Abstract
Mammalian orthoreoviruses (MRVs) are known to cause mild enteric and respiratory infections in humans. They are widespread and infect a broad spectrum of mammals. We report here the first case of an MRV detected in a child with acute gastroenteritis, which showed the highest similarity to an MRV reported recently in European bats. An examination of a stool sample from the child was negative for most common viral and bacterial pathogens. Reovirus particles were identified by electron microscopic examination of both the stool suspension and cell culture supernatant. The whole-genome sequence was obtained with the Ion Torrent next-generation sequencing platform. Prior to sequencing, the stool sample suspension and cell culture supernatant were pretreated with nucleases and/or the convective interaction medium (CIM) monolithic chromatographic method to purify and concentrate the target viral nucleic acid. Whole-genome sequence analysis revealed that the Slovenian SI-MRV01 isolate was most similar to an MRV found in a bat in Germany. High similarity was shared in all genome segments, with nucleotide and amino acid identities between 93.8 to 99.0% and 98.4 to 99.7%, respectively. It was shown that CIM monolithic chromatography alone is an efficient method for enriching the sample in viral particles before nucleic acid isolation and next-generation sequencing application.
INTRODUCTION
Reoviridae is a highly diverse virus family, including viruses capable of infecting various host species (mammals, reptiles, fish, birds, protozoa, fungi, plants, and insects). These viruses consist of an icosahedric capsid and a segmented genome of 10 to 12 double-stranded RNA (dsRNA) segments (1). They are unenveloped and relatively stable in the environment (2). Two subfamilies have been described within the Reoviridae family: Spinareovirinae and Sedoreovirinae, including 9 and 6 genera, respectively (3). In human medicine, the most recognized are Rotaviruses within the Sedoreovirinae subfamily and Orthoreoviruses within Spinoreovirinae. Orthoreoviruses, with mammalian orthoreovirus (MRV) as the type species, were already recognized in the 1950s as respiratory and enteric orphan viruses (4). They were found in hosts with or without clinical manifestations (1). MRVs have been reported to date in various mammalian hosts, including human and animal species. In the last few years, they have often been described as the sole pathogen in various hosts presenting severe clinical manifestations, such as hemorrhagic enteritis, acute respiratory infections, central nerve system implications, and others (5–10). There is consequently increasing concern about the widespread nature and pathogenesis of these viruses. A German group of researchers recently reported infections of bat species with MRVs, which resulted in evident pathological signs in the organs of infected bats (11). This was the first report of reoviruses in bats that were not clustered into the species Pteropine orthoreovirus but in MRVs. Almost at the same time, an Italian group published data on reovirus detection in various bat species, reporting nucleotide sequences of partial L1 and complete S1 segments, which showed the highest similarity to the German bat isolate (12). Both research groups speculated on bat-to-human interspecies transmission, but there was no evidence to support this hypothesis. A closely related MRV found in a dog with hemorrhagic enteritis had been previously described by another Italian group. The authors proposed that these viruses might be important zoonotic pathogens (13).
The zoonotic potential of reoviruses has already been described and discussed elsewhere (6, 7, 11). The transmission of reoviruses from one host to another is not limited to close contacts but extends to indirect transmission. Infection through contaminated food, water, or other factors in the environment is highly possible, since infective reovirus particles have been found in environmental samples (14–17). Viral persistence outside the host is one of the advantageous features that enables them to spread efficiently.
Researchers throughout the world have recently focused very intensively on zoonotic or potentially zoonotic viruses. Bats are of special interest because of their diversity, the wide spectrum of virus populations found in different bat samples, and a special virus-host interaction (18, 19). Screening of animals for potentially zoonotic viruses has resulted in the discovery of novel viruses. In the last few years, the pathogen discovery field has made a major step forward. Application of new technologies, such as next-generation sequencing (NGS), enables researchers to take a different approach to pathogen discovery, gathering a huge amount of genomic information from a sample of interest in a short period of time (20). In the last decade, the price of sequencing technology has decreased drastically, while the capacity of NGS data obtained has increased sharply (20). This research tool also has major potential as part of microbiological diagnostics in combination with classical or standard microbiological methodologies (21). In the future, NGS could help resolve clinical cases of infections with undetermined etiology. However, when using NGS in pathogen discovery or diagnostics, the preparation of high-quality and high-quantity nucleic acid is of major concern (22). Improved and optimized sample preparation, purification, and concentration of the target are essential for achieving the best results in NGS. Isolation of as pure as possible viral nucleic acid enables easier sequencing of the target of interest and faster bioinformatics workflow. Convective interaction medium (CIM) chromatography is the method of choice for virus concentration and purification from different samples (23, 24).
In this work, we discovered an MRV strain with high similarity to orthoreoviruses detected in European bats. The MRV was found in a child with acute gastroenteritis requiring hospitalization. This is an indication of zoonotic transmission. The whole virus genome was sequenced using the Ion Torrent platform. It is highly possible that this group of MRVs detected in bats in Europe is widespread and could easily cross species barriers. However, the detailed pathogenesis has yet to be determined. To the best of our knowledge, this is the first report of a bat MRV detected in a human with a clinical manifestation. In addition, a new approach for sample preparation prior to NGS is presented. A chromatographic method using CIM monoliths was used, which proved to be effective in enriching and purifying the sample of MRV prior to RNA extraction and NGS application.
CASE REPORT
A 17-month-old boy presented at the Department of Infectious Diseases, University Medical Centre Ljubljana, Ljubljana, Slovenia, with a 5-day history of nonbloody diarrhea. Stools were frequent; the child refused to drink and after 4 days developed fever. His previous medical history was unremarkable, with only a fever-related thrombocytopenia at the age of 3 months. He had been vaccinated against rotavirus. He attended a day care center at which no gastroenteritis cases were observed.
On admission, the child was mildly dehydrated and afebrile, and the abdomen was not sensitive to palpation. The C-reactive protein (CRP) concentration was 20 mg/liter, the white blood cell (WBC) count was 8.4 × 109/liter (with normal differential), the red blood cell (RBC) count was 4.94 × 1012/liter, the hemoglobin (Hb) level was 124 g/liter, the hematocrit (Hct) was 0.384, and the blood sugar, blood urea nitrogen, creatinine, and electrolytes were normal. The child was given 500 ml of intravenous fluids and discharged home. The next day he returned to the department because of ongoing fever, frequent nonbloody stools accompanied by colicky pains, and red and swollen gums with oral ulcers. Aphthous stomatitis was diagnosed, in addition to gastroenteritis, and the child was admitted for parenteral rehydration and discharged the next day. He made an uneventful recovery. The total duration of diarrhea was 8 days.
A stool sample was analyzed according to the standard diagnostic protocol, including classical bacterial examination with culturing techniques, checking for the most common bacterial pathogens (Campylobacter spp., Salmonella sp., pathogenic Escherichia coli, Yersinia sp., and Shigella sp.). A virological examination was carried out at the same time: negative-staining electron microscopy (EM) and enzyme-linked immunosorbent assays (ELISAs) for group A rotaviruses and adenoviruses 40 and 41 (Meridian Bioscience, Inc., Cincinnati, OH) and in-house real-time reverse transcription (RT)-PCR for noroviruses of genogroups I and II and astroviruses, as described previously (25, 26). Reoviruses were observed by electron microscopy in the stool sample taken at the first admission.
MATERIALS AND METHODS
Virus identification and cell culture propagation.
Cell line LLC-MK2 (rhesus monkey kidney epithelial cells) grown in modified Eagle's medium (MEM) supplemented with 10% fetal calf serum was used for virus propagation. A 10% stool suspension was prepared and clarified by centrifugation at 1,600 × g. Clarified supernatant of a 10% stool suspension was filtered through a 0.22-μm-pore filter (Millipore, Billerica, MA) and inoculated on a 48-h cell culture monolayer in 25-cm2 flasks (TPP, Trasadingen, Switzerland). After incubation for 1 h at 37°C, the inoculum was removed, and cells were supplemented with the original growth medium and incubated further at 37°C in an atmosphere with 5% CO2 until the appearance of a cytopathic effect (CPE). A 10-fold serial dilution of reovirus isolate was prepared to determine the 50% tissue culture infective dose (TCID50) value (27). A known concentration of reovirus isolate helped us to estimate the reovirus concentration in the child's stool sample using real-time RT-PCR, as described previously (11).
In addition, infected cells were prepared for thin sectioning. Briefly, the specimen was fixed with 2% (vol/vol) glutaraldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4) for 2 h, rinsed with 3 changes of PBS, and postfixed with 1% (vol/vol) OsO4 in PBS for 2 h. After being washed, the specimen was dehydrated in a graded series of ethanol and embedded in epoxy (agar low-viscosity) resin, following the standard protocol. Ultrathin sections were collected on carbon-coated 200 mesh copper grids and stained with 1% uranyl acetate and 1% lead citrate. In addition, clarified stool suspension was used for direct EM examination of the sample after negative staining with 2% phosphotungstic acid (pH 4.5). EM grids were screened at 80 kV in a JEM 1200 EXII transmission electron microscope (JEOL, Tokyo, Japan).
Sample preparation for NGS.
The RNA for the sequencing procedure was obtained from two sources: clarified stool suspension and cell culture supernatant after virus propagation. Two samples (6 ml of stool and 6 ml of cell culture supernatant) were incubated with 1,000 U/ml of Benzonase (Novagen, San Diego, CA) for 1 h at room temperature (samples 1 and 2) to digest nonencapsidated nucleic acid. Samples were then diluted 6× in chromatography running buffer (50 mM HEPES; pH 7) and left overnight at 4°C. An additional 6-ml sample of cell culture supernatant was diluted in the same way but without Benzonase treatment (sample 3). Ten milliliters of all three samples was loaded into a 0.34-ml-volume CIM-QA disk (BIA Separations, Ajdovščina, Slovenia), using an AKTA purifier chromatographic system (GE Healthcare, Uppsala, Sweden). After loading, nonbound material was washed with running buffer, and the elution of bound viruses proceeded in two different ways. The Benzonase-treated stool suspension and cell culture samples (samples 1 and 2) were eluted in a single step, by including 1 M NaCl in the running buffer. The Benzonase-untreated cell culture sample (sample 3) was eluted by using a gradient of 0 to 500 mM NaCl in ≈60 column volumes in order to purify the viruses as much as possible. In all three cases, the elution was collected in 1-ml fractions. The presence and approximate number of putative reoviruses in the elution fractions were estimated by the virus particle counting method under an electron microscope using a negative-staining technique (28). To have an indication of the presence of eukaryotic nucleic acid, RNA was extracted from the fractions using a QIAamp virus RNA kit (QIAgen, Valencia, CA) and applied to a eukaryotic 18S assay (Life Technologies, Applied Biosystems Division, Foster City, CA), in a one-step real-time RT-PCR format, using an Ag-Path master mix (Life Technologies) and an ABI 7900 HT real-time PCR system. To overcome the lower sensitivity of the electron microscopy technique and in order to confirm the presence of reovirus, the RNA from fractions 6 to 10 obtained in the stool sample run, in which no virus particles were observed, was applied to RT-PCR using a set of reovirus generic primers (5). Fractions that were selected for the sequencing procedure (fractions 6 to 10, 3 to 6, and 15 to 18 for runs involving samples 1, 2, and 3, respectively) were pooled, and RNA was extracted from the whole pooled volume using a TRIzol LS Plus RNA purification kit (Life Technologies, Invitrogen Division, Carlsbad, CA). The final purified RNA solution obtained from each of the three samples was further evaporated to a final volume of ≈100 μl in a GeneVac personal evaporator (Genevac, Ltd., Ipswitch, United Kingdom).
Ion Torrent library preparation and sequencing.
The RNA library was prepared using an Ion Total RNA-Seq kit v2 (Life Technologies, Invitrogen Division, Darmstadt, Germany) according to the manufacturer's protocol (4476286, revision D) for a low input of starting material (between 10 and 100 ng). The amount and size distribution of library DNA fragments were determined with a Labchip GX instrument (Caliper, Life Sciences, MA). Emulsion PCR and enrichment steps were carried out using an Ion OneTouch200 template kit v2 DL as described in the protocol (MAN0006957, revision 5.0). Assessment of the Ion Sphere particle quality was undertaken between the emulsion PCR and enrichment steps with an Ion Sphere quality control kit using a Qubit 2.0 fluorometer (Life Technologies). Each library was sequenced on a separate Ion 314 Chip (Life Technologies). Signal processing and base calling were performed with Torrent Suite software version 3.4.2. Adapter sequences were trimmed using the same software.
Complete genome sequence generation.
Trimmed reads were used for de novo assembly using CLC Genomic Workbench 6.0 (CLC Bio, Aarhus, Denmark), with default program parameters (see Table S2 in the supplemental material). The generated contigs were compared for similarity against all virus sequences deposited in the NCBI GenBank database using the Basic Local Alignment Search Tool (BLASTn and BLASTx). In all three samples, the majority of the contigs from all 10 viral genome segments showed the highest identity values with the MRV strain 342/08 genome, deposited in GenBank under accession no. JQ412755 to JQ412764 (11). This strain was therefore selected as the reference genome. Reads obtained from each sample were initially mapped separately to the reference sequence using default parameters (see Table S2). The generated consensus sequences were additionally compared, and no difference was observed between the consensus sequences of the three different samples. The reads of all three samples were subsequently mapped together to the reference. After removal of duplicate reads, all of the single nucleotide polymorphisms (SNPs) and indels (in comparison to the reference genome sequence) were explored visually. They were considered to be reliable if they were covered with at least 30 reads and were derived from both the positive and negative strands of RNA.
In order to compare the efficiencies of host (LLC-MK2 cell culture) nucleic acid removal between samples 2 and 3, reads were mapped to the Macaca mulatta genome (rheMac3; Beijing Genomics Institute, Shenzhen, China) (GenBank assembly ID GCA_000230795.1). To prevent unspecific mapping, reads smaller than 25 bp were removed, and highly stringent parameters were used for these mappings (given in Table S2 in the supplemental material).
Sequence analysis.
Consensus genome segments were further analyzed for open reading frames (ORFs), which were determined using Geneious 6.0.5 version (Biomatters, Ltd., Auckland, New Zealand), and deduced amino acid sequences were obtained.
For phylogenetic analysis, whole-genome MRV sequences available in GenBank were downloaded, and multiple sequence alignments (ClustalW) were carried out. A neighbor-joining phylogenetic tree (Kimura 2-parameter system) was made to show the phylogenetic relationships, using the MEGA 4 software system (29). Branch support was assessed by bootstrap analysis of 1,000 replicates. For reassortment or recombination events, the concatenated whole-genome sequence of the Slovenian MRV was compared to other whole-genome MRVs from GenBank. A similarity plot was constructed using SimPlot software, version 3.5.1 (30).
Nucleotide sequence accession numbers.
The nucleotide sequences obtained in this study have been deposited in GenBank under accession no. KF154724 to KF154733.
RESULTS
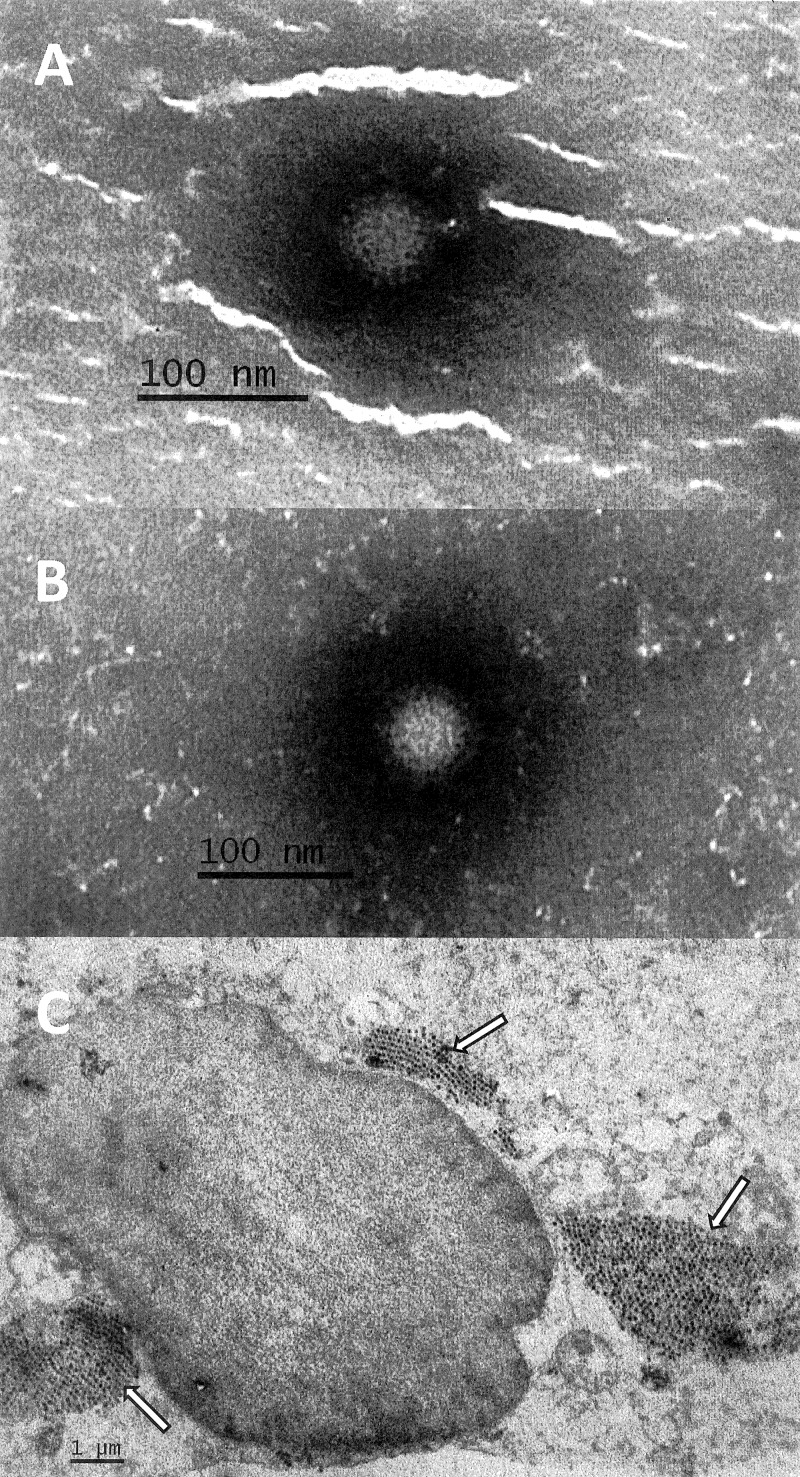
Bacterial laboratory examination of the patient's stool sample was negative at the first and second admissions. Virological examination was performed only on the sample at first admission and was negative for specific antigen detection tests for group A rotaviruses and adenoviruses 40 and 41, and molecular tests were negative for noroviruses in genogroups I and II and astroviruses, which are the most prevalent pathogens detected in the age group of the case. However, clear 75-nm reovirus particles were observed upon EM examination of the stool suspension (Fig. 1A). After inoculation of the filtered stool suspension on an LLC-MK2 cell culture, CPE appeared 48 h postinoculation, comprising cell rounding with a notable membrane and final cell lysis. Again, reovirus particles were observed under an electron microscope, on examination of the cell culture supernatant without a prior concentration step (Fig. 1B) and in a thin section of infected cell culture (Fig. 1C). In ultrathin sections, several cytoplasmic inclusions with densely packed virus particles were found. An electron-dense center and a clear outer rim were observed in all particles.
Fig 1.
Electron micrographs of reoviruses in stool suspension (A) (magnification, 100,000×) cell culture supernatant (B) (magnification, 100,000×), and ultrathin section of LLC-MK2 cells (C) (magnification, 10,000×) infected with the SI-MRV01 orthoreovirus strain. Arrows in Fig. 1C indicate reovirus particles.
The cell culture supernatant was cleared, and the reovirus concentration was determined to be 2.43 × 108 TCID50/ml. The regression equation was obtained from the real-time RT-PCR analysis of serial 10-fold dilutions of reovirus isolate (y = 40.73 − 3.49x; R2 = 0.999). The theoretical TCID50/ml was calculated for reoviruses in child's stool sample, using the quantification cycle (Cq) value of the reovirus real-time RT-PCR analysis. The concentration was estimated to 2.26 × 107 TCID50/ml, considering the 10% stool sample dilution.
Sample preparation and sequencing on the Ion Torrent PGM platform.
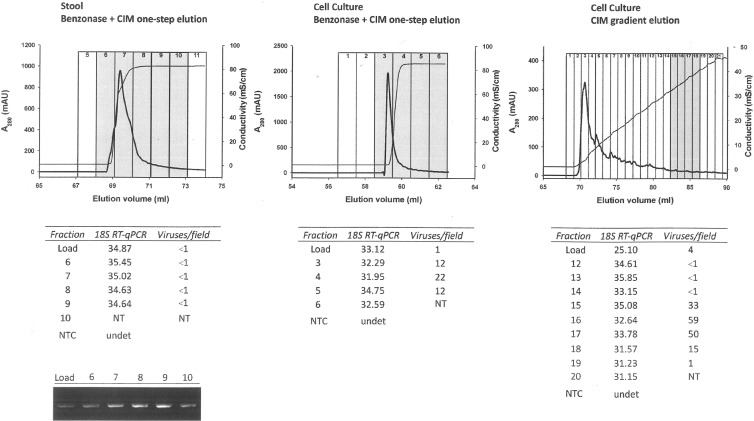
The high 18S real-time RT-PCR Cq values (≈34) observed in the load of the first two samples treated with nucleases (in comparison with nontreated sample 3; Cq ≈ 25) indicates that Benzonase degraded the nucleic acids present in the sample (Fig. 2). Comparing the 18S Cq values of the load and elution fractions in these two samples, it can be concluded that one-step elution did not further clean the sample from eukaryotic NA, although it allowed a single order of magnitude concentration of the viruses in sample 2 (1 virus per field of 400 mesh EM grid in the load compared to 12 to 22 in the eluted fractions). In sample 1 (stool suspension), the amount of virus was below the limit of detection (LOD) of the electron microscopy already in the load, and the presence of viruses was only detected by conventional RT-PCR (Fig. 2), in which a certain concentration was again observed when comparing the intensities of the agarose gel bands from load and elution fractions. Sample 3 showed the largest amount of virus in the eluted fractions (up to 59 viruses per field of 400 mesh EM grid), and it also allowed concentration of the viruses from 4 viruses per field to up to 59. Moreover, the gradient allowed more efficient separation of the virus-rich fraction, as seen from the chromatogram (Fig. 2) and from the 18S assay Cq values, which increased from 25 up to 35 in fraction 15. In this case, nonviral NA was removed physically from the sample, so a lower risk for the presence of small interfering fragments is expected in comparison to the Benzonase-treated ones.
Fig 2.
Elution peaks obtained in the chromatographic runs involving samples 1 (stool, Benzonase, CIM one-step elution), 2 (cell culture, Benzonase, CIM one-step elution), and 3 (cell culture, CIM gradient elution). mAU, milliabsorbance units. Fraction analysis for 18S rRNA (as indicative of eukaryotic RNA presence) and reovirus EM detection is shown below the graphs. In the case of sample 1, the presence of reovirus had to be confirmed by classic quantitative RT-PCR (RT-qPCR) (shown to the left below). NTC, negative template control; NT, not tested; undet, undetermined.
The joint data set from all three sequencing runs consisted of 861,215 reads. The average depth of coverage across all segments of the virus genome was 257.41×, with a standard deviation (SD) of 117.29×. After removal of duplicate reads, the average depth of coverage amounted to 101.88× (SD, 25.13×). The comparison of the sequencing outputs of the three samples pretreated differently is summarized in Table 1. In the case of sample 1, only 4.91% of reads aligned to the final consensus sequence used as a reference for comparisons. Much higher enrichment for viral sequences was seen in the case of samples isolated from tissue culture: 33.4% of reads aligned to the reference in case of sample 2. In the case of sample 3 with gradient elution, an even higher proportion of sequences aligned to the reference (40.14% of reads). Of all three samples, sample 3 had the highest N50 value for the de novo assembly of reads, and the contigs produced covered almost the entire virus genome. In addition, the percentage of high-quality reads that map to the M. mulatta genome (source species for cell culture cells) is much lower in sample 3 (2.5%) than in sample 2 (30.8%) (Table 1), indicating that the elution gradient was more efficient at removing background NA than the Benzonase treatment combined with single-step elution. Since the total number of reads was a lot higher for sample 2 than for sample 3, more data and better depth of coverage were obtained from the former. However, increased depth in the case of sample 2 was shown to be a consequence of a high number of duplicate reads (Table 1). Due to the small size of the viral genome, a very good average coverage depth was achieved even on the smallest PGM sequencing chip.
Table 1.
Quality and quantity of nucleotide sequences obtained from Ion Torrent sequencing for the three samples tested
| Parameter | Result fora: |
||
|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | |
| De novo assembly | |||
| N50 | 155 | 88 | 236 |
| No. of contigs | 885 | 2,556 | 371 |
| No. (total length) of regions not covered by contigsb | 73 (4,787 nt) | 96 (6,258 nt) | 9 (89 nt) |
| Mapping to new Orthoreovirus consensus sequence | |||
| % of reads mapped | 4.91 | 33.4 | 40.14 |
| Avg coverage | 11.47× (SD, 5.49×) | 151.77× (SD, 83.42×) | 119.07× (SD, 46.06×) |
| Avg coverage excluding duplicates | 10.08× (SD, 4.35×) | 39.34× (SD, 12.11×) | 76.74× (SD, 21.17×) |
| No. (total length) of zero coverage regions (all) | 6 (17 nt) | 4 (5 nt) | 4 (5 nt) |
| Mapping to Macaca mulatta genomec | |||
| % of reads mapped | 0.03 | 30.83 | 2.54 |
Sample 1, stool, Benzonase, CIM one-step elution; sample 2, cell culture, Benzonase, CIM one-step elution; sample 3, cell culture, no Benzonase, CIM gradient elution.
After they had been mapped to the new Orthoreovirus consensus sequence.
Only reads longer than 25 bp.
Sequence analysis.
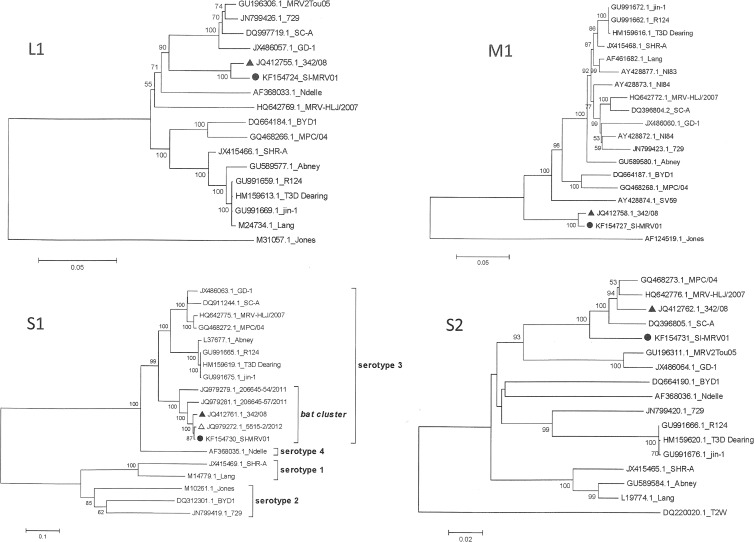
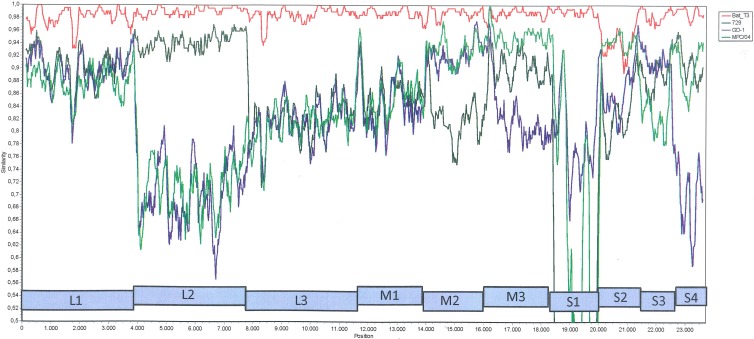
Nucleotide and deduced amino acid sequences were obtained and further analyzed. Following BLAST of the nucleotide sequence, the newly sequenced Slovenian reovirus isolate SI-MRV01 showed the highest nucleotide sequence identity with reovirus strain 342/08, isolated from an insectivorous bat in Germany, in all genome segments except S2, in which a slightly lower nucleotide identity was observed (see Table S1 in the supplemental material). The nucleotide identity with the 342/08 strain varied from 93.8% for the S2 segment to 99.0% for the L2 segment. The amino acid sequence identity consequently also showed the highest identity with the 342/08 strain in all genome segments (98.4 to 99.7%) (see Table S1). In addition, phylogenetic analysis confirmed the highest relationship of Slovenian human reovirus isolate SI-MRV01 with German bat reovirus isolate 342/08. Both isolates clustered together in a separate branch in all genome segments except S1 and S2 (Fig. 3; see Fig. S1 to S7 in the supplemental material). For the S1 segment, the Slovenian SI-MRV01 strain was found in a specific bat cluster formed within the mammalian reovirus serotype 3 group. For this segment, the highest identity was shown with the bat reovirus characterized recently in Italy (12). In the S2 segment, SI-MRV01 did not show relatedness to any specific strain but was clustered in the serotype 3 group, together with porcine strain SC-A, MRV-HLJ/2007, civet strain MPC/04, and bat strain 342/08. According to the results of the SimPlot analysis, there are some indications of possible genome reassortments. Most indications of a reassortment event were found for the L2 segment, comparing the SI-MRV01 strain with the porcine GD-1, 729, and civet MPC/04 strains (Fig. 4).
Fig 3.
Phylogenetic analysis of the L1, M1, S1, and S2 genome segments for the Slovenian strain and most related whole-genome strains from GenBank. The neighbor-joining algorithm was used for the construction of the phylogenetic tree with bootstrap values of 1,000 replicates shown at the branches. The scale bar represents the p-distance. ●, Slovenian MRV isolate SI-MRV01; △, Italian MRV strain identified in a bat; ▲, German MRV isolate 342/08 identified in a bat.
Fig 4.
Similarity plot analysis of the whole-genome nucleotide sequence comparing the Slovenian orthoreovirus strain with some of the most related strains from GenBank.
The SI-MRV01 strain's deduced amino acid sequence was analyzed for indicative amino acid positions related to pathogenesis. In previous studies, two amino acid positions, 350D and 419E, in the σ1 protein (S1 gene) were shown to be indicative of neurotropism (31, 32). However, in the SI-MRV01 strain, those sites were 350I and 419E. The significance of such an amino acid composition is not known and needs to be examined. It was also shown that 249I is required for protease resistance of the σ1 protein, which enables efficient viral spread and replication (33) and was also found in the SI-MRV01 strain. The S1 segment is also important in fusogenic reoviruses, since it encodes the fusion protein FAST (fusion-associated transmembrane protein) (34). However, the Slovenian SI-MRV01 strain does not have the FAST coding region. In the cell culture, no syncytia were observed, which is in concordance with molecular findings of the absent FAST coding region.
DISCUSSION
The case described in this work was presented with acute gastroenteritis requiring hospitalization in a 17-month-old boy, and MRVs were found in a stool sample as the only possible causative agent. There is a small possibility, though, of some other pathogen being involved in the disease (i.e., some parasites that were not tested on this occasion). However, the high concentration of MRV shedding in the stool of this child indicates acute infection and efficient replication of MRV in the gut. No additional stool samples were available for analysis to check the excretion's dynamic and possible asymptomatic carriage of reoviruses. Also, no acute-phase serum sample was stored for additional tests to serologically confirm the infection. As MRV infections are common in humans and the seroprevalence in early childhood from 1 to 5 years of age was shown to be 8 to 50% (35), we believe that serum reactivity to other MRVs besides the isolate SI-MRV01 should also be tested to exclude possible cross-reactivity. Although there was no serological proof of MRV infection causing gastroenteritis, we have presented data that demonstrate MRV as the possible causative agent of the disease.
It is well known that the disadvantage of electron microscopy is its low sensitivity. For successful detection of viruses with electron microscopy, the concentration of viral particles should be higher than 106 per milliliter of suspension (36). Bearing in mind the dilution factor of 10 (when preparing a 10% stool suspension), this means that the MRV concentration in the patient's stool sample was at least of 107 viruses per milliliter or even higher. Moreover, the viruses found in the stool sample were infective, since they were successfully propagated in a cell culture. The high concentration of reoviruses in stool sample was confirmed also by the theoretically calculated TCID50/ml, which was as high as 2.26 × 107. All these facts suggest high replication activity of MRV in the patient's gut.
After the MRV isolate was cultured and genotyped, the parents were contacted with regard to the child's potential animal exposure. The family lived in a renovated house in a village close to the city, where no bats had been observed. The only animal that had close contact with the child was a dog at the grandparents' house, but the animal was predominantly kept indoors. At the time of the child's illness, none of the family members experienced similar symptoms. The parents stated that, at the time of the disease, the child was known to ingest nonfood items (e.g., mild form of pica). According to the reovirus inactivation studies, showing their persistent stability in environment, indirect infection through contaminated surfaces could also be possible (2, 17, 37). The source of infection therefore remains unknown. Unfortunately, no bat stool samples from the child's residence were available for analysis to investigate the possible source of infection. It would be interesting in the future to screen bats in Slovenia for reoviruses, in order to have a clear picture of reovirus molecular epidemiology and to link possible zoonotic transmissions, such as the one described in our case.
MRVs were traditionally believed to be causative agents of mild respiratory and enteric infections, without significant clinical impact (1). However, in the last decade or two, increasing reports on severe human infections with reoviruses have been published, including central nervous system involvement (10, 38, 39). The pathogenesis of reoviruses is not yet elucidated in detail, but some parameters influencing tissue tropism, efficient spread within the host, and severe outcomes of infection are already known (31–33). It is thus important for diagnostic personnel to be aware of a possible reovirus etiology and to have tools to prove this infection. However, any reovirus-positive result should be interpreted carefully, with a broad spectrum of cotested pathogens and clinical picture of the patient. Whether or not zoonotic transmission of reoviruses is one of the possible factors associated with a severe clinical outcome is not yet clear. Nevertheless, there are reports in the literature of bat reoviruses in humans with severe clinical manifestations. Those reovirus isolates, though, all clustered to Pteropine reovirus species and not to MRV species (6–8). This study is the first description of a bat MRV found in humans. Probably MRVs are widely distributed among bat species, since they have been found in insectivorous bats in Germany and in Italy (11, 12).
Whole-genome sequence comparison of our strain to MRVs available in GenBank clearly shows that it is most closely related to MRVs found in bats in Germany. This was supported by high nucleotide and amino acid identities, phylogenetic analysis of separate genome segments, and whole-genome sequences, including SimPlot analysis. Genome reassortment is indicated by a similarity plot of reovirus genome sequences most closely related to the Slovenian SI-MRV01 strain (Fig. 4). A sharp decrease in nucleotide sequence identities for some of the genome segments was observed, like the L2 and S1 segments, comparing SI-MRV01 to the GD-1 and MPC/04 strains, and the S2 segment, comparing SI-MRV01 to the 342/8 strain. Unfortunately, no other strains with higher nucleotide identity were found in GenBank to explain this diversity or possible reassortment events.
Finding an MRV in the stool sample of a hospitalized child with diarrhea was surprising for us, since no such case had previously been described in our laboratory. In the cell culture unit, no previous work with reoviruses was performed, there was no reovirus nucleic acid isolated in the molecular laboratory, nor had a reovirus genome amplification project been performed. Thus, the possibility of sample contamination is negligible. In addition to antigen detection tests and real-time RT-PCR assays for the detection of the most common viral causes of gastroenteritis, electron microscopy is still an important diagnostic tool routinely used in our laboratory. It is possible with the electron microscope to detect various viruses associated with rare cases, not included in specific antigen and/or molecular testing procedures. It is a “catch-all” method, providing a broad-spectrum analysis of examined samples (40). For rapid and accurate diagnostics, combination of classical and new techniques in diagnostic virology is often needed. This study demonstrates a good case of such an interplay. Moreover, an improvement in sample preparation for successful downstream application was introduced with virus enrichment using CIM monolithic support. It was shown that gradient elution, used in sample 3 without Benzonase treatment, in principle allows more detailed separation between the eluted molecules, including viruses and nucleic acids. In addition, virus particles were concentrated in specific fractions after chromatographic separation. This was confirmed also by sequencing results. In sample 3, the total number of reads was the lowest, but the percentage of orthoreovirus reads was the highest.
Summarizing the results of our study, there are two major outputs of this work. First, the detection of an MRV with high identity to MRV isolates found in European bats is an important indicator of bat-to-human interspecies transmission, as was already speculated by German and Italian groups (11, 12). The real epidemiological situation regarding MRVs in bats and their transmission to other animal species should be further investigated in order to understand the full zoonotic potential and pathogenesis of these viruses. The second output is a tool for improving the sample pretreatment in the search for enrichment in viral particles and nucleic acid. The pretreatment step is essential in NGS application, in which the target nucleic acid is expected to be present with high background. The described CIM chromatographic approach demonstrates an excellent method combining concentration and purification of the target, providing a new potential shortcut for NGS application in diagnostic clinical virology.
Supplementary Material
ACKNOWLEDGMENTS
We thank Irena Šest for technical support with electron microscopy, diagnostics, and sample preparation and Snežana Kramar for assistance with cell culture propagation.
This work was financially supported by the Slovenian Research Agency (contracts no. L2-4314 and J3-4252).
Footnotes
Published ahead of print 11 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01531-13.
REFERENCES
- 1.Schiff LA, Nibert ML, Tyler KL. 2007. Orthoreoviruses and their replication, p 1854–1915 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2.Katz BD, Margolin AB. 2007. Inactivation of hepatitis A HM-175/18f, reovirus T1 Lang and MS2 during alkaline stabilization of human biosolids. J. Appl. Microbiol. 103:2225–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day JM. 2009. The diversity of the orthoreoviruses: molecular taxonomy and phylogenetic divides. Infection, genetics and evolution. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 9:390–400 [DOI] [PubMed] [Google Scholar]
- 4.Sabin AB. 1959. Reoviruses. Science 130:1387–1389 [DOI] [PubMed] [Google Scholar]
- 5.Ouattara LA, Barin F, Barthez MA, Bonnaud B, Roingeard P, Goudeau A, Castelnau P, Vernet G, Paranhos-Baccala G, Komurian-Pradel F. 2011. Novel human reovirus isolated from children with acute necrotizing encephalopathy. Emerg. Infect. Dis. 17:1436–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chua KB, Voon K, Crameri G, Tan HS, Rosli J, McEachern JA, Suluraju S, Yu M, Wang LF. 2008. Identification and characterization of a new orthoreovirus from patients with acute respiratory infections. PLoS One 3:e3803. 10.1371/journal.pone.0003803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua KB, Voon K, Yu M, Keniscope C, Abdul Rasid K, Wang LF. 2011. Investigation of a potential zoonotic transmission of orthoreovirus associated with acute influenza-like illness in an adult patient. PLoS One 6:e25434. 10.1371/journal.pone.0025434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng P, Lau CS, Lai A, Ho E, Leung P, Chan F, Wong A, Lim W. 2009. A novel reovirus isolated from a patient with acute respiratory disease. J. Clin. Virol. 45:79–80 [DOI] [PubMed] [Google Scholar]
- 9.Hermann L, Embree J, Hazelton P, Wells B, Coombs RT. 2004. Reovirus type 2 isolated from cerebrospinal fluid. Pediatr. Infect. Dis. J. 23:373–375 [DOI] [PubMed] [Google Scholar]
- 10.Tyler KL, Barton ES, Ibach ML, Robinson C, Campbell JA, O'Donnell SM, Valyi-Nagy T, Clarke P, Wetzel JD, Dermody TS. 2004. Isolation and molecular characterization of a novel type 3 reovirus from a child with meningitis. J. Infect. Dis. 189:1664–1675 [DOI] [PubMed] [Google Scholar]
- 11.Kohl C, Lesnik R, Brinkmann A, Ebinger A, Radonic A, Nitsche A, Muhldorfer K, Wibbelt G, Kurth A. 2012. Isolation and characterization of three mammalian orthoreoviruses from European bats. PLoS One 7:e43106. 10.1371/journal.pone.0043106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lelli D, Moreno A, Lavazza A, Bresaola M, Canelli E, Boniotti MB, Cordioli P. 2013. Identification of mammalian orthoreovirus type 3 in Italian bats. Zoonoses Public Health 60:84–92 [DOI] [PubMed] [Google Scholar]
- 13.Decaro N, Campolo M, Desario C, Ricci D, Camero M, Lorusso E, Elia G, Lavazza A, Martella V, Buonavoglia C. 2005. Virological and molecular characterization of a mammalian orthoreovirus type 3 strain isolated from a dog in Italy. Vet. Microbiol. 109:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodder WJ, de Roda Husman AM. 2005. Presence of noroviruses and other enteric viruses in sewage and surface waters in The Netherlands. Appl. Environ. Microbiol. 71:1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodder WJ, van den Berg HH, Rutjes SA, de Roda Husman AM. 2010. Presence of enteric viruses in source waters for drinking water production in The Netherlands. Appl. Environ. Microbiol. 76:5965–5971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spinner ML, Di Giovanni GD. 2001. Detection and identification of mammalian reoviruses in surface water by combined cell culture and reverse transcription-PCR. Appl. Environ. Microbiol. 67:3016–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irving LG, Smith FA. 1981. One-year survey of enteroviruses, adenoviruses, and reoviruses isolated from effluent at an activated-sludge purification plant. Appl. Environ. Microbiol. 41:51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong S, Lau S, Woo P, Yuen KY. 2007. Bats as a continuing source of emerging infections in humans. Rev. Med. Virol. 17:67–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang LF, Walker PJ, Poon LL. 2011. Mass extinctions, biodiversity and mitochondrial function: are bats ‘special’ as reservoirs for emerging viruses? Curr. Opin. Virol. 1:649–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipkin WI. 2013. The changing face of pathogen discovery and surveillance. Nat. Rev. Microbiol. 11:133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capobianchi MR, Giombini E, Rozera G. 2013. Next-generation sequencing technology in clinical virology. Clin. Microbiol. Infect. 19:15–22 [DOI] [PubMed] [Google Scholar]
- 22.Beerenwinkel N, Gunthard HF, Roth V, Metzner KJ. 2012. Challenges and opportunities in estimating viral genetic diversity from next-generation sequencing data. Front. Microbiol. 3:329. 10.3389/fmicb.2012.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutierrez-Aguirre I, Steyer A, Banjac M, Kramberger P, Poljsak-Prijatelj M, Ravnikar M. 2011. On-site reverse transcription-quantitative polymerase chain reaction detection of rotaviruses concentrated from environmental water samples using methacrylate monolithic supports. J. Chromatogr. A 1218:2368–2373 [DOI] [PubMed] [Google Scholar]
- 24.Kovac K, Gutierrez-Aguirre I, Banjac M, Peterka M, Poljsak-Prijatelj M, Ravnikar M, Mijovski JZ, Schultz AC, Raspor P. 2009. A novel method for concentrating hepatitis A virus and caliciviruses from bottled water. J. Virol. Methods 162:272–275 [DOI] [PubMed] [Google Scholar]
- 25.Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, Takeda N, Katayama K. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 41:1548–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svraka S, van der Veer B, Duizer E, Dekkers J, Koopmans M, Vennema H. 2009. Novel approach for detection of enteric viruses to enable syndrome surveillance of acute viral gastroenteritis. J. Clin. Microbiol. 47:1674–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed LJ, Muench H. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. (Lond.) 27:493–497 [Google Scholar]
- 28.Zheng YZ, Webb R, Greenfield PF, Reid S. 1996. Improved method for counting virus and virus like particles. J. Virol. Methods 62:153–159 [DOI] [PubMed] [Google Scholar]
- 29.Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 30.Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, Ingersoll R, Sheppard HW, Ray SC. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73:152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassel-Duby R, Spriggs DR, Tyler KL, Fields BN. 1986. Identification of attenuating mutations on the reovirus type 3 S1 double-stranded RNA segment with a rapid sequencing technique. J. Virol. 60:64–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaye KM, Spriggs DR, Bassel-Duby R, Fields BN, Tyler KL. 1986. Genetic basis for altered pathogenesis of an immune-selected antigenic variant of reovirus type 3 (Dearing). J. Virol. 59:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chappell JD, Barton ES, Smith TH, Baer GS, Duong DT, Nibert ML, Dermody TS. 1998. Cleavage susceptibility of reovirus attachment protein sigma1 during proteolytic disassembly of virions is determined by a sequence polymorphism in the sigma1 neck. J. Virol. 72:8205–8213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shmulevitz M, Duncan R. 2000. A new class of fusion-associated small transmembrane (FAST) proteins encoded by the non-enveloped fusogenic reoviruses. EMBO J. 19:902–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tai JH, Williams JV, Edwards KM, Wright PF, Crowe JE, Jr, Dermody TS. 2005. Prevalence of reovirus-specific antibodies in young children in Nashville, Tennessee. J. Infect. Dis. 191:1221–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hazelton PR, Gelderblom HR. 2003. Electron microscopy for rapid diagnosis of infectious agents in emergent situations. Emerg. Infect. Dis. 9:294–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward RL, Ashley CS. 1978. Heat inactivation of enteric viruses in dewatered wastewater sludge. Appl. Environ. Microbiol. 36:898–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyler KL. 1998. Pathogenesis of reovirus infections of the central nervous system. Curr. Top. Microbiol. Immunol. 233:93–124 [DOI] [PubMed] [Google Scholar]
- 39.Johansson PJ, Sveger T, Ahlfors K, Ekstrand J, Svensson L. 1996. Reovirus type 1 associated with meningitis. Scand. J. Infect. Dis. 28:117–120 [DOI] [PubMed] [Google Scholar]
- 40.Biel SS, Gelderblom HR. 1999. Diagnostic electron microscopy is still a timely and rewarding method. J. Clin. Virol. 13:105–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.