarrhea, malnourishment, iron deficiency, and iron deficiency anemia in young children. Breath and stool samples were obtained at intervals of at least 3 months from infants and children of ages 6 to 36 months.
The [13C]UBT was performed after at least 4 h of fasting by the subjects. The Brazilian children drank 100 ml of orange juice and Peruvian children 100 ml of water containing 50 mg of [13C]urea (≥99.00% chemical purity; Eurisotop, Paris, France) through a straw. Breath samples, collected by trained staff, using a face mask with unidirectional valve into a breath bag, were sent to the Laboratory of Research in Bacteriology (LPB), Belo Horizonte, Brazil, where they were analyzed within 1 month after sample collection with a nondispersive infrared spectrometer (Wagner Analysen Tecnik, Bremen, Germany).
An increase in the ratio of carbon-13 dioxide to carbon-12 dioxide between the baseline sample and the 30-min sample (delta-over-baseline [DOB]) values of ≥4‰ is considered indicative of active infection and <4‰ negative, according to the manufacturer's recommendations. We also analyzed the data considering cutoff DOB values of 3.5‰, 5‰, and 6‰.
For the stool antigen assay, stool samples, collected by parents or caretakers, were maintained at 4°C if there was a refrigerator in the household or at room temperature for up to 8 h before being sent at 4°C to local laboratories, where they were maintained at −80°C. The stool samples were transported in dry ice to LPB, Belo Horizonte, Brazil, where they were tested by using a commercial ELISA Premier Platinum HpSA Plus assay (Meridian Bioscience Europe from Launch Diagnostics Ltd., Longfield, United Kingdom) that uses multiple murine monoclonal anti-H. pylori capture antibodies adsorbed to microwells, according to the manufacturer's recommendations. A 10-μl disposable loop was used to dilute stool samples in 500 μl of sample diluent. In the case of liquid of semisolid stools, 100 μl of stools were added to the sample diluent. Optical densities (OD) of ≥0.140 at 450 nm were considered positive, and an OD of <0.140 was considered negative, according to the manufacturer's recommendations. The samples were randomly selected among those in which the time interval between stool and breath sample collection was equal to or less than 30 days.
To evaluate the effect on the stool antigen test results of maintaining the stool samples at increased temperature, positive samples classified as having high (1.449 and 1.620) and low (0.149 and 0.183) ODs were retested in parallel after being maintained at 25°C and 37°C for 6 h, 12 h, 24 h, and 48 h.
Statistical analysis.
The kappa coefficient with 95% confidence interval (CI) (19) was used to assess agreement between the two assay methods. In order to investigate variables associated with discordant results between the two tests, the association of independent variables (the age of the children in months, gender, country of birth, unformed/watery stools, and time interval in days between breath and stool collections) and a dependent variable (disagreement between the tests, ascribed as absent or present) was tested in univariate analysis. All variables with P values of ≤0.25 were included in the full model of logistic regression. Odds ratio (OR) and 95% CI were used as an estimate of the risk. The Hosmer-Lemeshow goodness-of-fit test was used to evaluate the fit of the model.
Because [13C]UBT DOB and stool test OD values do not have a Gaussian distribution, data were compared by using the two-tailed χ2 test, Fisher's test, Student's t test, and one-way analysis of variance (ANOVA) after natural log transformation. Correlations were analyzed by using Pearson's correlation coefficient. The level of significance was set at a P value of <0.05. Analyses were performed using the Statistical Package for the Social Sciences version 17 (SPSS Inc., Chicago, IL).
RESULTS
A total of 1,323 breath and stool samples were tested. For 28 (2.1%) children, only one sample was tested. The mean of samples tested per child was 3.9. No difference in the gender balance (P = 0.41; OR = 1.1; 95% CI = 0.87 to 1.40) was observed between Peru (453/908; 49.9% girls) and Brazil (197/415; 47.5% girls), but, the mean age (± standard deviation [SD]) in months of the Peruvian children (17.84 ± 6.14) was lower (P = 0.001) than that of the Brazilian children (19.86 ± 12.16).
Concordance between [13C]UBT and stool antigen test.
Because the best concordance, even in each country separately, was obtained by adopting the cutoff point of 4‰, all analyses were done with this cutoff value (Table 1). The [13C]UBT and stool test results concurred with each other for 1,255 (94.9%) breath/stool samples with a kappa coefficient of 0.90 (95% CI = 0.87 to 0.92). [13C]UBT results concurred with stool test results for 878/908 (96.7%) of the Peruvian samples (kappa coefficient of 0.93; 95% CI = 0.91 to 0.96) and for 377/415 (90.8%) of the Brazilian samples (kappa coefficient of 0.75; 95% CI = 0.68 to 0.83), being significantly higher in the former (P < 0.001; OR = 2.95; 95% CI = 1.75 to 4.97) (Table 2).
Table 1.
Agreement between [13C]UBT and monoclonal stool test results for diagnosis of H. pylori infection in infants and toddlers according to the different [13C]UBT cutoff pointsa
| Cutoff (‰) | No. of discordant results |
No. (%) of results in agreement | Kappa coefficient | 95% CI | |
|---|---|---|---|---|---|
| +UBT/−SAT | −UBT/+SAT | ||||
| 3.5 | 77 | 21 | 1,225 (92.59) | 0.85 | 0.82–0.88 |
| 4.0 | 45 | 23 | 1,255 (94.86) | 0.90 | 0.87–0.92 |
| 5.0 | 120 | 18 | 1,185 (89.57) | 0.79 | 0.76–0.82 |
| 6.0 | 191 | 14 | 1,118 (84.50) | 0.69 | 0.65–0.73 |
n = 1,323. ‰, DOB (delta over baseline); +UBT, positive urea breath test; −UBT, negative urea breath test; +SAT, positive stool antigen test; −SAT, negative stool antigen test. Kappa coefficient was calculated according to Cohen's statistic (18).
Table 2.
[13C]Urea breath test and stool antigen monoclonal test results for samples from Brazilian and Peruvian infants and toddlers
| Result categorya | No. of results |
||||
|---|---|---|---|---|---|
| Brazil | Peru | Boys | Girls | Total | |
| All | 415 | 908 | 673 | 650 | 1,323 |
| Concordant | |||||
| HP+ | 81 | 546 | 287 | 340 | 627 |
| HP− | 296 | 332 | 352 | 276 | 628 |
| Total | 377 | 878 | 639 | 616 | 1,255 |
| Discordant | |||||
| HPSA+ UBT− | 14 | 9 | 6 | 17 | 23 |
| HPSA− UBT+ | 24 | 21 | 26 | 19 | 45 |
| Total | 38 | 30 | 32 | 36 | 68 |
HP+, H. pylori positive; HP−, H. pylori negative; HpSA, H. pylori stool antigen assay. The DOB (delta over baseline) of 4‰ was adopted.
The concordance between the tests did not differ (P = 0.43; OR = 2.25; 95% CI = 0.53 to 13.50) when unformed/watery stool samples (80 of 82, concordance of 97.6%) were compared with normal stool samples (1,175 of 1,241, concordance of 94.7%) and when boys were compared with girls (P = 0.62; OR = 0.86; 95% CI = 0.51 to 1.43). The mean age (± SD) in months (18.47 ± 8.47 and 19.21 ± 9.87; P = 0.48) and the mean time interval (days) between breath and stool sample collection (13.97 ± 10.35 and 14.16 ± 12.09; P = 0.88) did not differ between concordant and discordant results, respectively.
The positivity of the tests was higher (P < 0.001; OR = 6.01; 95% CI = 4.50 to 8.04) for samples from Peru (546/878; 62.2%) than for those from Brazil, (81/377; 21.5%). In Brazil, no difference was observed between the genders (41/138 girls versus 40/158 boys; P = 0.61; OR = 1.17; 95% CI = 0.70 to 1.98), whereas in Peru, samples from girls (299/437; 68.4%) were more frequently positive than those from boys (247/441; 56.0%) (P < 0.001; OR = 1.70; 95% CI = 1.28 to 2.26), even after Bonferroni adjustment for age. A greater number of positive samples that became negative by the two tests from the first year to the second year of age was observed for Peruvian females (84.5 to 62.9%) than for males (67.0 to 59.6%; P = 0.02; OR = 1.2; 95% CI = 1.02 to 1.39). The prevalence of the infection increased with increasing age in Brazil (P = 0.02), whereas in Peru it decreased with increasing age (P < 0.001) (Fig. 1).
Fig 1.
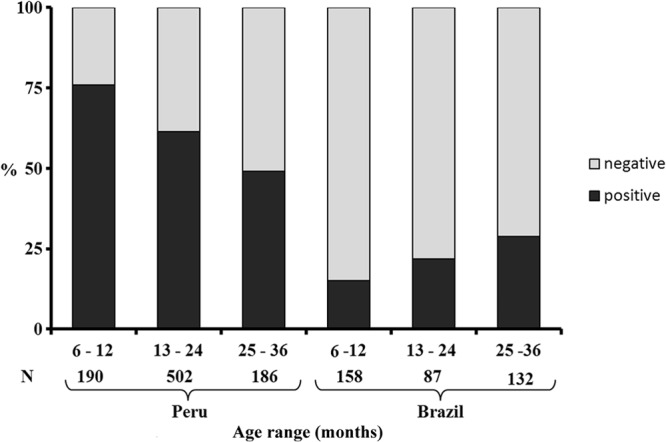
H. pylori prevalences based on concomitant positive results of [13C]UBT and the monoclonal stool antigen test according to age range in Peru (P < 0.001) and Brazil (P = 0.001). The numbers at the bottom refer to the number of samples tested in each age group (total number = 1,255). Statistical analysis was done using the chi-square test.
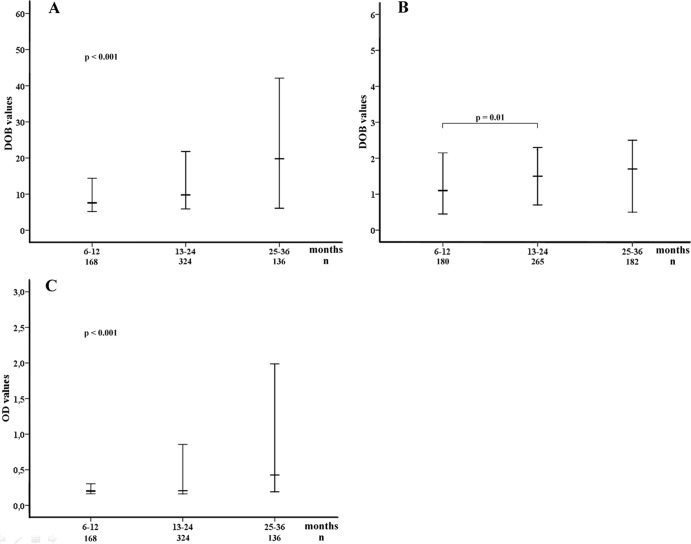
In the group of H. pylori-positive (r = 0.62; P < 0.001) but not H. pylori-negative (r = 0.02; P = 0.67) children, DOB and OD values were correlated. In the positive group, both the DOB and OD values increased with increasing age (Fig. 2A and C). In the negative group, a significant increase in DOB values occurred from the first to the second year of life (Fig. 2B). Significantly higher DOB values were observed for Brazilian than for Peruvian positive children (Table 3). No differences in the mean DOB (P = 0.80) and OD (P = 0.58) values were observed between watery (20.19 ± 25.55 and 0.72 ± 1.09, respectively) and normal (19.63 ± 24.09 and 0.76 ± 1.04, respectively) positive stool samples. No other difference in respect to DOB and OD values was observed.
Fig 2.
Results of [13C]UBT and monoclonal stool antigen tests according to age range. The [13C]UBT and stool antigen test results are shown in DOB and OD values (median and interquartile range, 25 to 75). The analyses were done after log transformation. (A) [13C]UBT positive results (n = 671). Statistical analysis was done using ANOVA (P < 0.001). (B) [13C]UBT negative results (n = 652). Statistical analysis was done using Student's t test (P = 0.01; comparison between the first and the second year of age). (C) Stool antigen test positive results (n = 671). Statistical analysis was done using ANOVA (P < 0.001).
Table 3.
Mean values of [13C]UBT DOBs and HpSA Plus ODs for Brazilian and Peruvian children who had concordant results between the two testsa
| Result | Mean value ± SD for group or comparison |
|||||
|---|---|---|---|---|---|---|
| Country |
Gender |
|||||
| Peru | Brazil | P value | Boys | Girls | P value | |
| UBT DOB | ||||||
| Negative | 1.37 ± 1.63 | 1.36 ± 1.30 | 0.38 | 1.30 ± 1.36 | 1.48 ± 1.67 | 0.23 |
| Positive | 18·87 ± 23.36 | 25.20 ± 28.24 | 0.05 | 18·37 ± 21.44 | 19.45 ± 25.38 | 0.52 |
| HpSA OD | ||||||
| Negative | 0.07 ± 0.02 | 0.07 ± 0.03 | 0.35 | 0.07 ± 0.03 | 0.07 ± 0.02 | 0.54 |
| Positive | 0.76 ± 0.99 | 0.78 ± 0.93 | 0.26 | 0.77 ± 1.06 | 0.76 ± 1.03 | 0.85 |
DOB, delta over baseline, mean ± SD (‰); OD, optical density, mean ± SD; cutoff values adopted were those recommended by the manufacturers (n = 1,255). Data were analyzed by using Student's t test after natural log transformation.
Discrepant results between [13C]UBT and stool antigen test and associated factors.
The results of the tests were discrepant in 68/1,323 (5.1%) samples (23 [33.8%] negative-[13C]UBT and positive-stool-test samples and 45 [66.2%] positive-[13C]UBT and negative-stool-test samples) (Table 2).
Only birth in Brazil (P < 0.001; OR = 3.14; 95% CI = 1.92 to 5.16) was associated with the disagreement between the tests. We then constructed two other models of analysis. First, the positive-[13C]UBT/negative-stool-test group was compared with the concordant group, and only the country of birth remained associated in the multivariate analysis (Table 4). Second, the negative-[13C]UBT/positive-stool-test group was compared with the concordant group. Female gender, increased time interval between stool and breath sample collection, and birth in Brazil remained directly and independently associated (Table 4).
Table 4.
Variables associated with discrepant results between [13C]UBT and HpSA Plus for Brazilian and Peruvian children
| Variablea | Univariate analysis, P value | Multivariate analysis |
||
|---|---|---|---|---|
| OR | 95% CI | P value | ||
| Positive UBT/negative HpSA | ||||
| Increasing age | 0.53 | |||
| Female gender | 0.60 | |||
| Unformed/watery stools | 0.65 | |||
| Time intervalb | 0.15 | 0.99 | 0.96–1.02 | 0.35 |
| Country of birth | <0.001 | 2.81 | 1.53–5.17 | 0.001 |
| Negative UBT/positive HpSA | ||||
| Unformed/watery stools | 0.75 | |||
| Increasing age | 0.89 | |||
| Female gender | 0.02 | 3.12 | 1.21–8.00 | 0.02 |
| Time interval | 0.03 | 1.03 | 1.01–1.05 | 0.04 |
| Country of birth | 0.001 | 3.70 | 1.57–8.70 | 0.003 |
A Hosmer-Lemeshow test showed good fitness of the model (8 degrees of freedom, P ≥ 0.48, and 10 steps). OR, odds ratio; CI, confidence interval.
Time interval, interval of time in days between breath and stool sample collection (≤30 days).
Effect of temperature on performance of stool antigen test.
When stool samples with initial OD values of 1.449 and 1.620 maintained at 25°C were retested, the OD values dropped to 1.305 and 1.356, respectively, after 6 h and to 0.944 and 0.921, respectively, after 48 h. The decrease of the OD values was more pronounced when the samples were maintained at 37°C, dropping to 1.225 and 1.210, respectively, in the first 6 h and to 0.159 and 0.173, respectively, after 48 h. Remarkably, when the stool samples with OD values of 0.149 and 0.183 were retested after 6 h of incubation at 37°C, the OD dropped to values below the cutoff (0.125 and 0.120, respectively).
DISCUSSION
Although there are studies demonstrating high accuracy of the monoclonal stool antigen test (13, 14) for the diagnosis of H. pylori infection in young children, there is not concordance among studies evaluating another noninvasive test, the [13C]UBT (9, 11, 12). Furthermore, since most validation studies to date have been undertaken in developed countries, where the prevalence of H. pylori infection is low, only a small number of young infected children have been included. Concomitant H. pylori infection and diarrheal disease might also influence the performance of the noninvasive diagnostic tests in developing countries, also indicating the need to test the reliability of the [13C]UBT and stool antigen test in developing countries.
Notably, by evaluating a large number of samples obtained from infants and toddlers in two developing countries, we demonstrated that the results of the [13C]UBT were highly in agreement with those of the monoclonal stool antigen test, which points to a good accuracy of each test for the diagnosis of H. pylori infection in very young children. Reinforcing this hypothesis, the positive correlation observed between [13C]UBT DOB and stool test OD values in the group of H. pylori-positive children is in agreement with our previous report for older children (20).
In the H. pylori-positive infants and toddlers, the DOB values increased with age, which is in contrast to observations of older children by others (21, 22) and our group (20). In the present study, the OD values in the stool antigen assay also increased with age. No association between OD values and age has been observed in older children (14, 20). Taken together, these results suggest that DOB and OD values increase with age only in very young children and represent a progressive increase in the gastric bacterial load at this age. In [13C]UBT-negative children, however, the DOB values increase only from the first to the second year of life.
Clear differences between the two studied populations in South America were observed. First, H. pylori positivity increased with increasing age in Fortaleza but decreased in Lima. Spontaneous clearance of the infection as observed in Lima has been questioned. Some authors argue that the DOB values are higher in H. pylori-negative infants, which could lead to [13C]UBT false positivity at this age (21). However, in the [13C]UBT-negative Lima population, the lowest DOB values were observed in the first year of life, in parallel with the highest H. pylori positivity, also observed in the first year of life. Furthermore, we could not demonstrate any cause of false-positive results in both tests in Peruvian children. Differences in respect to the prevalence of the infection, which was higher in Lima than in Fortaleza, were also observed. One explanation is that Peruvian children live in an area of very prevalent H. pylori infection, being highly exposed to the bacterium (23), which contributes to very early infection. Ethnic differences could also account for the differences observed, since it has been demonstrated that the population living in Lima has a predominantly Amerindian ancestry (>70%) (24), whereas in people from Fortaleza, European ancestry predominates (>70%) (25).
In this study, a small but significant discordant result between the two noninvasive tests was observed. Explanations include the lower prevalence of the infection in Fortaleza than in Lima, leading to a lower positive predictive value for the former, different cultural habits between the two countries, and the storage of stool samples, which is considered a critical step in the performance of the stool antigen test (11, 12, 21). Positive [13C]UBT and negative stool test results may have occurred in Brazilian children due to the higher mean annual temperature in Fortaleza (30°C) than in Lima (23°C), which may result in false-negative results for a stool test with low positive OD values. In this study, the relevance of adequate storage of the stool samples to the performance of the stool test in regions of high ambient temperature was demonstrated. By experimentally keeping stool samples at 37°C for more than 6 h, stool tests with OD values slightly above the cutoff limit turned negative. Transient gastric infection with urease-producing Streptococcus (26) and other gastric Helicobacter species may also have contributed to positive [13C]UBT and negative stool test results. However, the prevalence of other gastric Helicobacter species is less than 1.0% (27).
A large time interval between stool and breath sample collection was also independently associated with a negative [13C]UBT and a positive stool test. In young children, the infection may be transient (3, 6) and H. pylori may be cleared from the gastric mucosa in the interval between the collection of stool and breath samples.
Discrepant results were also more frequently observed for girls. In accordance with the results of another study in Peru (28), we also observed that the prevalence of H. pylori in Peru was higher in females and decreased from the first to the second year of age more frequently in females. A more frequent loss of infection might also explain a greater rate of disagreement between the two tests for girls, because the elimination of H. pylori antigens in the stool may take longer.
Importantly, having a diarrheic stool sample was not associated with test result disagreement. High concordance between the two tests was observed for children both with and without diarrhea. One might speculate that the concentration of H. pylori antigens would be lower in watery stools than in normal stools. To avoid this possibility, we adopted the manufacturer's recommendations using a high volume of stool. A false-positive stool test result, due to cross-reactivity between antigens of H. pylori-related bacteria, has also been suggested (12). Against this possibility, we used a highly specific monoclonal HpSA Plus enzyme immunoassay (EIA) for detection of H. pylori antigens in stool samples. Reinforcing the reliability of our results, we observed positive results of the stool antigen test for 98.3% of the diarrheic children with positive-[13C]UBT. Furthermore, we are unaware of causes of false-positive [13C]UBT results for diarrheic children.
In conclusion, this study showed excellent agreement between the results of the [13C]UBT and the stool antigen test for infants and toddlers in both high- and moderate-H. pylori prevalence developing countries in South America. This indicates that both noninvasive tests are reliable methods for the diagnosis of H. pylori infection in very young children, which will facilitate robust epidemiological studies in infants and toddlers in developing countries.
ACKNOWLEDGMENTS
This work was supported by the Sixth Framework Programme of the European Union, Project CONTENT (grant number INCO-DEV-3-032136), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil), and Instituto de Biomedicina do Semi-Árido (INCT-IBISAB/Brazil).
We thank the CONTENT external advisor Guillermo Perez-Perez for his expert advice at the commencement of the project, the members of the communities of the Pampas de San Juan and Parque Universitário for their collaboration, Lilia Cabrera for her assistance in field work in Lima, and Maria Luiza Scarabelli for her assistance in reading results of [13C]UBT assays of this study.
Footnotes
Published ahead of print 4 September 2013
REFERENCES
- 1.Neale KR, Logan RP. 1995. The epidemiology and transmission of Helicobacter pylori infection in children. Aliment. Pharmacol. Ther. 9:77–84 [PubMed] [Google Scholar]
- 2.Rocha GA, Rocha AM, Silva LD, Santos A, Bocewicz AC, Queiroz RDM, Bethony J, Gazzinelli A, Corrêa-Oliveira R, Queiroz DM. 2003. Transmission of Helicobacter pylori infection in families of preschool-aged children from Minas Gerais, Brazil. Trop. Med. Int. Health 8:987–989 [DOI] [PubMed] [Google Scholar]
- 3.Rowland M, Daly L, Vaughan M, Higgins A, Bourke B, Drumm B. 2006. Age-specific incidence of Helicobacter pylori. Gastroenterology 130:65–72 [DOI] [PubMed] [Google Scholar]
- 4.Weyermann M, Rothenbacher D, Brenner H. 2009. Acquisition of Helicobacter pylori infection in early childhood: independent contributions of infected mothers, fathers and siblings. Am. J. Gastroenterol. 104:182–189 [DOI] [PubMed] [Google Scholar]
- 5.Queiroz DM, Carneiro JG, Braga-Neto MB, Fialho AB, Fialho AM, Goncalves MH, Rocha GA, Rocha AM, Braga LL. 2012. Natural history of Helicobacter pylori infection in childhood: eight-year follow-up cohort study in an urban community in northeast of Brazil. Helicobacter 17:23–29 [DOI] [PubMed] [Google Scholar]
- 6.Nurgalieva Z, Goodman KJ, Phillips CV, Fischbach L, de la Rosa JM, Gold BD. 2008. Correspondence between Helicobacter pylori antibodies and urea breath test results in a US-Mexico birth cohort. Paediatr. Perinat. Epidemiol. 22:302–312 [DOI] [PubMed] [Google Scholar]
- 7.Windle HJ, Kelleher D, Crabtree JE. 2007. Childhood Helicobacter pylori infection and growth impairment in developing countries: a vicious cycle? Pediatrics 119:e754–e759. 10.1542/peds.2006-2196 [DOI] [PubMed] [Google Scholar]
- 8.Sýkora J, Rowland M. 2011. Helicobacter pylori in pediatrics. Helicobacter 16:59–64 [DOI] [PubMed] [Google Scholar]
- 9.Rowland M, Lambert I, Gormally S, Daly LE, Thomas JE. 1997. Carbon13-labeled urea breath test for the diagnosis of Helicobacter pylori infection in children. J. Pediatr. 131:815–820 [DOI] [PubMed] [Google Scholar]
- 10.Bazzoli F, Cecchini L, Corvaglia L, Dall'Antonia M, De Giacomo C, Fossi S, Casali LG, Gullini S, Lazzari R, Leggeri G, Lerro P, Valdambrini V, Mandrioli G, Marani M, Martelli P, Miano A, Nicolini G, Oderda G, Pazzi P, Pozzato P, Ricciardiello L, Roda E, Simoni P, Sottili S, Zagari RM. 2000. Validation of the 13C-urea breath test for the diagnosis of Helicobacter pylori infection in children: a multicenter study. Am. J. Gastroenterol. 95:646–650 [DOI] [PubMed] [Google Scholar]
- 11.Imrie C, Rowland M, Bourke B, Drumm B. 2001. Limitations to carbon13-labeled urea breath testing for Helicobacter pylori in infants. J. Pediatr. 139:734–737 [DOI] [PubMed] [Google Scholar]
- 12.Mégraud F, European Pediatric Task Force on Helicobacter pylori 2005. Comparison of non-invasive tests to detect Helicobacter pylori infection in children and adolescents: results of a multicenter European study. J. Pediatr. 146:198–203 [DOI] [PubMed] [Google Scholar]
- 13.Koletzko S, Konstantopoulos N, Bosman D, Feydt-Schmidt A, van der Ende A, Kalach N, Raymond J, Rüssmann H. 2003. Evaluation of a novel monoclonal antibody enzyme immunoassay for detection of Helicobacter pylori antigen in stool from children. Gut 52:804–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolho KL, Klemola T, Koivusalop A, Rautellin H. 2006. Stool antigen tests for the detection of Helicobacter pylori in children. Diagn. Microbial. Infect. Dis. 55:269–273 [DOI] [PubMed] [Google Scholar]
- 15.Nguyen TV, Bengtsson C, Nguyen GK, Granström M. 2008. Evaluation of two triple-therapy regimens with metronidazole or clarithromycin for the eradication of H. pylori infection in Vietnamese children: a randomized, double-blind clinical trial. Helicobacter 13:550–556 [DOI] [PubMed] [Google Scholar]
- 16.Kindermann A, Demmelmair H, Koletzko B, Krauss-Etschmann S, Wiebecke B, Koletzko S. 2000. Influence of age on 13C-urea breath test results in children. J. Pediatr. Gastroenterol. Nutr. 30:85–91 [DOI] [PubMed] [Google Scholar]
- 17.Elitsur Y, Tolia V, Gilger MA, Reeves-Garcia J, Schmidt-Sommerfeld E, Opekun AR, El-Zimaity H, Graham DY, Enmei K. 2009. Urea breath test in children: the United States prospective, multicenter study. Helicobacter 14:134–140 [DOI] [PubMed] [Google Scholar]
- 18.Goodman KJ, Correa P. 2000. Transmission of Helicobacter pylori among siblings. Lancet 355:358–362 [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. 1960. A coefficient of agreement for nominal scales. Educ. Psychol. Measurement 20:37–46 [Google Scholar]
- 20.de Carvalho Costa Cardinali L, Rocha GA, Rocha AM, de Moura SB, de Figueiredo Soares T, Esteves AM, Nogueira AM, Cabral MM, de Carvalho AS, Bitencourt P, Ferreira A, Queiroz DM. 2003. Evaluation of [13C]urea breath test and Helicobacter pylori stool antigen test for diagnosis of H. pylori infection in children from a developing country. J. Clin. Microbiol. 41:3334–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koletzko S, Feydt-Schmidt A. 2001. Infants differs from teenagers: use of non-invasive tests for detection of Helicobacter pylori infection in children. Eur. J. Gastroenterol. Hepatol. 13:1047–1052 [DOI] [PubMed] [Google Scholar]
- 22.Zevit N, Niv Y, Shirin H, Shamir R. 2011. Age and gender differences in urea breath test results. Eur. J. Clin. Invest. 41:767–772 [DOI] [PubMed] [Google Scholar]
- 23.Herrera PM, Mendez M, Velapatiño B, Santivañez L, Balqui J, Finger SA, Sherman J, Zimic M, Cabrera L, Watanabe J, Rodríguez C, Gilman RH, Berg DE. 2008. DNA-level diversity and relatedness of Helicobacter pylori strains in shantytown families in Peru and transmission in a developing-country setting. J. Clin. Microbiol. 46:3912–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira L, Zamudio R, Soares-Souza G, Herrera P, Cabrera L, Hooper CC, Cok J, Combe JM, Vargas G, Prado WA, Schneider S, Kehdy F, Rodrigues MR, Chanock SJ, Berg DE, Gilman RH, Tarazona-Santos E. 2012. Socioeconomic and nutritional factors account for the association of gastric cancer with Amerindian ancestry in a Latin American admixed population. PLoS One 7:e41200. 10.1371/journal.pone.0041200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pena SD, Di Pietro G, Fuchshuber-Moraes M, Genro JP, Hutz MH, Kehdy Fde S, Kohlrausch F, Magno LA, Montenegro RC, Moraes MO, de Moraes ME, de Moraes MR, Ojopi EB, Perini JA, Racciopi C, Ribeiro-Dos-Santos AK, Rios-Santos F, Romano-Silva MA, Sortica VA, Suarez-Kurtz G. 2011. The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS One 62:e17063. 10.1371/journal.pone.0017063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang I, Nell S, Suerbaum S. 2013. Survival in hostile territory: the microbiota of the stomach. FEMS Microbial. Rev. 37:736–761 [DOI] [PubMed] [Google Scholar]
- 27.Boyanova L, Lazarova E, Jelev C, Gergova G, Mitov I. 2007. Helicobacter pylori and Helicobacter heilmannii in untreated Bulgarian children over a period of 10 years. J. Med. Microbiol. 56:1081–1085 [DOI] [PubMed] [Google Scholar]
- 28.Klein PD, Gilman RH, Leon-Barua R, Diaz F, Smith EO, Graham DY. 1994. The epidemiology of Helicobacter pylori in Peruvian children between 6 and 30 months of age. Am. J. Gastroenterol. 89:2196–2200 [PubMed] [Google Scholar]