Abstract
This meta-analysis evaluated preoperative aspiration culture for diagnosing prosthetic joint infection (PJI) in total hip arthroplasty (THA) and total knee arthroplasty (TKA). The pooled sensitivity and specificity were 0.72 (95% confidence interval, 0.65 to 0.78) and 0.95 (0.93 to 0.97), respectively. Subgroup analyses revealed nonsignificant worse diagnostic performance for THA than for TKA (sensitivity, 0.70 versus 0.78; specificity, 0.94 versus 0.96). Preoperative aspiration culture has moderate to high sensitivity and very high specificity for diagnosing PJI.
TEXT
Prosthetic joint infection (PJI) is a common and challenging complication for both patients and surgeons (1–4). The incidence of PJI after total joint arthroplasty (TJA) is 1 to 12% (5, 6). A multitude of preoperative tests are available to clinicians for diagnosing PJI, including preoperative laboratory testing and radiological examination (7). However, the limited sensitivity and specificity of these tests pose difficulties in distinguishing between PJI and other causes of joint failure, such as aseptic loosening (1, 8). Guidelines by the American Academy of Orthopaedic Surgeons (AAOS) and Infectious Diseases Society of America (IDSA) strongly recommend preoperative aspiration culture for assessment for PJI (9, 10). In recent years, several studies have assessed the diagnostic value of preoperative aspiration culture for PJI. However, the sensitivities (range, 0 to 1) and specificities (range, 0.54 to 1) among studies are inconsistent (7, 11–43). We therefore performed a meta-analysis for evaluating the detection validity of preoperative aspiration culture in the diagnosis of PJI.
We searched Medline, Embase, and Ovid from 1 January 1990 through 1 May 2013 with combined search terms using medical subject headings (MeSH) or free-text words: (i) “aspiration,” “aspirate,” or “synovial fluid” and (ii) “joint prosthesis,” “prosthesis infection,” “septic loosening,” “aseptic loosening,” “replacement,” or “arthroplasty.” We also manually searched related review articles and the reference lists of eligible studies. The reviewers independently evaluated the selected studies using the following inclusion criteria: (i) accuracy of preoperative aspiration culture, in comparison with visible purulence of the surgical site, presence of a sinus tract (fistula) communicating with the prosthesis, acute inflammation in histopathology sections of periprosthetic tissue, or simultaneously obtained microbiologic cultures from at least two periprosthetic tissue samples (the reference standard), for the diagnosis of joint infection; (ii) sufficient data to allow us to calculate the true-positive (TP), false-negative (FN), false-positive (FP), and true-negative (TN) values; and (iii) ≥10 patients with data extraction using a standardized data collection form (X.Q., Z.Z., and C.W.). If different studies included the same patients, we used the one that was the most detailed. Discrepancies were resolved by discussion with other investigators and consulting the original articles (Z.Z. and K.D.).
We estimated the sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the curve (AUC) of summary receiver-operating characteristic curves to evaluate the capability of preoperative aspiration culture assays in diagnosing PJI. For each study, we constructed a 2-by-2 contingency table consisting of TP, FN, FP, and TN results. We then calculated sensitivity as TP/(TP + FN), specificity as TN/(FP + TN), DOR as (TP × TN)/(FP × FN), PLR as sensitivity/(1 − specificity), and NLR as (1 − sensitivity)/specificity. We performed subgroup analyses to assess potential heterogeneity using the following stratification: type of arthroplasty (total hip arthroplasty versus total knee arthroplasty), publication year (<2002 versus ≥2002), geographical location (United States versus Europe), number of patients (<100 versus ≥100), study design (prospective versus retrospective), patient enrollment (consecutive versus not provided). We also constructed Deeks' funnel plot asymmetry test to evaluate potential publication bias. All statistical analyses were performed using STATA version 11 (StataCorp, College Station, TX, USA). P values of <0.05 were considered statistically significant.
We scanned 2,179 titles and abstracts, of which we excluded 1,970 studies during the first phase of our selection strategy. During the second phase (full-text review), we excluded 175 studies. A total of 34 articles, comprising 3,332 patients, fulfilled all inclusion criteria and were subjected to analysis (see Table S1 in the supplemental material). Twenty-one studies detected PJI in total hip arthroplasty (THA), 4 in total knee arthroplasty (TKA), and 9 in both THA and TKA. Thirteen studies enrolled patients prospectively. Patient enrolments were consecutive in 11 studies and were not documented in 23. We found significant heterogeneity for all test performances.
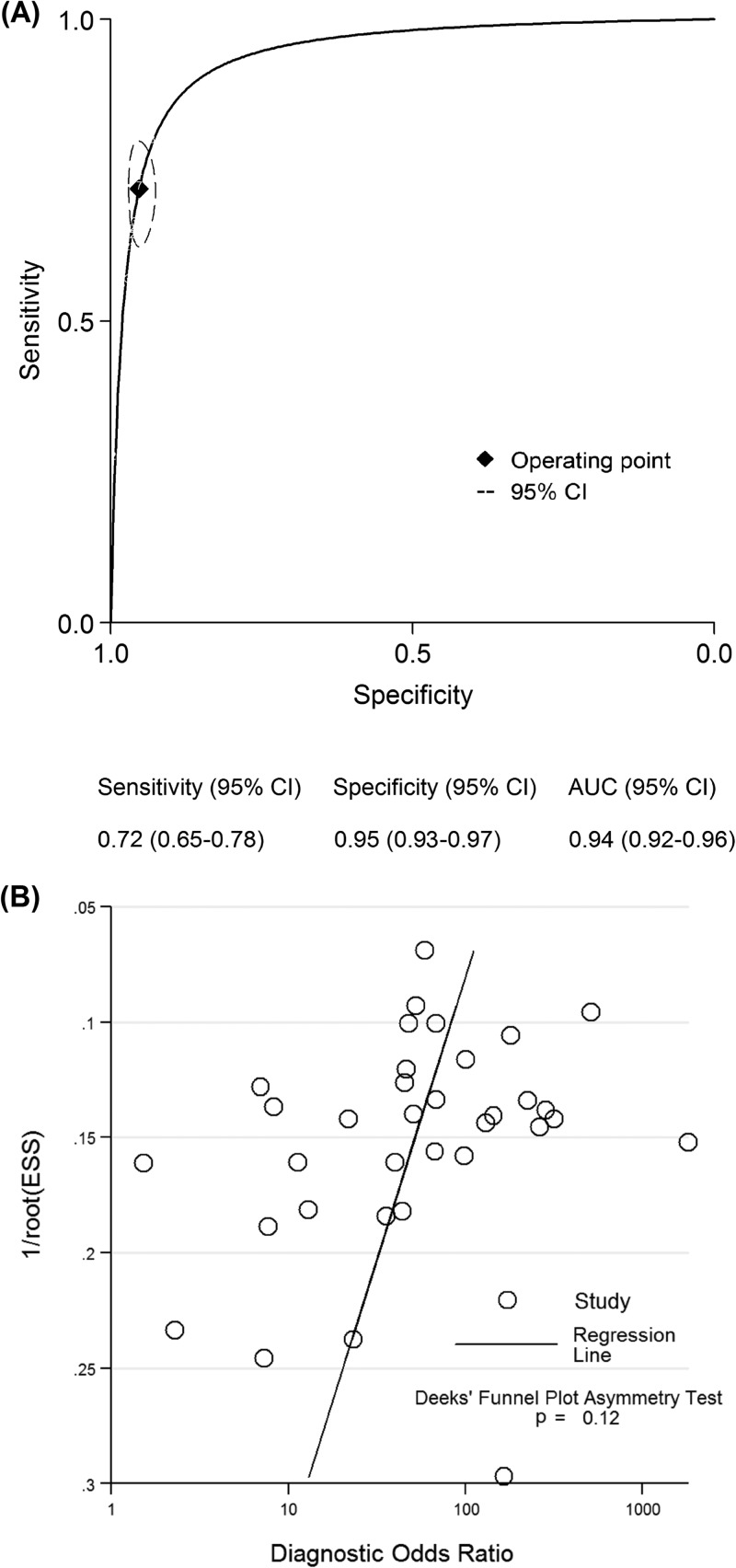
The pooled sensitivity, specificity, PLR, NLR, DOR, and AUC estimates for the detection of PJI using preoperative aspiration culture were 0.72 (95% confidence interval [CI], 0.65 to 0.78), 0.95 (95% CI, 0.93 to 0.97), 15.3 (95% CI, 10.6 to 22.1), 0.29 (95% CI, 0.23 to 0.38), 52 (95% CI, 31 to 86), and 0.94 (95% CI, 0.92 to 0.96), respectively (Fig. 1A). The Deeks' funnel plot asymmetry test found no evidence of a small-study effect for preoperative aspiration culture (P = 0.12) (Fig. 1B). In subgroup analyses, test performances varied by the type of arthroplasty, publication year, geographical location, patient number, study design, and patient enrollment (Table 1). The sensitivity and specificity of THA were 0.70 (95% CI, 0.59 to 0.79) and 0.94 (95% CI, 0.91 to 0.96), and those of TKA were 0.78 (95% CI, 0.60 to 0.90) and 0.96 (95% CI, 0.70 to 1.00), respectively. Prospective studies revealed a nonsignificantly lower sensitivity of 0.69 (95% CI, 0.58 to 0.78) compared to retrospective studies.
Fig 1.
Summary ROC curves (A) and funnel plots (B) for preoperative aspiration culture. Curves include a summary operating point for sensitivity and specificity on the curve and a 95% confidence contour ellipsoid.
Table 1.
Accuracy estimates from subgroup analyses
| Targeted study characteristic | No. of studies | No. of patients | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | PLR (95% CI) | NLR (95% CI) | DOR |
|---|---|---|---|---|---|---|---|---|
| Overall studies | 34 | 3,332 | 0.72 (0.65–0.78) | 0.95 (0.93–0.97) | 0.94 (0.92–0.96) | 15.3 (10.6–22.1) | 0.29 (0.23–0.38) | 52 (31–86) |
| Type of arthroplasty | ||||||||
| Total hip arthroplasty | 21 | 2,134 | 0.70 (0.59–0.79) | 0.94 (0.91–0.96) | 0.94 (0.91–0.95) | 11.3 (7.7–16.7) | 0.32 (0.23–0.45) | 35 (19–66) |
| Total knee arthroplasty | 4 | 332 | 0.78 (0.60–0.90) | 0.96 (0.70–1.00) | 0.90 (0.88–0.93) | 21.9 (1.8–262.1) | 0.22 (0.11–0.47) | 97 (5–2010) |
| Publication year | ||||||||
| <2002 | 17 | 1,613 | 0.74 (0.60–0.84) | 0.95 (0.93–0.96) | 0.96 (0.93–0.97) | 14.3 (9.7–21.1) | 0.28 (0.18–0.44) | 51 (25–104) |
| ≥2002 | 17 | 1,719 | 0.71 (0.63–0.78) | 0.96 (0.92–0.98) | 0.90 (0.87–0.92) | 17.1 (8.2–35.8) | 0.30 (0.23–0.40) | 56 (23–136) |
| Geographical location | ||||||||
| United States | 10 | 1,264 | 0.73 (0.57–0.85) | 0.96 (0.92–0.98) | 0.96 (0.93–0.97) | 19.2 (9.5–38.6) | 0.28 (0.17–0.47) | 69 (26–178) |
| Europe | 20 | 1,776 | 0.73 (0.65–0.80) | 0.95 (0.92–0.97) | 0.93 (0.91–0.95) | 15.1 (9.0–25.6) | 0.28 (0.21–0.37) | 54 (27–108) |
| No. of patients | ||||||||
| <100 | 21 | 1,261 | 0.70 (0.59–0.79) | 0.95 (0.92–0.97) | 0.94 (0.91–0.95) | 14.7 (8.5–25.5) | 0.31 (0.22–0.45) | 47 (21–102) |
| ≥100 | 13 | 2,071 | 0.76 (0.67–0.83) | 0.95 (0.92–0.97) | 0.95 (0.92–0.96) | 15.9 (10.1–25.0) | 0.26 (0.19–0.35) | 62 (36–107) |
| Study design | ||||||||
| Prospective | 13 | 1,285 | 0.69 (0.58–0.78) | 0.96 (0.92–0.98) | 0.93 (0.90–0.95) | 15.8 (8.6–28.9) | 0.33 (0.23–0.45) | 49 (23–104) |
| Retrospective | 15 | 1,421 | 0.71 (0.56–0.83) | 0.96 (0.92–0.98) | 0.95 (0.93–0.97) | 17.6 (8.7–35.6) | 0.30 (0.19–0.49) | 58 (21–161) |
| Patients enrollment | ||||||||
| Consecutive | 11 | 1,356 | 0.71 (0.52–0.85) | 0.94 (0.91–0.96) | 0.95 (0.92–0.96) | 11.6 (7.3–18.6) | 0.31 (0.17–0.55) | 38 (15–97) |
| Not provided | 23 | 1,967 | 0.72 (0.65–0.78) | 0.96 (0.93–0.98) | 0.91 (0.89–0.93) | 18.3 (10.2–32.8) | 0.29 (0.23–0.37) | 63 (31–127) |
This meta-analysis showed that preoperative aspiration culture had moderate to high sensitivity (72%) and very high specificity (95%) for diagnosing PJI, which is acceptable for clinical practice (Fig. 2).
Fig 2.
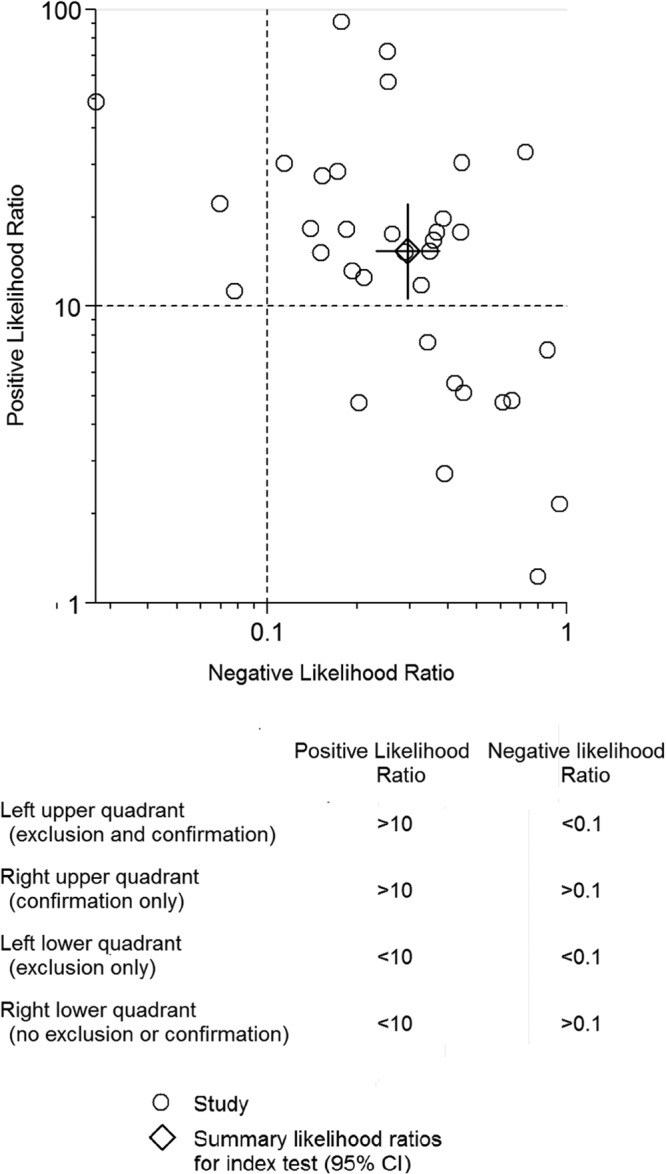
Likelihood ratio scattergram for preoperative aspiration culture. The likelihood ratio profile shows that preoperative aspiration culture is a potent tool for ruling out PJI in this patient population.
The diagnosis of PJI after TJA remains a challenge (1, 7, 10). Of the numerous preoperative tests available—including white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels—no test has perfect sensitivity and specificity (1, 7, 10, 44). However, their diagnostic ability is not entirely reliable; a recent meta-analysis showed that the sensitivity and specificity of WBC count, ESR, and CRP levels were 45%, 75%, and 88% and 87%, 70%, and 74%, respectively (44). While fluorodeoxyglucose positron emission tomography (FDG-PET) (sensitivity, 82%; specificity, 87%) and antigranulocyte scintigraphy with 99mTc-labeled monoclonal antibodies (sensitivity, 83%; specificity, 80%) show good diagnostic capabilities (45, 46), these tests are expensive, complex, and need special operators, limiting their clinical application.
Moreover, we must highlight that with a joint aspiration sample, culture and leukocyte counts and percentages of neutrophils can be realized. Several studies have assessed the diagnostic value of preoperative aspiration leukocyte count and percentages of neutrophils for PJI. The sensitivity of aspiration leukocyte count ranges from 36% to 100%, with specificity from 60% to 99% (35, 47–50). And the sensitivity of aspiration percentages of neutrophils ranges from 71% to 98%, with specificity from 62% to 98% (35, 47, 48, 51, 52). Furthermore, low-grade infections caused by low-virulent microorganisms usually have normal values of inflammatory markers. So it is important to perform preoperative aspiration culture if there is a high suspicion of PJI even though values of inflammatory markers are normal.
Guidelines by the AAOS and IDSA strongly recommend preoperative aspiration culture for detecting PJI. Our results demonstrate that preoperative aspiration culture is a diagnostic method with very high specificity, in agreement with the AAOS and IDSA guidelines. However, the true diagnostic ability of preoperative aspiration cultures depends on whether bacteria are accurately recovered from synovial fluid aspirate (8, 53), which is influenced by various factors, including synovial fluid volume, antibiotic use, and specimen contamination. Therefore, occasional false-positive results may induce a moderate sensitivity.
Our study has certain limitations. First, the reference standards in the included studies varied, with no established gold standard. Misclassification bias resulting from imperfect reference standards may affect the estimates of diagnostic accuracy of a tested method (45). Second, 13 studies were prospectively designed. Study design was assessed as a potential source of heterogeneity; however, subgroup analysis showed that prospective study design did not significantly influence the sensitivity. Third, the summary results of this meta-analysis had high statistical heterogeneity. Although thorough subgroup analyses were included to investigate possible sources of heterogeneity, no causes of heterogeneity were revealed. These issues may reduce the strength of the conclusions drawn from this meta-analysis.
In conclusion, the meta-analysis indicates that preoperative aspiration culture may play a role in the diagnosis of PJI; however, identifying the optimal combination of diagnostic tests for PJI needs further studies.
Supplementary Material
ACKNOWLEDGMENTS
We have no conflicts of interest to declare.
This work was supported by the Fund for Key National Basic Research Program of China (grant no. 2012CB619101), Major Basic Research of Science and Technology Commission of Shanghai Municipality (grant no. 11DJ1400303), and Key Disciplines of Shanghai Municipal Education Commission (grant no. J50206).
Footnotes
Published ahead of print 14 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01467-13.
REFERENCES
- 1.Bozic KJ, Ward DT, Lau EC, Chan V, Wetters NG, Naziri Q, Odum S, Fehring TK, Mont MA, Gioe TJ, Della Valle CJ. 2013. Risk factors for periprosthetic joint infection following primary total hip arthroplasty: a case control study. J. Arthroplasty. [Epub ahead of print.] 10.1016/j.arth.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 2.Cazanave C, Greenwood-Quaintance KE, Hanssen AD, Karau MJ, Schmidt SM, Gomez Urena EO, Mandrekar JN, Osmon DR, Lough LE, Pritt BS, Steckelberg JM, Patel R. 2013. Rapid molecular microbiologic diagnosis of prosthetic joint infection. J. Clin. Microbiol. 51:2280–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez E, Cazanave C, Cunningham SA, Greenwood-Quaintance KE, Steckelberg JM, Uhl JR, Hanssen AD, Karau MJ, Schmidt SM, Osmon DR, Berbari EF, Mandrekar J, Patel R. 2012. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J. Clin. Microbiol. 50:3501–3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parvizi J, Ghanem E, Menashe S, Barrack RL, Bauer TW. 2006. Periprosthetic infection: what are the diagnostic challenges? J. Bone Joint Surg. Am. 88(Suppl 4):138–147 [DOI] [PubMed] [Google Scholar]
- 5.Alijanipour P, Bakhshi H, Parvizi J. 2013. Diagnosis of periprosthetic joint infection: the threshold for serological markers. Clin. Orthop. Relat. Res. 471:3186–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Kakis A, Nichols A, Ries MD, Vail TP, Bozic KJ. 4 May 2013. Targeted use of vancomycin as perioperative prophylaxis reduces periprosthetic joint infection in revision TKA. Clin. Orthop. Relat. Res. 10.1007/s11999-013-3029-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N. Engl. J. Med. 357:654–663 [DOI] [PubMed] [Google Scholar]
- 8.Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. 2013. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin. Orthop. Relat. Res. 471:3002–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal VK, Higuera C, Deirmengian G, Parvizi J, Austin MS. 2013. Swab cultures are not as effective as tissue cultures for diagnosis of periprosthetic joint infection. Clin. Orthop. Relat. Res. 471:3196–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zmistowski B, Tetreault MW, Alijanipour P, Chen AF, Della Valle CJ, Parvizi J. 12 April 2013. Recurrent periprosthetic joint infection: persistent or new infection? J. Arthroplasty. [Epub ahead of print.] 10.1016/j.arth.2013.02.021 [DOI] [PubMed] [Google Scholar]
- 11.Ali F, Wilkinson JM, Cooper JR, Kerry RM, Hamer AJ, Norman P, Stockley I. 2006. Accuracy of joint aspiration for the preoperative diagnosis of infection in total hip arthroplasty. J. Arthroplasty 21:221–226 [DOI] [PubMed] [Google Scholar]
- 12.Barrack RL, Harris WH. 1993. The value of aspiration of the hip joint before revision total hip arthroplasty. J. Bone Joint Surg. Am. 75:66–76 [DOI] [PubMed] [Google Scholar]
- 13.Barrack RL, Jennings RW, Wolfe MW, Bertot AJ. 1997. The coventry award. The value of preoperative aspiration before total knee revision. Clin. Orthop. Relat. Res. 1997:8–16 [PubMed] [Google Scholar]
- 14.Battaglia M, Vannini F, Guaraldi F, Rossi G, Biondi F, Sudanese A. 2011. Validity of preoperative ultrasound-guided aspiration in the revision of hip prosthesis. Ultrasound Med. Biol. 37:1977–1983 [DOI] [PubMed] [Google Scholar]
- 15.Bernard L, Lubbeke A, Stern R, Bru JP, Feron JM, Peyramond D, Denormandie P, Arvieux C, Chirouze C, Perronne C, Hoffmeyer P. 2004. Value of preoperative investigations in diagnosing prosthetic joint infection: retrospective cohort study and literature review. Scand. J. Infect. Dis. 36:410–416 [DOI] [PubMed] [Google Scholar]
- 16.Duff GP, Lachiewicz PF, Kelley SS. 1996. Aspiration of the knee joint before revision arthroplasty. Clin. Orthop. Relat. Res.:132–139 [DOI] [PubMed] [Google Scholar]
- 17.Eisler T, Svensson O, Engstrom CF, Reinholt FP, Lundberg C, Wejkner B, Schmalholz A, Elmstedt E. 2001. Ultrasound for diagnosis of infection in revision total hip arthroplasty. J. Arthroplasty 16:1010–1017 [DOI] [PubMed] [Google Scholar]
- 18.Fehring TK, Cohen B. 1996. Aspiration as a guide to sepsis in revision total hip arthroplasty. J. Arthroplasty 11:543–547 [DOI] [PubMed] [Google Scholar]
- 19.Fink B, Gebhard A, Fuerst M, Berger I, Schafer P. 2013. High diagnostic value of synovial biopsy in periprosthetic joint infection of the hip. Clin. Orthop. Relat. Res. 471:956–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fink B, Makowiak C, Fuerst M, Berger I, Schafer P, Frommelt L. 2008. The value of synovial biopsy, joint aspiration and C-reactive protein in the diagnosis of late peri-prosthetic infection of total knee replacements. J. Bone Joint Surg. Br. 90:874–878 [DOI] [PubMed] [Google Scholar]
- 21.Glithero PR, Grigoris P, Harding LK, Hesslewood SR, McMinn DJ. 1993. White cell scans and infected joint replacements. Failure to detect chronic infection. J. Bone Joint Surg. Br. 75:371–374 [DOI] [PubMed] [Google Scholar]
- 22.Itasaka T, Kawai A, Sato T, Mitani S, Inoue H. 2001. Diagnosis of infection after total hip arthroplasty. J. Orthop. Sci. 6:320–326 [DOI] [PubMed] [Google Scholar]
- 23.Klatte TO, Meinicke R, O'Loughlin P, Rueger JM, Gehrke T, Kendoff D. 21 March 2013. Incidence of bacterial contamination in primary THA and combined hardware removal: analysis of preoperative aspiration and intraoperative biopsies. J. Arthroplasty. [Epub ahead of print.] 10.1016/j.arth.2013.02.017 [DOI] [PubMed] [Google Scholar]
- 24.Kraemer WJ, Saplys R, Waddell JP, Morton J. 1993. Bone scan, gallium scan, and hip aspiration in the diagnosis of infected total hip arthroplasty. J. Arthroplasty 8:611–616 [DOI] [PubMed] [Google Scholar]
- 25.Lachiewicz PF, Rogers GD, Thomason HC. 1996. Aspiration of the hip joint before revision total hip arthroplasty. Clinical and laboratory factors influencing attainment of a positive culture. J. Bone Joint Surg. Am. 78:749–754 [DOI] [PubMed] [Google Scholar]
- 26.Levitsky KA, Hozack WJ, Balderston RA, Rothman RH, Gluckman SJ, Maslack MM, Booth RE., Jr 1991. Evaluation of the painful prosthetic joint. Relative value of bone scan, sedimentation rate, and joint aspiration. J. Arthroplasty 6:237–244 [DOI] [PubMed] [Google Scholar]
- 27.Malhotra R, Morgan DA. 2004. Role of core biopsy in diagnosing infection before revision hip arthroplasty. J. Arthroplasty 19:78–87 [DOI] [PubMed] [Google Scholar]
- 28.Meermans G, Haddad FS. 2010. Is there a role for tissue biopsy in the diagnosis of periprosthetic infection? Clin. Orthop. Relat. Res. 468:1410–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulcahy DM, Fenelon GC, McInerney DP. 1996. Aspiration arthrography of the hip joint. Its uses and limitations in revision hip surgery. J. Arthroplasty 11:64–68 [DOI] [PubMed] [Google Scholar]
- 30.Muller M, Morawietz L, Hasart O, Strube P, Perka C, Tohtz S. 2008. Diagnosis of periprosthetic infection following total hip arthroplasty—evaluation of the diagnostic values of pre- and intraoperative parameters and the associated strategy to preoperatively select patients with a high probability of joint infection. J. Orthop. Surg. Res. 3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panousis K, Grigoris P, Butcher I, Rana B, Reilly JH, Hamblen DL. 2005. Poor predictive value of broad-range PCR for the detection of arthroplasty infection in 92 cases. Acta Orthop. 76:341–346 [PubMed] [Google Scholar]
- 32.Pons M, Angles F, Sanchez C, Matamala A, Cuchi E, Salavert M, Forcada P, Ferrer H. 1999. Infected total hip arthroplasty—the value of intraoperative histology. Int. Orthop. 23:34–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts P, Walters AJ, McMinn DJ. 1992. Diagnosing infection in hip replacements. The use of fine-needle aspiration and radiometric culture. J. Bone Joint Surg. Br. 74:265–269 [DOI] [PubMed] [Google Scholar]
- 34.Somme D, Ziza JM, Desplaces N, Chicheportiche V, Chazerain P, Leonard P, Lhotellier L, Jacquenod P, Mamoudy P. 2003. Contribution of routine joint aspiration to the diagnosis of infection before hip revision surgery. Joint Bone Spine 70:489–495 [DOI] [PubMed] [Google Scholar]
- 35.Spangehl MJ, Masri BA, O'Connell JX, Duncan CP. 1999. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J. Bone Joint Surg. Am. 81:672–683 [DOI] [PubMed] [Google Scholar]
- 36.Taylor T, Beggs I. 1995. Fine needle aspiration in infected hip replacements. Clin. Radiol. 50:149–152 [DOI] [PubMed] [Google Scholar]
- 37.Teller RE, Christie MJ, Martin W, Nance EP, Haas DW. 2000. Sequential indium-labeled leukocyte and bone scans to diagnose prosthetic joint infection. Clin. Orthop. Relat. Res. 2000:241–247 [DOI] [PubMed] [Google Scholar]
- 38.Tigges S, Stiles RG, Meli RJ, Roberson JR. 1993. Hip aspiration: a cost-effective and accurate method of evaluating the potentially infected hip prosthesis. Radiology 189:485–488 [DOI] [PubMed] [Google Scholar]
- 39.Tohtz SW, Muller M, Morawietz L, Winkler T, Perka C. 2010. Validity of frozen sections for analysis of periprosthetic loosening membranes. Clin. Orthop. Relat. Res. 468:762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trampuz A, Piper KE, Hanssen AD, Osmon DR, Cockerill FR, Steckelberg JM, Patel R. 2006. Sonication of explanted prosthetic components in bags for diagnosis of prosthetic joint infection is associated with risk of contamination. J. Clin. Microbiol. 44:628–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van den Bekerom MP, Stuyck J. 2006. The value of pre-operative aspiration in the diagnosis of an infected prosthetic knee: a retrospective study and review of literature. Acta Orthop. Belg. 72:441–447 [PubMed] [Google Scholar]
- 42.Virolainen P, Lahteenmaki H, Hiltunen A, Sipola E, Meurman O, Nelimarkka O. 2002. The reliability of diagnosis of infection during revision arthroplasties. Scand. J. Surg. 91:178–181 [DOI] [PubMed] [Google Scholar]
- 43.Williams JL, Norman P, Stockley I. 2004. The value of hip aspiration versus tissue biopsy in diagnosing infection before exchange hip arthroplasty surgery. J. Arthroplasty 19:582–586 [DOI] [PubMed] [Google Scholar]
- 44.Gollwitzer H, Dombrowski Y, Prodinger PM, Peric M, Summer B, Hapfelmeier A, Saldamli B, Pankow F, von Eisenhart-Rothe R, Imhoff AB, Schauber J, Thomas P, Burgkart R, Banke IJ. 2013. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J. Bone Joint Surg. Am. 95:644–651 [DOI] [PubMed] [Google Scholar]
- 45.Aggarwal VK, Rasouli MR, Parvizi J. 2013. Periprosthetic joint infection: current concept. Indian J. Orthop. 47:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrack RL, Berend KR, Cui Q, Fehring TK, Della Valle CJ, Gehrke T, Lombardi AV, Jr, Mont MA, Parvizi J, Springer BD. 2013. Cement spacers in periprosthetic joint infection. Clin. Infect. Dis. 57:328–329 [DOI] [PubMed] [Google Scholar]
- 47.Della Valle CJ, Sporer SM, Jacobs JJ, Berger RA, Rosenberg AG, Paprosky WG. 2007. Preoperative testing for sepsis before revision total knee arthroplasty. J. Arthroplasty 22:90–9317823024 [Google Scholar]
- 48.Ghanem E, Parvizi J, Burnett RS, Sharkey PF, Keshavarzi N, Aggarwal A, Barrack RL. 2008. Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J. Bone Joint Surg. Am. 90:1637–1643 [DOI] [PubMed] [Google Scholar]
- 49.Kusuma SK, Ward J, Jacofsky M, Sporer SM, Della Valle CJ. 2011. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin. Orthop. Relat. Res. 469:1002–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mason JB, Fehring TK, Odum SM, Griffin WL, Nussman DS. 2003. The value of white blood cell counts before revision total knee arthroplasty. J. Arthroplasty 18:1038–1043 [DOI] [PubMed] [Google Scholar]
- 51.Lee SC, Jung KA, Yoon JY, Nam CH, Hwang SH, Park IS. 2010. Analysis of synovial fluid in culture-negative samples of suspicious periprosthetic infections. Orthopedics 33:725. [DOI] [PubMed] [Google Scholar]
- 52.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. 2004. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am. J. Med. 117:556–562 [DOI] [PubMed] [Google Scholar]
- 53.Janz V, Wassilew GI, Hasart O, Matziolis G, Tohtz S, Perka C. 2013. Evaluation of sonicate fluid cultures in comparison to histological analysis of the periprosthetic membrane for the detection of periprosthetic joint infection. Int. Orthop. 37:931–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.