Abstract
The ultraviolet circular dichroism spectra of human lysozyme are presented. Effects of pH and added inhibitor (N-acetyl-D-glucosamine) were examined and the results were compared with similar measurements of hen egg-white lysozyme. The near-ultraviolet CD spectral bands are substantially different in the human and hen egg-white enzymes. In addition to marked dissimilarities in the spectral interval 260-300 nm, an unusual CD band occurs at an anomalous wavelength (313 nm) in human lysozyme. The pH dependence of the latter suggests a possible interaction, absent in hen egg-white lysozyme, between a tryptophan and a tyrosine residue. Analysis of the spectra furthermore suggests lesser net rotational strengths of tryptophan bands in hen egg-white lysozyme than in human lysozyme, although the latter has one less tryptophan residue. The relationship between the CD spectra and the sequence differences of the proteins is discussed, as well as the CD spectra (published by others) of a closely related protein, bovine α-lactalbumin. Contributions of cystine residues to the spectra are examined in the light of possible differences in chirality of one of the four disulfide bridges.
The far-ultraviolet CD spectra of human and egg-white lysozyme are quite similar, though not identical. In view of the pronounced differences in side-chain optical activity, and of the effect of pH variation on the far-ultraviolet CD spectrum of human lysozyme, it is likely that at least part of the observed difference in spectra is due to nonpeptide optical activity, and that the proteins have a secondary structure in common.
Full text
PDF
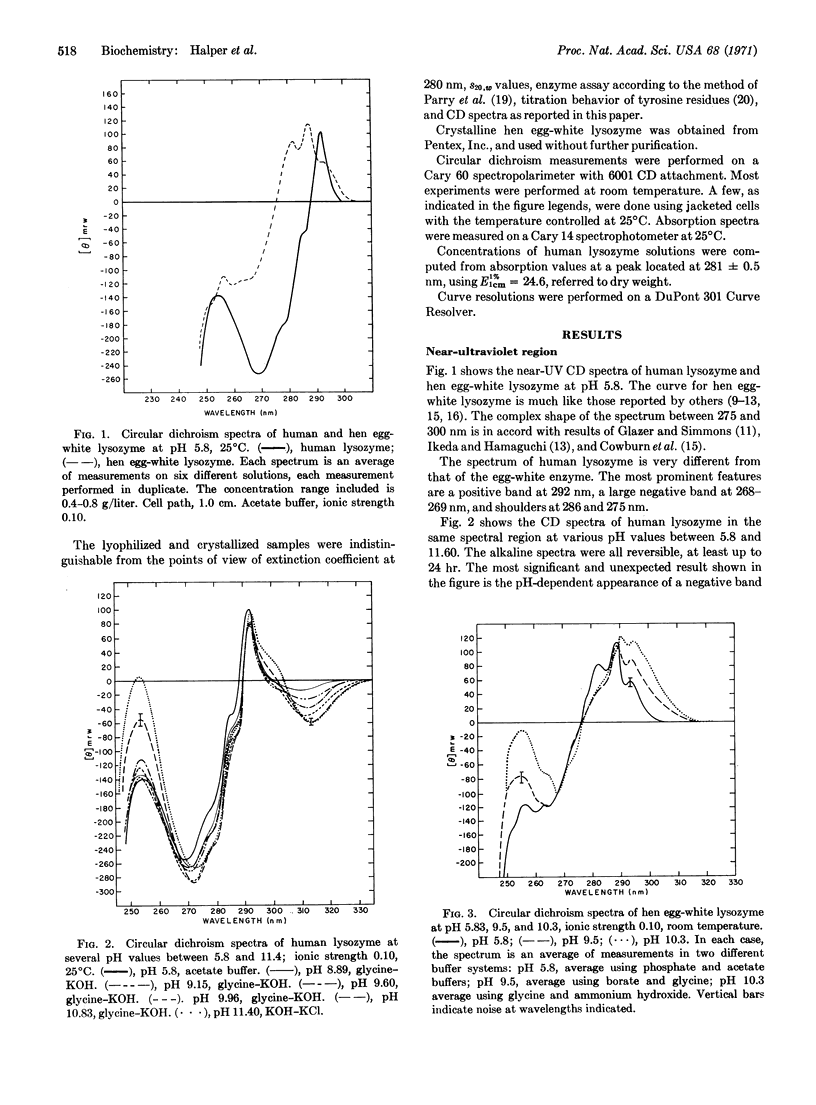
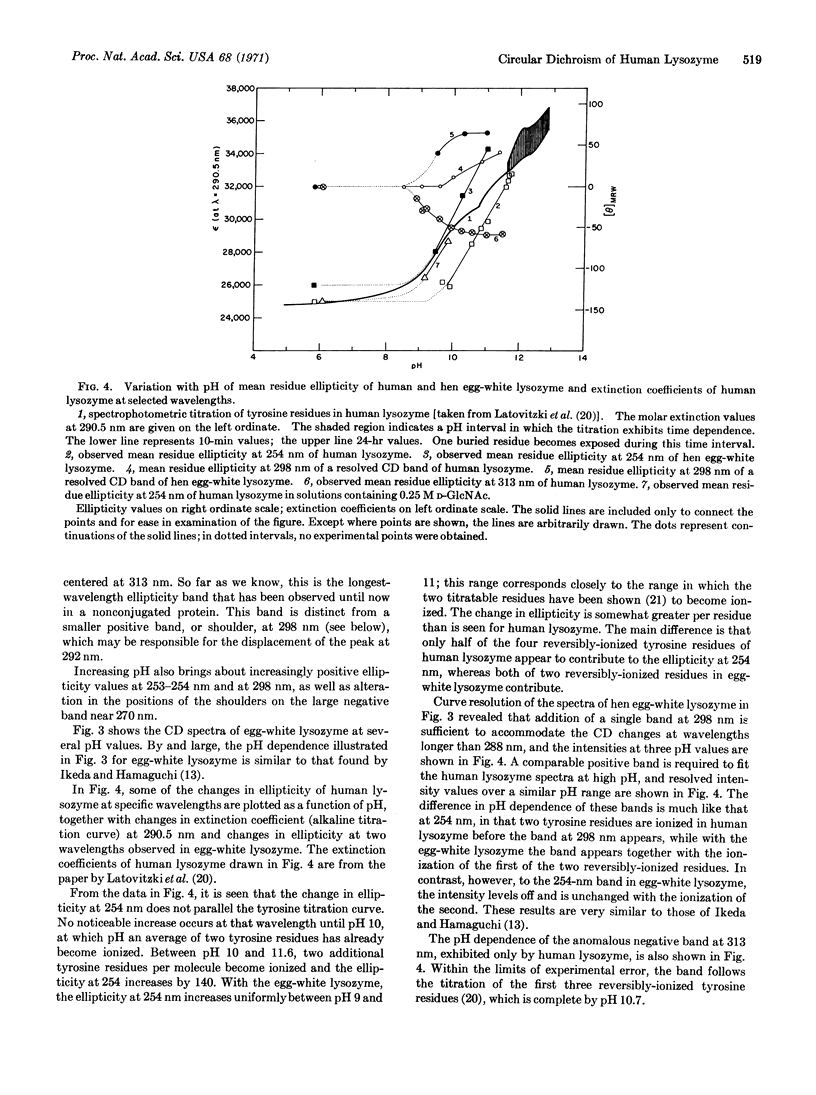
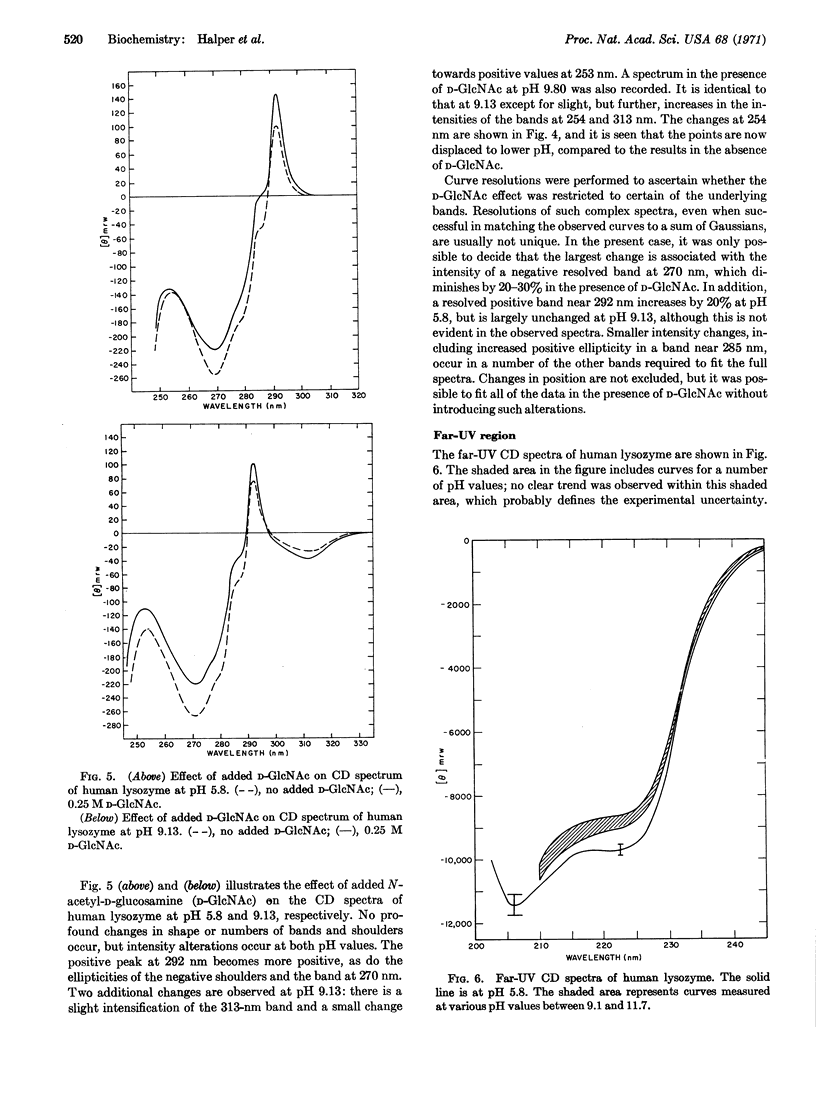


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beychok S., Breslow E. Circular dichroism of oxytocin and several oxytocin analogues. J Biol Chem. 1968 Jan 10;243(1):151–154. [PubMed] [Google Scholar]
- Beychok S. Side-chain optical activity in cystine-containing proteins: circular dichroism studies. Proc Natl Acad Sci U S A. 1965 May;53(5):999–1006. doi: 10.1073/pnas.53.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake C. C., Mair G. A., North A. C., Phillips D. C., Sarma V. R. On the conformation of the hen egg-white lysozyme molecule. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):365–377. doi: 10.1098/rspb.1967.0034. [DOI] [PubMed] [Google Scholar]
- Breslow E. Optical activity of bovine neurophysins and their peptide complexes in the near ultraviolet. Proc Natl Acad Sci U S A. 1970 Oct;67(2):493–500. doi: 10.1073/pnas.67.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Castellino F. J., Vanaman T. C., Hill R. L. The complete amino acid sequence of bovine alpha-lactalbumin. J Biol Chem. 1970 Sep 10;245(17):4570–4582. [PubMed] [Google Scholar]
- Browne W. J., North A. C., Phillips D. C., Brew K., Vanaman T. C., Hill R. L. A possible three-dimensional structure of bovine alpha-lactalbumin based on that of hen's egg-white lysozyme. J Mol Biol. 1969 May 28;42(1):65–86. doi: 10.1016/0022-2836(69)90487-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. S. Proton magnetic resonance studies of human lysozyme. Nature. 1969 Jul 5;223(5201):43–46. doi: 10.1038/223043a0. [DOI] [PubMed] [Google Scholar]
- Cowburn D. A., Bradbury E. M., Crane-Robinson C., Gratzer W. B. An investigation of the conformation of bovine alpha-lactalbumin by proton magnetic resonance and optical spectroscopy. Eur J Biochem. 1970 May 1;14(1):83–93. doi: 10.1111/j.1432-1033.1970.tb00264.x. [DOI] [PubMed] [Google Scholar]
- Edelhoch H., Lippoldt R. E., Wilchek M. The circular dichroism of tryosyl and tryptophanyl diketopiperazines. J Biol Chem. 1968 Sep 25;243(18):4799–4805. [PubMed] [Google Scholar]
- Fasman G. D., Foster R. J., Beychok S. The conformational transition associated with the activation of chymotrypsinogen to chymotrypsin. J Mol Biol. 1966 Aug;19(2):240–253. doi: 10.1016/s0022-2836(66)80002-5. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Simmons N. S. The cotton effect associated with certain tyrosine residues in ribonuclease. J Am Chem Soc. 1965 Sep 5;87(17):3991–3993. doi: 10.1021/ja01095a043. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Hamaguchi K., Imanishi M., Amano T. Effect of pH on the ultraviolet optical rotatory dispersion and circular dichroism of lysozyme. J Biochem. 1967 Sep;62(3):315–320. [PubMed] [Google Scholar]
- Ikeda K., Hamaguchi K. The binding of N-acetylglucosamine to lysozyme. Studies on circular dichroism. J Biochem. 1969 Oct;66(4):513–520. doi: 10.1093/oxfordjournals.jbchem.a129176. [DOI] [PubMed] [Google Scholar]
- Johnson L. N., Phillips D. C., Rupley J. A. The activity of lysozyme: an interim review of crystallographic and chemical evidence. Brookhaven Symp Biol. 1968 Jun;21(1):120–138. [PubMed] [Google Scholar]
- Jollès P., Saint Blancard J., Charlemagne D., Dianoux A. C., Jollès J., Le Baron J. L. Comparative behavior of six different lysozymes in the presence of an inhibitor. Biochim Biophys Acta. 1968 Feb 5;151(2):532–534. doi: 10.1016/0005-2744(68)90123-x. [DOI] [PubMed] [Google Scholar]
- Kronman M. J. Similarity in backbone conformation of egg white lysozyme and bovine gamma lactalbumin. Biochem Biophys Res Commun. 1968 Nov 25;33(4):535–541. doi: 10.1016/0006-291x(68)90327-6. [DOI] [PubMed] [Google Scholar]
- Osserman E. F., Cole S. J., Swan I. D., Blake C. C. Preliminary crystallographic data on human lysozyme. J Mol Biol. 1969 Nov 28;46(1):211–212. doi: 10.1016/0022-2836(69)90070-9. [DOI] [PubMed] [Google Scholar]
- Osserman E. F. Crystallization of human lysozyme. Science. 1967 Mar 24;155(3769):1536–1537. doi: 10.1126/science.155.3769.1536. [DOI] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARRY R. M., Jr, CHANDAN R. C., SHAHANI K. M. A RAPID AND SENSITIVE ASSAY OF MURAMIDASE. Proc Soc Exp Biol Med. 1965 Jun;119:384–386. doi: 10.3181/00379727-119-30188. [DOI] [PubMed] [Google Scholar]
- Peggion E., Cosani A., Verdini A. S., Del Pra A., Mammi M. Conformational studies on poly-L-tryptophan: circular dichroism and x-ray diffraction studies. Biopolymers. 1968 Oct;6(10):1477–1486. doi: 10.1002/bip.1968.360061010. [DOI] [PubMed] [Google Scholar]
- Strickland E. H., Horwitz J., Billups C. Fine structure in the near-ultraviolet circular dichroism and absorption spectra of tryptophan derivatives and chymotrypsinogen A at 77 degrees K. Biochemistry. 1969 Aug;8(8):3205–3213. doi: 10.1021/bi00836a012. [DOI] [PubMed] [Google Scholar]
- Strickland E. H., Wilchek M., Horwitz J., Billups C. Low temperature circular dichroism of tyrosyl and tryptophanyl diketopiperazines. J Biol Chem. 1970 Aug 25;245(16):4168–4177. [PubMed] [Google Scholar]
- Teichberg V. I., Kay C. M., Sharon N. Separation of contributions of tryptophans and tyrosines to the ultraviolet circular dichroism spectrum of hen egg-white lysozyme. Eur J Biochem. 1970 Sep;16(1):55–59. doi: 10.1111/j.1432-1033.1970.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Zuclich J. Triplet-state electron spin resonance of the aromatic amino acids and proteins. J Chem Phys. 1970 Apr 1;52(7):3586–3591. doi: 10.1063/1.1673527. [DOI] [PubMed] [Google Scholar]



