Abstract
A combined-disk method using imipenem and cloxacillin was evaluated to discriminate between carbapenemase-producing (n = 56) and nonproducing (n = 62) strains of Pseudomonas aeruginosa. With a cloxacillin load of 4,000 μg/disk, this very simple method showed a sensitivity and a specificity of 100%, irrespective of the type of carbapenemase produced.
TEXT
Development of carbapenem resistance is common in Pseudomonas aeruginosa, as it now concerns about 15 to 20% of clinical strains isolated in Europe (EARS-Net Database) and the United States (1). This resistance, which is due mainly to alteration of the OprD porin, the specific uptake pathway of carbapenems in P. aeruginosa (2), may also result from acquisition of foreign genes encoding Ambler class A, class B, or class D β-lactamases able to hydrolyze carbapenems at various degrees (3–6). Over the last decade, strains producing metallo-β-lactamases (MBLs) of different types (e.g., AIM, FIM, GIM, IMP, NDM, SPM, VIM) have been reported with an increasing frequency in P. aeruginosa worldwide (3–7). In order to improve the detection of carbapenemase producers, various inhibitor-based tests and enzymatic assays (e.g., NP-Carba) have been proposed as a first screening step prior to the use of confirmatory molecular techniques (8–11). However, because most of imipenem-nonsusceptible strains are just OprD-deficient mutants, these phenotypic or enzymatic tests usually yield low rates of positivity. In this study, we evaluated the performance of a very simple, inexpensive detection method applicable in medical laboratories that combines imipenem and cloxacillin, a strong inhibitor of intrinsic cephalosporinase AmpC, in order to discriminate carbapenemase-producing strains from nonproducers. This test is based on the observation that imipenem resistance resulting from OprD deficiency requires constitutive and/or carbapenem-induced overproduction of AmpC (12). Therefore, inhibition of AmpC by cloxacillin is expected to restore partial or complete sensitivity to imipenem in OprD-deficient strains but not in carbapenemase-positive strains.
A panel of 118 imipenem-nonsusceptible (MIC > 4 mg/liter) non-cystic fibrosis clinical strains of P. aeruginosa characterized by the French National Reference Centre for Antibiotic Resistance was investigated. Fifty-six of these strains produced GES (n = 5)-, KPC (n = 3)-, IMP (n = 16)-, NDM (n = 1)-, SPM (n = 1)-, and/or VIM (n = 32)-type carbapenemases, with one isolate coexpressing enzymes GES-4 and VIM-1, and another one expressing IMP-7 and VIM-4 (see Table S1 in the supplemental material). In parallel, 62 strains of P. aeruginosa were used as carbapenemase-negative controls. These isolates overproduced the efflux pump MexAB-OprM (n = 7), MexXY/OprM (n = 4), or MexEF-OprN (n = 3) and/or showed increased levels of intrinsic β-lactamase AmpC (n = 48). Some strains contained additional narrow (n = 4)- or broad (n = 14)-spectrum β-lactamases. Sequencing of the porin OprD gene (oprD) in 20 randomly selected strains belonging to this group showed that all were OprD deficient as a result of various genetic events (see Table S2 in the supplemental material). All the β-lactamase genes were characterized by PCR and DNA sequencing (13). Overexpression of genes encoding AmpC and efflux systems was assessed by real-time quantitative PCR after reverse transcription, as described previously (14–16). Combined imipenem-cloxacillin disks were prepared daily by adding 10 μl of cloxacillin solution (sodium salt monohydrate; Sigma-Aldrich) at 100 g liter−1, 200 g liter−1, 300 g liter−1, and 400 g liter−1 to commercially available imipenem disks (10 μg; Bio-Rad) in order to obtain a cloxacillin load of 1,000, 2,000, 3,000, and 4,000 μg per disk, respectively. Preliminary experiments demonstrated the absence of an inhibition zone around disks impregnated with 4,000 μg cloxacillin alone (i.e., without imipenem). Diffusion tests on Mueller-Hinton agar were performed according to the EUCAST recommendations, with bacterial suspensions calibrated at a 0.5 McFarland standard and plate inoculation with swabs. After 18 to 20 h of incubation at 35 to 37°C, the difference between the zone diameters around the imipenem disks with and without cloxacillin was calculated and expressed as Δd in millimeters.
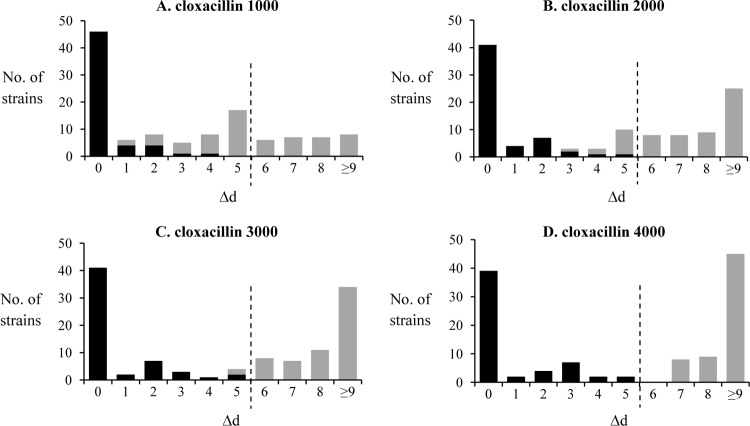
As indicated by Fig. 1, Δd was ≤5 mm with all of the carbapenemase-producing strains, even with the highest load (4,000 μg) of cloxacillin. No inhibition zone was visible around the imipenem-cloxacillin disk in 70% of the strains, of which most produced VIM-1,VIM-2, or VIM-4 enzymes, known to have a strong activity on imipenem (17). The cutoff value of 5 mm was also found to apply to all the carbapenemase-producing strains exhibiting moderate resistance to imipenem, with inhibition zones between 11 and 19 mm (e.g., IMP-1, -7, -13, -15, or -19 or VIM-11). Interestingly, all these latter isolates produced wild-type porin OprD, as deduced from the sequence analysis of their oprD genes. Demonstrating that the combined-disk method using imipenem and 4,000 μg cloxacillin (named I-C4000) is not influenced by OprD-dependent membrane impermeability, 12 out of 12 carbapenemase-producing isolates known to harbor disrupted oprD genes also tested positive (see Table S1 in the supplemental material).
Fig 1.
Application of the I-C test to characterize carbapenemase-producing (black bars) and carbapenemase-negative strains (gray bars). The difference (Δd) between the zone diameters around imipenem disks supplemented or not with cloxacillin at 1,000 (A), 2,000 (B), 3,000 (C), or 4,000 μg (D) per disk is indicated in millimeters. The dashed line indicates the finally chosen cutoff point.
In noncarbapenemase producers, the Δd value increased with the cloxacillin load in combined disks. As already reported (8), 1,000 μg per disk was not sufficient to inhibit the AmpC contribution to imipenem resistance in the OprD-deficient isolates. Comparative assays with 1,000 μg, 2,000 μg, 3,000 μg, and 4,000 μg resulted in 34, 11, 2, and 0 false positives, respectively, when considering a cutoff value of 5 mm (i.e., absence of carbapenemase activity if Δd is >5 mm). The complete results are detailed in Tables S1 and S2 in the supplemental material, and an illustration of the test is given in Fig. 2.
Fig 2.
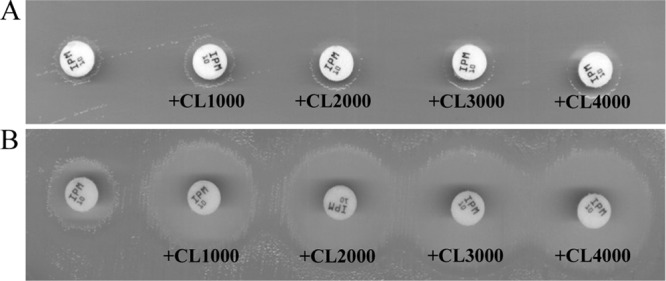
Representative results obtained with the I-C test on a carbapenemase-producing (GES-5) strain (A) and a carbapenemase-negative (AmpC-overproducing, OprD-deficient) strain (B). Combined disks contained 10 μg imipenem and 1,000, 2,000, 3,000, or 4,000 μg cloxacillin (CL). A >5-mm increase in the inhibition zone in the presence of cloxacillin relative to the imipenem disk without inhibitor (left) is indicative of the absence of carbapenemase activity.
In conclusion, the I-C4000 test is a simple and inexpensive presumptive method for routine detection of carbapenemase-positive P. aeruginosa. When systematically added to the standard antibiogram by diffusion, the imipenem-cloxacillin disk may allow the detection of potentially epidemic carbapenemase producers with 100% specificity and sensitivity the same day the drug susceptibility data are obtained. Thus, it may reveal useful to rapidly implement infection control measures and also to optimize the use of more sophisticated and expensive methods (e.g., PCR, chips) necessary to detect and characterize carbapenemase genes. The performance of the I-C4000 test with cystic fibrosis strains of P. aeruginosa is currently being investigated.
Supplementary Material
ACKNOWLEDGMENTS
This work was financially supported by the French Ministry of Health through the InVS agency and by the Besançon University-affiliated hospital.
We thank Patrice Nordmann and Laurent Poirel for providing the IMP-1-, KPC-, and SPM-positive isolates.
Footnotes
Published ahead of print 21 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01299-13.
REFERENCES
- 1.Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 22:582–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trias J, Dufresne J, Levesque RC, Nikaido H. 1989. Decreased outer membrane permeability in imipenem-resistant mutants of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 33:1202–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornaglia G, Giamarellou H, Rossolini GM. 2011. Metallo-beta-lactamases: a last frontier for beta-lactams? Lancet Infect. Dis. 11:381–393 [DOI] [PubMed] [Google Scholar]
- 4.El Garch F, Bogaerts P, Bebrone C, Galleni M, Glupczynski Y. 2011. OXA-198, an acquired carbapenem-hydrolyzing class D beta-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 55:4828–4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirel L, Weldhagen GF, Naas T, De Champs C, Dove MG, Nordmann P. 2001. GES-2, a class A beta-lactamase from Pseudomonas aeruginosa with increased hydrolysis of imipenem. Antimicrob. Agents Chemother. 45:2598–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villegas MV, Lolans K, Correa A, Kattan JN, Lopez JA, Quinn JP. 2007. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing beta-lactamase. Antimicrob. Agents Chemother. 51:1553–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollini S, Maradei S, Pecile P, Olivo G, Luzzaro F, Docquier JD, Rossolini GM. 2013. FIM-1, a new acquired metallo-beta-lactamase from a Pseudomonas aeruginosa clinical isolate from Italy. Antimicrob. Agents Chemother. 57:410–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasteran F, Veliz O, Faccone D, Guerriero L, Rapoport M, Mendez T, Corso A. 2011. A simple test for the detection of KPC and metallo-beta-lactamase carbapenemase-producing Pseudomonas aeruginosa isolates with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin. Clin. Microbiol. Infect. 17:1438–1441 [DOI] [PubMed] [Google Scholar]
- 9.Pasteran F, Veliz O, Rapoport M, Guerriero L, Corso A. 2011. Sensitive and specific modified Hodge test for KPC and metallo-beta-lactamase detection in Pseudomonas aeruginosa by use of a novel indicator strain, Klebsiella pneumoniae ATCC 700603. J. Clin. Microbiol. 49:4301–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dortet L, Poirel L, Nordmann P. 2012. Rapid detection of carbapenemase-producing Pseudomonas spp. J. Clin. Microbiol. 50:3773–3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picao RC, Andrade SS, Nicoletti AG, Campana EH, Moraes GC, Mendes RE, Gales AC. 2008. Metallo-beta-lactamase detection: comparative evaluation of double-disk synergy versus combined disk tests for IMP-, GIM-, SIM-, SPM-, or VIM-producing isolates. J. Clin. Microbiol. 46:2028–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livermore DM. 1992. Interplay of impermeability and chromosomal beta-lactamase activity in imipenem-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 36:2046–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fournier D, Richardot C, Muller E, Robert-Nicoud M, Llanes C, Plésiat P, Jeannot K. 2013. Complexity of resistance mechanisms to imipenem in intensive care unit strains of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 68:1772–1780 [DOI] [PubMed] [Google Scholar]
- 14.Cabot G, Ocampo-Sosa AA, Tubau F, Macia MD, Rodriguez C, Moya B, Zamorano L, Suárez C, Peña C, Martínez-Martínez L, Oliver A. 2011. Overexpression of AmpC and efflux pumps in Pseudomonas aeruginosa isolates from bloodstream infections: prevalence and impact on resistance in a Spanish multicenter study. Antimicrob. Agents Chemother. 55:1906–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumas JL, van Delden C, Perron K, Köhler T. 2006. Analysis of antibiotic resistance gene expression in Pseudomonas aeruginosa by quantitative real-time-PCR. FEMS Microbiol. Lett. 254:217–225 [DOI] [PubMed] [Google Scholar]
- 16.Jeannot K, Sobel ML, El Garch F, Poole K, Plésiat P. 2005. Induction of the MexXY efflux pump in Pseudomonas aeruginosa is dependent on drug-ribosome interaction. J. Bacteriol. 187:5341–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassaux P, Traore DA, Loisel E, Favier A, Docquier JD, Sohier JS, Laurent C, Bebrone C, Frère JM, Ferrer JL, Galleni M. 2011. Biochemical and structural characterization of the subclass B1 metallo-beta-lactamase VIM-4. Antimicrob. Agents Chemother. 55:1248–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.