Abstract
Staphylococcus aureus causes a broad range of life-threatening diseases in humans. This bacterium produces a large number of extracellular virulence factors that are closely associated with specific diseases which are controlled by quorum sensing. In this study, we show that azithromycin was active against methicillin-resistant Staphylococcus aureus (MRSA) strains with MICs ranged from 32 to 64 μg/mL. Azithromycin at subinhibitory concentration, markedly reduced the production of α-hemolysin at (1/16MIC, 1/8MIC) and biofilm formation at (1/16MIC, 1/8MIC), respectively. The results indicated that sub-inhibitory concentrations of azithromycin decreased the production of α-hemolysin and biofilm formation in MRSA in a dose-dependent manner. Therefore, azithromycin may be useful in the treatment of α-hemolysin producing and biofilm formation MRSA infections.
Keywords: Staphylococcus aureus, Azithromycin, Biofilm, α-hemolysin
Staphylococcus aureus is found among the normal human skin flora and it is an important pathogen of humans, causing diseases ranging from superficial skin and wound infections to severe illnesses such as septicaemia, endocarditis and toxic shock syndrome [1]. S. aureus is particularly a problem in hospitals because it spreads easily in these environments and causes potentially fatal infections in immunocompromised hospital patients. S. aureus cause disease through the production of virulence factors. These factors include adhesins, exotoxins, enterotoxins, hemolysins, and leukocidin, as well as proteases that enable the bacteria to spread within the host [2, 3]. Quorum sensing (QS) regulates expression of genes encoding these virulence factors and biofilm formation. Strains defective in their ability to form a biofilm or produce toxins show diminished virulence [4], suggesting that a novel approach for therapy development would be to interfere with the production of virulence factors [5–7]. Azithromycin (AZM) is one of the macrolides antibiotics, and it is a broad-spectrum macrolide antibiotic effective against Gram-positive, Gram-negative, and atypical bacteria and has anti-inflammatory characteristics [8]. The purpose of this study is to assess the effect of azithromycin, used as a quorum-sensing inhibitor, for decrease the production of QS associated virulence factors and biofilm formation in Staphylococcus aureus.
Two different clinical bacterial strains of methicillin-resistant Staphylococcus aureus (MRSA) 10 and 21 were collected from different patients hospitalized at Lishui People’s Hospital, Zhejiang province. S. aureus strains was identified biochemically from routinely obtained specimens by means of the Vitek ATB Expression System, version 2.7.8 (BioMe’rieux Deutschland GmbH, Nu¨rtingen, Germany), which uses 32 biochemical reactions. Bacterial isolates were stored as suspensions in LB medium containing 12.5 % (vol/vol) glycerol at −70 °C until tests were performed. S. aureus MRSA 10, 21 single colony was inoculated into LB media and cultured overnight at 37. Overnight culture was subcultured (1:100) into test tubes with fresh LB medium in triplicate. Optical density readings at 600 nm were obtained with spectrophotometer (UV-721, Shanghai optical instrument factory, Chian) at 30 min intervals. Experiments were repeated three times to obtain the mean and standard error of means. Standardized planktonic antibiotic MICs were determined by the broth microdilution method outlined by the Clinical and Laboratory Standards Institute (formerly the National Committee for Clinical Laboratory Standards) [9]. Briefly, the test antibiotic was serially diluted twofold in tubes containing 5 mL of LB. The S. aureus inoculum for the series of tubes was 100 μL of a 5.0 × 106 CFU/mL dilution in LB. The MIC was the lowest concentration of antibiotic that prevented turbidity after 20 h of incubation at 37 °C. For detection of hemolysin production, S. aureus strains were grown in LB in the absence or presence of sub-inhibitory concentrations of azithromycin until reaching the post-exponential growth phase. Bacterial supernatants were collected by centrifugation (5,000×g, 4 °C, 5 min), the supernatant was collected, and the residual cells were removed using a 0.2 μm filter. Prior to the addition of 25 μL of defibrinated rabbit blood, a 0.1 mL volume of culture supernatant was brought up to a volume of 1 mL through the addition of PBS buffer. After incubation for 15 min at 37 °C, the unlysed blood cells were pelleted by centrifugation (5,000×g, room temperature, 2 min). The hemolytic activity of the supernatant was detected by measuring the optical density at 543 nm. The supernatant of culture without azithromycin served as the 100 % hemolysis, and the percent hemolysis was calculated by comparison with the control culture [10]. For screening biofilm producing strains, Congo red Agar method was used. This method is based on the characteristic cultural morphology of biofilm-forming bacteria on Congo red medium. The medium was composed of brain heart infusion broth (BHI) (Oxoid Ltd, Hampshire, England) 37 g/L, sucrose 50 g/L, agar 10 g/L and Congo red (BDH Chemical Ltd, Poole, England) 0.8 g/L. Congo red stain was made ready as a strong aqueous solution and sterilized (121 °C for 15 min) separate from the rest of the medium components and supplemented to the agar when the temperature reached 55 °C. Agar plates were prepared and inoculated and kept in the incubator for 24 h at 37 °C. The production of black colonies with a dry crystalline consistency by the organisms was taken to indicate biofilm production as non-biofilm-producing strains develop red colonies [11].
Biofilm formation was determined as previously described by Christensen et al. [12]. LB (10 mL) was inoculated with loopful of microorganism from overnight culture plates and incubated for 24 h at 37 °C. The tubes were decanted and washed with PBS (pH 7.3), and fixed with 2.5 % glutaraldehyde, washed once with water, and stained with a 0.4 % crystal violet solution. After solubilization of the crystal violet with ethanol-acetone (80:20 vol/vol) the absorbance at 590 nm was determined.
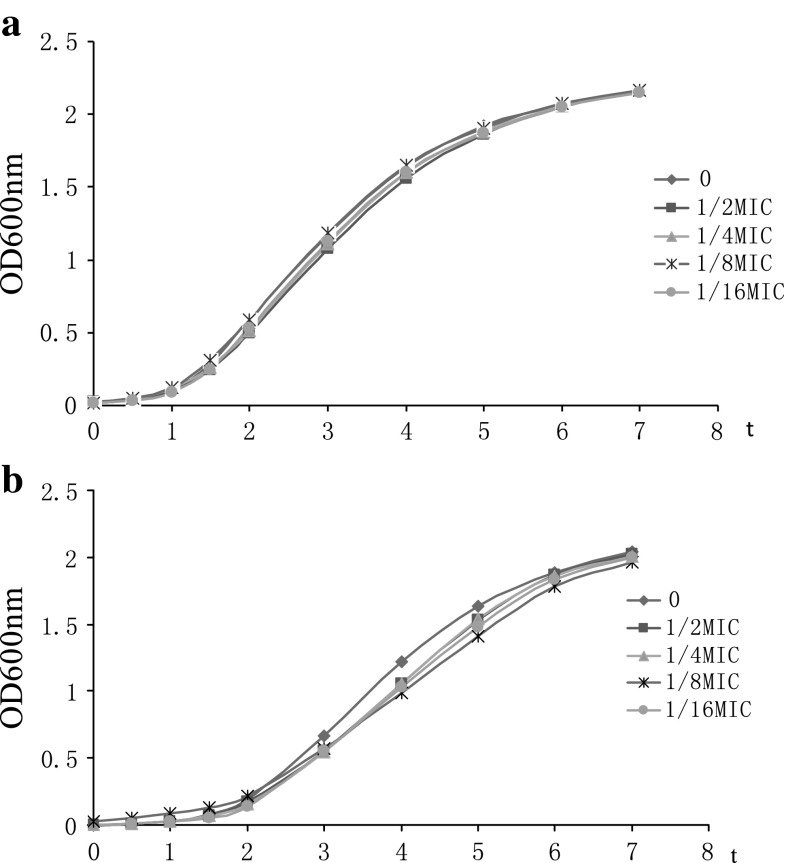
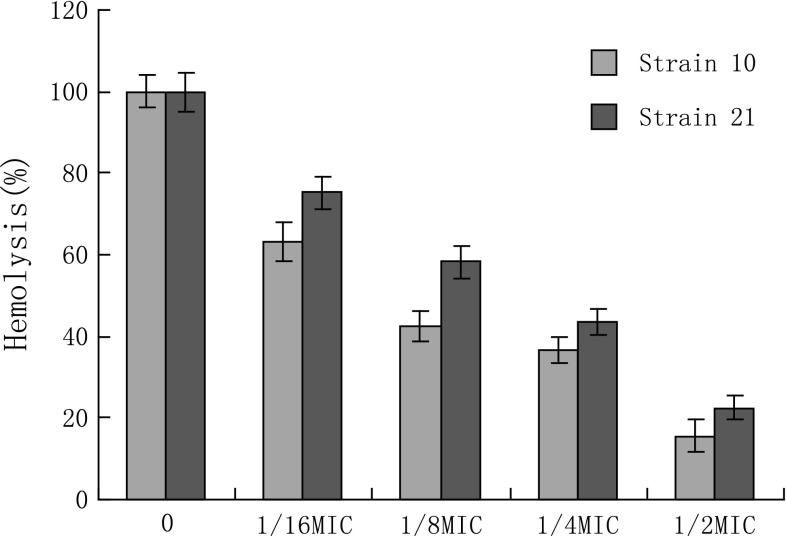
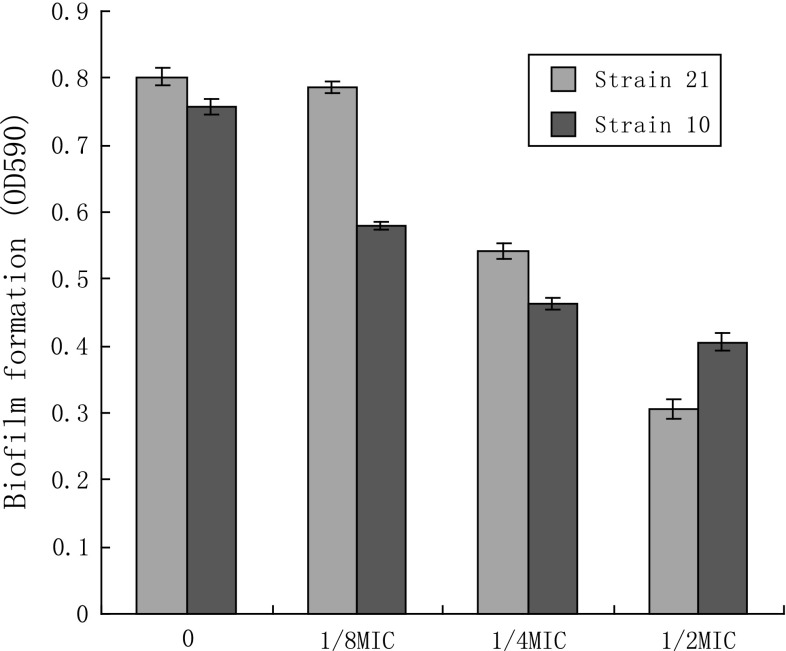
The MICs of azithromycin against S. aureus strains MRSA 10 and MRSA 21 were 128 and 64 μg/mL, respectively. Growth curves for S. aureus isolates MRSA 10 and MRSA 21 grown in the presence of increasing concentrations of azithromycin at sub-MIC concentrations are shown in Fig. 1. For both isolates, growth in the presence of azithromycin at sub-MIC were not markedly different from controls. S. aureus strains were exposed to graded sub-inhibitory concentrations of azithromycin to the post-exponential phase. The haemolytic activities of the culture supernatants are detected. The results shown that the reducing of α-hemolysin levels in S. aureus was dose-dependent. When grown in the presence of 1/16MIC of azithromycin, the haemolysis values of 10 and 21 culture supernatants were 63.5, 75.3 %, respectively, compared with a azithromycin-free culture Fig. 2 Notably, supplementation with 1/2MIC of azithromycin led to 15.6 and 22.5 % hemolysin production. On Congo red agar plate, slime producing strains formed black (complete) colonies to slightly black, whereas non producing strains develop strong red colonies. Strains MRSA 10 and 21 formed strong black pigmentation, indicated that they can form biofilm. To determine if azithromycin could be prophylactically used to prevent biofilm formation, we tested the effect of azithromycin at sub-inhibitory concentrations (1/2×, 1/4× and 1/8×) against biofilm formation (Fig. 3). On both strains, azithromycin at 1/2× and 1/4×MIC caused a significantly higher reduction in biofilm-forming ability of S. aureus.
Fig. 1.
Growth curves of S. aureus strains MRSA 10 (a) and MRSA21 (b) treated with or without azithromycin. (black rhombus), untreated S. aureus; (black square), S. aureus plus azithromycin at 1/2 MIC; (black triangle), S. aureus plus azithromycin at 1/4 MIC; (*), S. aureus plus azithromycin at 1/8 μg/mL; and (black circle), S. aureus plus azithromycin at 1/16 μg/mL
Fig. 2.
Relative culture supernatant hemolytic ability assay. The culture supernatants without azithromycin served as the 100 % haemolysis control
Fig. 3.
Effect of azithromycin on S. aureus biofilm formation
Researches shows that strains defective in the ability to form biofilm or produce toxins show diminished virulence [13], suggesting that a novel approach for therapy development would be to interfere with the production of virulence factors. Some antibiotics display an anti-virulence activity at concentrations below the MIC based on quorum sensing inhibitory ability. Macrolide antibiotics have been shown to act as QS inhibitors at subinhibitory concentrations. For example, erythromycin has been reported to suppress production of P. aeruginosa hemagglutinins, protease, hemolysin and AHL signals [14], and studies have shown that azithromycin at sub-MIC concentrations affects QS-regulated virulence genes and biofilm formation in vitro [15–17] and in in vivo models of disease [18]. In the present study, we demonstrate that sub-inhibitory concentrations of azithromycin could dose-dependently reduce the haemolytic activity and biofilm formation in S. aureus. More recent studies by Parra-Ruiz et al. [19] have shown that low concentrations of Clarithromycin could inhibit the biofilm formation of S. aureus, but Kumar and Ting [20] found that the biofilm formation by S. aureus was enhanced under sub-inhibitory norfloxacin stress. Targeting bacterial quorum sensing is an alternative strategy that is now gaining great interest in antimicrobial therapy and offers promising opportunities to impede pathogenesis and its consequences without placing immediate life-or-death pressure on the target bacterium [21]. However, once QSI concentration gets below the threshold level, bacteria may be free to express its virulence and evidence is accumulating that bacteria may develop resistance to QSIs [22]. Our results showing that azithromycin significantly decreases α-hemolysin secretion and biofilm formation in S. aureus suggest that azithromycin may be may be useful in the treatment of infections with MRSA S. aureus.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Ohlsen K, Koller KP, Hacker J. Analysis of expression of the alpha-toxin gene (hla) of Staphylococcus aureus by using a chromosomally encoded hla: lacZ gene fusion. Infect Immun. 1997;65:3606–3614. doi: 10.1128/iai.65.9.3606-3614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong-Geller E, Gupta G. Therapeutic approaches to superantigen-based diseases: a review. J Mol Recognit. 2003;16:91–101. doi: 10.1002/jmr.612. [DOI] [PubMed] [Google Scholar]
- 4.Gov Y, Borovok I, Korem M, Singh VK, Jayaswal RK, Wilkinson BJ, Rich SM, Balaban N. Quorum sensing in staphylococci is regulated via phosphorylation of three conserved histidine residues. J Biol Chem. 2004;279:14665–14672. doi: 10.1074/jbc.M311106200. [DOI] [PubMed] [Google Scholar]
- 5.Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31:224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Kalia VC, Purohit HJ. Quenching the quorum sensing system: potential antibacterial drug targets. Crit Review Microbiol. 2011;37:121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 7.Annapoorani A, Jabbar AKKA, Musthafa SKS, Pandian SK, Ravi AV. Inhibition of quorum sensing mediated virulence factors production in urinary pathogen serratia marcescens ps1 by marine sponges. Indian J Microbiol. 2012;52:160–166. doi: 10.1007/s12088-012-0272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parra-Ruiz J, Vidaillac C, Rybak MJ. Macrolides and staphylococcal biofilms. Rev Esp Quimioter. 2012;25:10–16. [PubMed] [Google Scholar]
- 9.Performance standards for antimicrobial susceptibility testing; 20th informational supplement. Wayne: Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 10.Larzábal PAM, Mercado EC, Vilte DA, Salazar-González H, Cataldi A, Navarro-Garcia F. Designed coiled-coil peptides inhibit the type three secretion system of enteropathogenic Escherichia coli. PLoS ONE. 2010;5:e9046. doi: 10.1371/journal.pone.0009046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathur T, Singhal S, Khan S, Upadhyay DJ, Fatma T, Rattan A. Detection of biofilm formation among the clinical isolates of staphylococci: an evaluation of three different screening methods. Indian J Med Microbiol. 2006;24:25–29. doi: 10.4103/0255-0857.19890. [DOI] [PubMed] [Google Scholar]
- 12.Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of slime–producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gov Y, Borovok I, Korem M, Singh VK, Jayaswal RK, Wilkinson BJ, Rich SM, Balaban N. Quorum sensing in staphylococci is regulated via phosphorylation of three conserved histidine residues. J Biol Chem. 2004;279:14665–14672. doi: 10.1074/jbc.M311106200. [DOI] [PubMed] [Google Scholar]
- 14.Sofer D, Gilboa-Garber N, Belz A, Garber NC. ‘Subinhibitory’ erythromycin represses production of Pseudomonas aeruginosa lectins, autoinducer and virulence factors. Chemotherapy. 1999;45:334–335. doi: 10.1159/000007224. [DOI] [PubMed] [Google Scholar]
- 15.Tateda K, Comte R, Pechere JC, Kohler T, Yamaguchi K, Van Delden C. Azithromycin inhibits quorum sensing in pseudomonas aeruginosa. Antimicrob Agents Chemother. 2001;45:1930–1933. doi: 10.1128/AAC.45.6.1930-1933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillis RJ, Iglewski BH. Azithromycin retards Pseudomonas aeruginosa biofilm formation. J Clin Microbiol. 2004;42:5842–5845. doi: 10.1128/JCM.42.12.5842-5845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nalca Y, Jansch L, Bredenbruch F, Geffers R, Buer J, Haussler S. Quorum-sensing antagonistic activities of azithromycin in pseudomonas aeruginosa PAO1: a global approach. Antimicrob Agents Chemother. 2006;50:1680–1688. doi: 10.1128/AAC.50.5.1680-1688.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann N, Lee B, Hentzer M, Rasmussen TB, Song Z, Johansen HK, Givskov M, Høiby N. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr−/− mice. Antimicrob Agents Chemother. 2007;51:3677–3687. doi: 10.1128/AAC.01011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parra-Ruiz J, Vidaillac C, Rybak MJ. Macrolides and staphylococcal biofilms. Rev Esp Quimioter. 2012;25:10–16. [PubMed] [Google Scholar]
- 20.Kumar A, Ting YP. Effect of sub-inhibitory antibacterial stress on bacterial surface properties and biofilm formation. Colloids Surf B Biointerfaces. 2013;12:747–754. doi: 10.1016/j.colsurfb.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol. 2008;6:17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalia VC, Wood TK, Kumar P. Evolution of resistance to quorum-sensing inhibitors. Microb Ecol. 2013 doi: 10.1007/s00248-013-0316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]