Abstract
A loop-mediated isothermal amplification (LAMP) assay targeting the mpb64 gene for the diagnosis of intraocular tuberculosis was highly specific (100%), sensitive (85.7%), rapid, and easy to perform. The LAMP assay can be an alternative to conventional PCR for the diagnosis of ocular tuberculosis in resource-limited settings.
TEXT
Intraocular tuberculosis (TB) is a disease with an extremely paucibacillary nature that is difficult to diagnose with conventional tests such as microscopy and culture. Molecular techniques such as PCR are vital for definitive diagnosis of this condition (1). However, PCR requires expensive instrumentation, which hinders its widespread use. The loop-mediated isothermal amplification (LAMP) assay relies on autocycling strand displacement DNA synthesis in the presence of Bst DNA polymerase under isothermal conditions and can be conducted in the laboratory water bath or heating block (2). We report the application of a LAMP assay targeting the mpb64 gene for rapid detection of intraocular tuberculosis.
The study was approved by the institutional review board. A set of six LAMP primers (Table 1) targeting the mpb64 gene in the Mycobacterium tuberculosis H37Rv strain (GenBank accession number NC_018143) were designed using primer explorer V4 software (http://primerexplorer.jp/elamp4.0.0/index.html). The mpb64 gene was chosen since significant number of clinical isolates of M. tuberculosis in India may have low numbers of copies (1, 2) or no copies (3) of the IS6110 gene element. Also, the sensitivity of mpb64 detection was found to be much higher than that of IS6110 detection during PCR analysis of ocular samples in our laboratory (unpublished data). The LAMP assay was standardized according to a previously published protocol (4), except that 2.0 μl instead of 1.5 μl of 50 mM MgSO4 was used. Nondenatured DNA was used as the template. The reaction mixture was incubated at 65°C for 1 h and heated at 80°C for 2 min to terminate the reaction in a thermal cycler (Veriti; Applied Biosystems), though the same could be achieved with a water bath. M. tuberculosis H37Rv DNA was used as the positive control and double-autoclaved Milli-Q water as the negative control. LAMP-positive amplicons were confirmed by adding 1 μl of (1,000×) SYBR green Ι dye (Sigma-Aldrich) to each reaction tube and incubating in the dark for 10 min, as previously described (5). A yellowish green color (under natural light conditions) indicated a positive reaction, while reddish orange indicated a negative reaction (Fig. 1). LAMP products were verified by 2% agarose gel electrophoresis with UV light transillumination. The analytical sensitivity of the LAMP assay was found to be 10 fg per reaction (2 copies/reaction), while analytical specificity was confirmed using DNA from different microorganisms, including Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Proteus mirabilis ATCC 12453, Pseudomonas aeruginosa ATCC 27853, Candida albicans ATCC 90028, and clinical isolates of Streptococcus pneumoniae, Klebsiella pneumoniae, Nocardia asteroides, Corynebacterium striatum, Mycobacterium fortuitum, Fusarium solani, Acanthamoeba species, herpes simplex virus 1, cytomegalovirus, human adenovirus (kindly provided by Clinical Virology Laboratory, Christian Medical College, Vellore, India), and varicella zoster virus Oka strain (Varilix live attenuated vaccine; Smith Kline Beecham, Belgium) and human leukocytes.
Table 1.
Primers used for M. tuberculosis LAMP assay targeting the mpb64 gene
| Primer name | Primer sequence (5′–3′) |
|---|---|
| MTB-F3 | CCCCCGGGTTGAAGAAGA |
| MTB-B3 | GGCCTATCGCAAGCCAAT |
| MTB-FIP | GTATCGATAGCGCCGAATGCCGTTTTCGTTCGTGACTGCGAAGT |
| MTB-BIP | TGCTTGCTCAGTTCACCTTGCATTTTCACCTATGACACGCTGTGG |
| MTB-FLP | GCTTGGACCCGGTGAATTATCAGA |
| MTB-BLP | AGCGGATCGGTGTCAGCCT |
Fig 1.
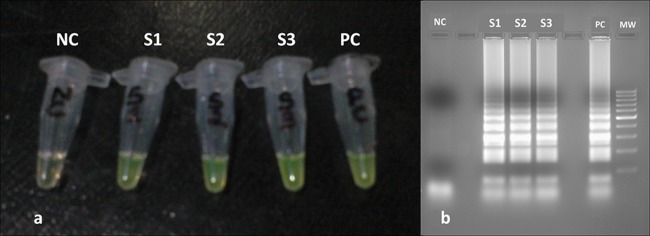
(a) Visual detection of LAMP-amplified products. Positive reactions indicated by a yellowish green color are seen in tubes S1, S2, and S3 (patient samples) and tube PC (positive control), while a reddish orange color indicates a negative reaction in tube NC (negative control). (b) Agarose gel electrophoresis (2%) picture of LAMP assays showing DNA amplification (ladder-like banding pattern) in lanes S1, S2, and S3 (patient samples) and lane PC (positive control). No amplification was detected in lane NC (negative control). MW (molecular weight), 100-bp DNA ladder.
For clinical verification, we included 14 intraocular samples (10 aqueous humor and 4 vitreous humor) collected from patients with presumed ocular TB (criteria described previously) (1). Twenty aqueous humor samples from patients undergoing cataract surgery were used as negative controls. DNA was extracted from all the samples using a QIAamp DNA minikit (Qiagen, Germany) and tested for M. tuberculosis DNA by the LAMP assay and multitarget PCR as described earlier (6). Of the 14 tested samples from patients with presumed ocular TB, 12 (85.7%) were positive by the LAMP assay and 9 (64.3%) by multitarget PCR (Table 2). The distribution of results between multitarget PCR and LAMP (PCR/LAMP) was as follows: positive/positive, 9; negative/negative, 2; positive/negative, 0; and negative/positive, 3. Considering clinical presentation as the gold standard, the sensitivity, specificity, and positive and negative predictive values of the LAMP assay were 85.7%, 100%, 100%, and 90.9%, respectively. No amplification was observed in 20 negative controls.
Table 2.
Results of multitarget PCR and LAMP assay
| Sample no. | Clinical diagnosis | Sample type | Multitarget PCR result | LAMP result |
|---|---|---|---|---|
| 1 | Anterior uveitis | Aqueous | Negative | Negative |
| 2 | Panuveitis | Vitreous | Positive | Positive |
| 3 | Retinal vasculitis | Aqueous | Negative | Positive |
| 4 | Panuveitis | Aqueous | Positive | Positive |
| 5 | Panuveitis | Aqueous | Positive | Positive |
| 6 | Anterior uveitis | Aqueous | Negative | Positive |
| 7 | Retinal vasculitis | Aqueous | Negative | Positive |
| 8 | Multifocal serpiginoid choroiditis | Aqueous | Negative | Negative |
| 9 | Intermediate uveitis | Vitreous | Positive | Positive |
| 10 | Retinal vasculitis | Aqueous | Positive | Positive |
| 11 | Retinal vasculitis | Vitreous | Positive | Positive |
| 12 | Retinal vasculitis | Vitreous | Positive | Positive |
| 13 | Panuveitis | Aqueous | Positive | Positive |
| 14 | Multifocal serpiginoid choroiditis | Aqueous | Positive | Positive |
LAMP assays have been used for detection of several types of infections, including ocular infections (4, 7). Our report is the first on its application in diagnosis of intraocular TB. We believe that LAMP assays can have wide application in cases of ocular TB, considering its extreme paucibacillary nature and the limited access to thermal cyclers in most countries where TB is endemic.
ACKNOWLEDGMENTS
We declare that have no proprietary or commercial interest in any materials discussed in this article.
This work was supported by the Department of Science and Technology, government of India, New Delhi, India, grant no. SR/FT/LS-15/2011, awarded to S.B.
P.K.B. is supported by a senior research fellowship from the Council of Scientific and Industrial Research (CSIR), Government of India, New Delhi, India.
Footnotes
Published ahead of print 21 August 2013
REFERENCES
- 1.Gupta V, Gupta A, Rao NA. 2007. Intraocular tuberculosis—an update. Surv. Ophthalmol. 52:561–587 [DOI] [PubMed] [Google Scholar]
- 2.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. 2000. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28:E63. 10.1093/nar/28.12.e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chauhan DS, Sharma VD, Prashar D, Chauhan A, Singh D, Singh HB, Das R, Aggarwal BM, Malhotra B, Jain A, Sharma M, Kataria VK, Aggarwal JK, Hanif M, Shahani A, Katoch VM. 2007. Molecular typing of Mycobacterium tuberculosis isolates from different parts of India based on IS6110 element polymorphism using RFLP analysis. Indian J. Med. Res. 125:577–581 [PubMed] [Google Scholar]
- 4.Reddy AK, Balne PK, Reddy RK, Mathai A, Kaur I. 2010. Development and evaluation of loop-mediated isothermal amplification assay for rapid and inexpensive detection of cytomegalovirus DNA in vitreous specimens from suspected cases of viral retinitis. J. Clin. Microbiol. 48:2050–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwamoto T, Sonobe T, Hayashi K. 2003. Loop-mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare in sputum samples. J. Clin. Microbiol. 41:2616–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma K, Gupta V, Bansal R, Sharma A, Sharma M, Gupta A. 2013. Novel multi-targeted polymerase chain reaction for diagnosis of presumed tubercular uveitis. J. Ophthalmic Inflamm. Infect. 3:25. 10.1186/1869-5760-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy AK, Balne PK, Reddy RK, Mathai A, Kaur I. 2011. Loop-mediated isothermal amplification assay for the diagnosis of retinitis caused by herpes simplex virus-1. Clin. Microbiol. Infect. 17:210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]


