Abstract
Moderate alcohol consumption (one to two drinks per day) has been associated with better cognitive function and lower risk of developing dementia in the elderly. In light of alcohol’s well-known neurotoxic properties, more evidence from well-controlled population-based studies is required. The objective of this study was to examine whether self-reported alcohol intake at age 70 is linked to cognitive function (assessed by trail making tests (TMTs) A and B, which are measures of attention, mental speed, and flexibility) in a population-based cohort consisting of 652 cognitively healthy elderly men. Linear regression models were used to assess both cross-sectional (i.e., age 70) and prospective (i.e., age 77) associations between alcohol intake and cognitive function. The analyses were adjusted for education, body mass index, energy intake, self-reported physical activity, smoking, a history of hypertension or diabetes, apolipoprotein E ε4 status, and cholesterol levels at the age of 70. Baseline data were obtained from 1990 to 1996. Self-reported alcohol intake (mean 6.9 ± 7.1 g/day) was associated with better performance on TMT-B at ages 70 and 77 (β = −0.87, p < 0.001). In contrast, alcohol intake was not predictive of the difference in performance on these tests between ages 70 and 77. Despite cross-sectional associations with performance in a test of executive functioning, moderate intake of alcohol was not linked to differences in cognitive performance between ages 70 and 77 in the present study. Thus, our findings do not support the view that daily moderate alcohol consumption is a recommendable strategy to slow cognitive aging in elderly populations.
Keywords: Alcohol intake, Cognitive impairment, Cognition, Education
Introduction
Previous studies have shown that moderate alcohol consumption (one to two drinks per day) is linked to improved cognitive functions (e.g., Launer et al. 1996; Bond et al. 2005; Reid et al. 2006), increased gray matter volume (Sachdev et al. 2008), and a reduced risk of developing dementia in late life (Huang et al. 2002; Ruitenberg et al. 2002; Weyerer et al. 2011). Possible mechanisms underlying these findings may include, e.g., elevations in HDL cholesterol (Moore and Pearson 1986) and preservation of the brain vasculature (Sacco et al. 1999); factors that may also account for a lower risk of cardiovascular events among moderate alcohol drinkers (Rimm et al. 1996). Such results have led researchers to include moderate alcohol intake in dietary guidelines as a useful and evidence-based strategy to slow the processes of brain aging (e.g., Neafsey and Collins 2011). However, there is also evidence to the contrary, varying from no effects up to negative health effects of moderate alcohol intake on brain health (Dent et al. 1997; Cervilla et al. 2000; de Bruin et al. 2005). For instance, in a recent brain magnetic resonance imaging study, habitual intake of low to moderate amounts of alcohol in middle-aged men has been linked to lower gray matter volume in regions typically affected during the early stages of dementia, such as the frontal lobe (de Bruin et al. 2005).
Based on these controversial findings, it is obvious that more evidence from population-based studies is required before health professionals can recommend moderate alcohol intake as a strategy to slow cognitive aging to the public. Against this background, in the present study, both cross-sectional and prospective associations between alcohol consumption and cognitive function (using trail making tests, which are measures of age-related cognitive decline (Giovagnoli et al. 1996)) were examined in a cohort of cognitively healthy elderly Swedish men.
Methods
Sample
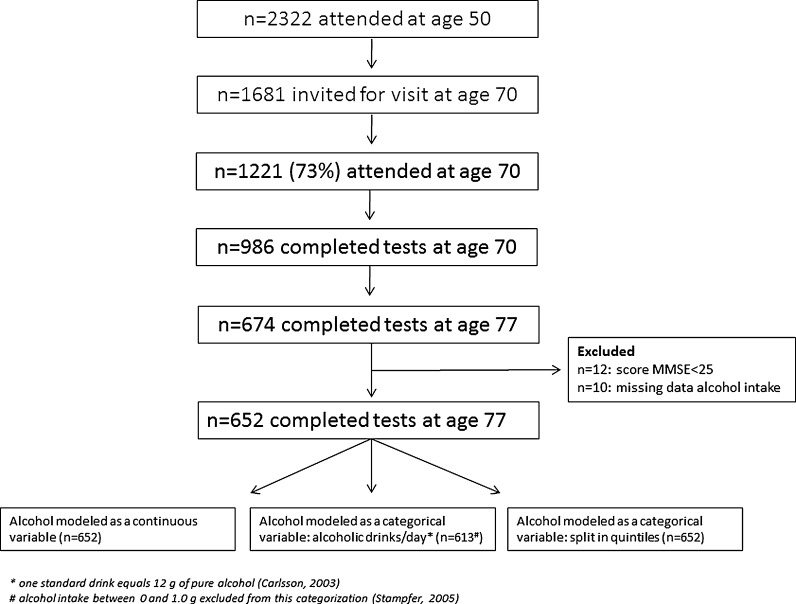
The study is based on the cohort of the Uppsala Longitudinal Study of Adult Men (http://www.pubcare.uu.se/ULSAM/), an epidemiological study that aims to identify metabolic risk factors for CVD, to which all 50-year-old men living in Uppsala County were invited between 1970 and 1974. From this initial cohort of 50-year-old men (n = 2,322), 1,221 men were restudied 20 years later, which is the baseline of the current study. Importantly, at age 50, neither cognitive functions nor dietary intake was measured. Of the 70-year-old men that completed the cognitive function tests (n = 986), 674 completed the cognitive tests at the age of 77 (Fig. 1). The 235 men who did not complete the cognitive tests reported lower alcohol intake (4.7 ± 5.6 g/day) as compared to the 986 who did complete the cognitive tests (6.8 ± 7.8 g/day); self-reported alcohol intake of those lost to follow-up between ages 70 and 77 did not differ from those included. We excluded individuals of whom data on self-reported alcohol intake were missing (n = 10) and of whom scores on the mini-mental state examination (MMSE) were below 25 points (n = 12) to study only those with normal cognition (n = 652) (Mungas 1991). All subjects gave written consent, and the regional ethics committee of Uppsala (EPN) approved the study.
Fig. 1.
Flow chart of subject recruitment and selection
Assessments
The baseline investigation (Table 1) included questionnaires (to assess education, current smoking status, physical activity, food record) and physical examinations including anthropometric measures to assess body mass index (BMI); diabetes prevalence, defined according to the American Diabetes Association criteria (American Diabetes Association 1997); cholesterol concentrations, assessed using IL Test Cholesterol Trinder’s Method; hypertension prevalence, defined as a supine blood pressure of 140/90 mm Hg or greater and/or treatment with antihypertensive drugs; and apolipoprotein (APO) E genotype—a predictor of Alzheimer’s disease (Stampfer et al. 2005), genotyped by mini-sequencing.
Table 1.
Subject characteristics (mean ± SD) at age 70, self-reported alcohol intake, and performance on the TMT-A and TMT-B and associations with alcohol intake
| Self-reported alcohol intake | |||||
|---|---|---|---|---|---|
| g/day | drinks/daya | ||||
| 0 | 1 | 2 | >3 | ||
| N | 652 | 88 | 399 | 99 | 27 |
| MMSE score | 28.6 ± 1.2 | 27.0 ± 2.1 | 27.7 ± 2.3 | 28.1 ± 2.2 | 29.2 ± 0.8 |
| Alcohol (g/day) | 6.9 ± 7.1 | 0 | 5.4 ± 3.1 | 16.7 ± 3.2 | 28.9 ± 5.3 |
| Total dietary energy intake (kcal/day) | 1,786 ± 474 | 1,729 ± 468 | 1,795 ± 472 | 1,769 ± 456 | 1,749 ± 412 |
| BMI (kg/m2) | 26.2 ± 3.1 | 26.9 ± 3.7 | 26.1 ± 2.9 | 26.4 ± 3.2 | 26.4 ± 3.4 |
| Education (primary/secondary/university; %) | 50/32/18 | 63/27/10 | 52/33/15 | 34/39/26 | 9/35/57 |
| Smoking (yes; n) | 105 (16 %) | 15 | 17 | 14 | 13 |
| Physical activity level (1/2/3/4: 1 = low, 4 = high; %) | 2/31/60/7 | 1/36/51/8 | 3/39/60/6 | 0/35/55/9 | 0/26/52/17 |
| Hypertension prevalence (yes; %) | 30 | 28 | 29 | 32 | 52 |
| Diabetes prevalence (yes; %) | 12 | 11 | 11 | 16 | 17 |
| HDL cholesterol (mmol/L) | 1.30 ± 0.35 | 1.22 ± 0.34 | 1.27 ± 0.32 | 1.43 ± 0.41 | 1.53 ± 0.43 |
| LDL cholesterol (mmol/L) | 3.88 ± 0.90 | 3.90 ± 1.07 | 3.84 ± 0.85 | 3.99 ± 0.88 | 4.11 ± 0.87 |
| APO-E allele ε4 present (yes; %) | 30.5 | 26.4 | 31.5 | 33.4 | 43.5 |
Abbreviations: MMSE mini-mental state examination, BMI body mass index, HDL high-density lipoprotein, LDL low-density lipoprotein, APO-E apolipoprotein E
aOne standard drink equals 12 g of pure alcohol (Carlsson et al. 2003), and alcohol intake between 0 and 1.0 g is excluded from this categorization (Stampfer et al. 2005)
Alcohol intake
Subjects completed five questions regarding alcohol intake on a standardized questionnaire, formulated as follows: How much light beer/cider (number of bottles) do you usually drink per week?; How much medium-alcohol beer (number of bottles) do you usually drink per week?; How much high-alcohol beer (number of bottles) do you usually drink per week?; How much wine (number of glasses) do you usually drink per week?; and How much liquor (number of milliliters) do you usually drink per week? The answers were transformed into grams of alcohol per week. These responses have been validated by a 7-day precoded dietary record that the subjects were instructed to complete (Risérus and Ingelsson 2007). For men, moderate alcohol intake is considered to be no more than two drinks per day (e.g., Carlsson et al. 2003; Panza et al. 2012).
Cognitive function tests
The MMSE (Folstein et al. 1975) and trail making tests (TMTs) A and B (Lezak 1995) were used. The MMSE is a frequently used screening test for dementia and cognitive decline. It is easy to administer and has high replicability but is insensitive to minor dysfunction. We used MMSE scores to define a cognitively healthy study population, including only those with scores ≥25 points indicating normal cognition. Lower scores indicate mild (21–24 points), moderate (10–20 points), or severe (≤9 points) cognitive impairment (Mungas 1991). In the TMT-A, the subject is asked to draw lines between a set of numbers (digits 1–25) that is distributed over a paper in the right order as fast as possible. In the TMT-B, a set of letters is added (A–L). The subject draws lines to connect the characters in an ascending pattern but with the added task of alternating between the numbers and letters (i.e., 1–A–2–B–3–C–… etc.). Scores of the TMTs are equal to the time of completion of the test. One of the authors (L.K.) and two specially trained occupational therapists administered all the cognitive tests.
Data analyses
Data are presented as means ± SD and frequencies. Linear mixed models were used to assess whether self-reported daily alcohol consumption (assessed at the age of 70) was linked to the performance on the TMT-A and TMT-B at the ages of 70 and 77 as well as to their 7-year difference. Alcohol intake was modeled as a continuous variable (g/day; n = 652) and as two categorical variables: (1) drinks per day—with one standard drink equaling 12 g of pure alcohol (Carlsson et al. 2003) and excluding n = 39 with intake between 0 and 1.0 g (Stampfer et al. 2005) (leaving n = 613), and (2) quintiles—to be able to compare equally sized groups (n = 652) (Fig. 1). Alcohol intake per quintile was as follows: 0–0.96, 0.97–3.29, 3.30–6.42, 6.43–11.63, and >11.64 g/day. The higher education level as observed in individuals reporting three or more alcoholic drinks per day (Table 1) may suggest residual confounding by education. We therefore repeated the analyses modeling alcohol as a categorical variable excluding the individuals reporting three or more drinks per day. All analyses were adjusted for potential confounding variables, i.e., those possibly related to both cognitive function and alcohol intake: highest educational degree, level of physical activity (four levels), total dietary energy intake (kcal/day), smoking (yes/no), BMI (kg/m2), a history of hypertension (yes/no), history of diabetes (yes/no), HDL and LDL cholesterol levels (mmol/L), and APO-E ε4 status. Data were analyzed using SPSS software (SPSS Inc, Chicago, IL, USA). Results at a p value of <0.05 were considered significantly different.
Results
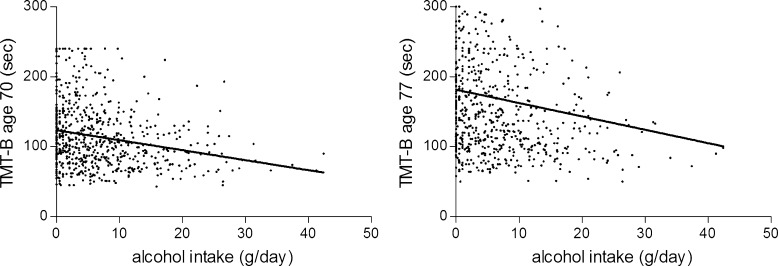
Table 2 shows the results of the TMT-A and TMT-B at the ages of 70 and 77 and associations with self-reported alcohol intake. Alcohol intake at the age of 70 was associated with better cognitive performance on the TMT-B on ages 70 and 77. Alcohol intake did, however, not modulate the difference in performance on the cognitive tests at ages 70 and 77 (Table 2). Association patterns did not change when excluding individuals who reported three or more alcoholic drinks per day (data not shown) or when analyzing alcohol intake as a continuous variable (Fig. 2) or in quintiles (Table 2). Also, stratification by APO-E ε4 presence did not reveal significant effects of alcohol intake on cognitive function in non-carriers vs. carriers (data not shown).
Table 2.
Performance on the TMT-A and TMT-B (mean ± SD) on both ages 70 and 77 and associations with self-reported alcohol intake at age 70 modeled as a categorical variable (drinks/day, n = 613) and as a continuous variable (g/day, n = 652)
| Self-reported alcohol intake (drinks/day)a | Test for linear trend (drinks/day) | Self-reported alcohol intake (g/day) | Test for linear trend (quintiles) | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1b | 2 | >3 | β (p value) | β (p value) | β (p value) | |
| n | 88 | 399 | 99 | 27 | 652 | 652 | |
| TMT-A (age 70; s) | 48 ± 18 | 46 ± 18 | 41 ± 16 | 38 ± 15 | |||
| TMT-A (age 77; s) | 58 ± 22 | 57 ± 27 | 51 ± 23 | 47 ± 25 | |||
| ΔTMT-A (s) | 10 ± 19 | 11 ± 23 | 10 ± 20 | 8 ± 15 | |||
| Cross-sectionalb | −1.785 (0.103) | −0.149 (0.147) | −0.575 (0.248) | ||||
| Longitudinalc | 0.140 (0.921) | −0.020 (0.878) | −0.119 (0.852) | ||||
| TMT-B (age 70; s) | 125 ± 47 | 117 ± 46 | 97 ± 33 | 89 ± 34 | |||
| TMT-B (age 77; s) | 184 ± 90 | 173 ± 94 | 147 ± 84 | 132 ± 81 | |||
| ΔTMT-B (s) | 60 ± 73 | 58 ± 78 | 51 ± 71 | 42 ± 60 | |||
| Cross-sectionalb | −7.964 (0.002) | −0.869 (<0.001) | −4.087 (0.001) | ||||
| Longitudinalc | −3.642 (0.454) | −0.325 (0.471) | −0.743 (0.736) | ||||
Adjusted for the highest educational degree, current smoking, physical activity, total energy intake, BMI, hypertension prevalence, diabetes prevalence, HDL and LDL cholesterol, and APO-E genotype
aOne standard drink equals 12 g of pure alcohol (Carlsson et al. 2003), and alcohol intake between 0 and 1.0 g is excluded from this categorization (Stampfer et al. 2005)
bLinear mixed model regression was used to assess associations between alcohol intake and performance on the TMTs
cLongitudinal associations refer to ΔTMT performance, i.e., difference in trail making tests between age 70 and age 77
Fig. 2.
Associations (unadjusted data) between self-reported alcohol intake (g/day, n = 652) and performance on the TMT-B at age 70 (left panel: β = −1.43, p < 0.001) and age 77 (right panel: β = −1.91, p < 0.001)
Discussion
Moderate alcohol consumption is a widely accepted behavior. For instance, at scientific conferences, social evenings are typically announced as an important activity during which one to two glasses of wine will not only help build scientific collaborations, but also benefit participants’ hearts and minds, as suggested by various studies (e.g., Rimm et al. 1996; Neafsey and Collins 2011). However, in terms of brain health, it is also important to note that there is also evidence to the contrary. For instance, when consuming low to moderate amounts of alcohol, middle-aged men show signs of accelerated brain aging as compared to non-drinkers (de Bruin et al. 2005). Further, alcohol has neurotoxic properties and induces neuroinflammatory processes (Alfonso-Loeches and Guerri 2011) and increases the oxidative burden (Collins and Neafsey 2012) in the central nervous system. This, in conjunction with alcohol’s strong addictive potential, makes it necessary to demonstrate in additional elderly populations that moderate alcohol consumption is a valid lifestyle factor to slow the process of brain aging. In the present population-based study involving 652 elderly men, moderate intake of alcohol showed cross-sectional associations with enhanced executive function at ages 70 and 77, suggesting at first glance that alcohol when consumed in moderate amounts may benefit brain functions in the elderly. However, in contrast to these findings, moderate intake of alcohol was not linked to age-related decline in cognitive functioning, i.e., it was not linked to differences in performance on the TMT tests between ages 70 and 77. With this result in mind, our findings do not confirm the view that daily moderate alcohol consumption is a recommendable strategy to slow the loss of cognitive functioning in elderly populations.
The current results are in line with findings of previous similar-sized population-based cross-sectional studies (e.g., Carmelli et al. 1999; Lindeman et al. 2005; Reid et al. 2006). These associations were observed in study populations of, e.g., US veterans (Reid et al. 2006), Mexican elderly (Lindeman et al. 2005), or elderly people (showing recent instability in cognitive functioning) from the south of France (Leibovici et al. 1999). We add to these findings showing the same associations in another relatively small cohort—from Northern Europe. However, in the current study, self-reported alcohol intake was not predictive of the difference in performance on these tests between ages 70 and 77. While this finding is in line with some previous reports in which alcohol consumption was not related to cognitive decline (Launer et al. 1996; Bond et al. 2005), there are other studies suggesting the contrary, i.e., that moderate alcohol intake may help deter cognitive aging in the elderly (Elias et al. 1999; Ngandu et al. 2007). Possible reasons that may account for this discrepancy between study results include differences in cognitive tests administered to the study participants, lifestyle (e.g., combination of lifestyle factors that may result in a “survivor bias”), follow-up period, genetic polymorphisms (e.g., alcohol dehydrogenase, APO-E ε4), or drinking practices (e.g., daily drink for lunch vs. intoxication at weekends). Further, some studies included women only (Stampfer et al. 2005) or observed that the association between moderate alcohol intake and cognitive function was significant in females but not in males (e.g., McGuire et al. 2007). Finally, in the current study, moderate alcohol intake was linked to the performance on the TMT-B at age 70 but not to its change between ages 70 and 77. One possible explanation for this discrepancy is residual confounding, as moderate drinkers may have more moderate, and perhaps healthier, lifestyles than abstainers or heavy drinkers. As our study findings are based on correlational analyses, we cannot examine whether the effect of moderate alcohol consumption on cognitive functioning is of causal nature.
Strengths and limitations
Results from self-reported alcohol intake have been shown to be consistent with independent 7-day dietary records (Risérus and Ingelsson 2007). In addition, modeling alcohol intake as a continuous or as a categorical variable did not change the results. Finally, the trail making tests assessing cognitive processing speed are considered sensitive measures of age-related cognitive decline (Giovagnoli et al. 1996). In this context, it is important to note that this cognitive test does not encompass all aspects of cognitive function. Further, we previously showed that poor performance on the TMT-B was an independent predictor of brain infarcts in ULSAM (Wiberg et al. 2010). However, there are also several limitations that may apply to our study findings. The individuals that did not complete the cognitive tests at age 70 reported lower alcohol intake than those who completed the tests, and no information was available on drinking patterns or on alcohol consumption before the age of 70. The group that reported to drink no alcohol may, e.g., include former drinkers—and some of these people may abstain because of illness or former alcohol abuse (“sick quitter effect”). In addition, some associations between alcohol consumption and a number of disorders have been found to be J-shaped or U-shaped (Ronksley et al. 2011). However, in our population of 652 elderly men, only 4 % reported an alcohol intake greater than 24 g/day. Thus, we cannot exclude that daily alcohol intake may show a non-linear association with cognitive functions in elderly subjects. Finally, the cohort included cognitively healthy elderly men only. Thus, generalization to the effects of alcohol intake on cognitive functions and cognitive aging on females, other age groups, and cognitively impaired individual is not appropriate.
Conclusion
The current results demonstrate that self-reported alcohol intake of elderly men is positively associated with executive functioning, a cognitive modality that shows the greatest decline during late life (Giovagnoli et al. 1996). However, in contrast to these findings, moderate alcohol consumption did not affect the age-related decline in cognitive performance on the trail making tests. Although current findings do not indicate a harmful effect of alcohol on executive functioning, they do not support the view that daily moderate alcohol consumption is a recommendable strategy to slow cognitive aging in elderly Swedish populations.
Acknowledgments
This work was supported by the Swedish Research Council, Åhlens stiftelse, Swedish Brain Research Foundation, Tore Nilsons Foundation, Fredrik och Ingrid Thurings Foundation, Brain Foundation, Åke Wiberg Foundation, and Novo Nordisk Foundation.
Footnotes
Pleunie S Hogenkamp and Christian Benedict contributed equally to this work.
Contributor Information
Pleunie S. Hogenkamp, Email: pleunie.hogenkamp@neuro.uu.se
Christian Benedict, Email: christian.benedict@neuro.uu.se.
References
- Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit Rev Clin Lab Sci. 2011;48(1):19–47. doi: 10.3109/10408363.2011.580567. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Clinical practice recommendations 1997. Diabetes Care. 1997;20:S1–S70. [PubMed] [Google Scholar]
- Bond GE, Burr RL, McCurry SM, Rice MM, Borenstein AR, Larson EB. Alcohol and cognitive performance: a longitudinal study of older Japanese Americans. The Kame Project. Int Psychogeriatr. 2005;17(04):653–668. doi: 10.1017/S1041610205001651. [DOI] [PubMed] [Google Scholar]
- Carlsson S, Hammar N, Grill V, Kaprio J. Alcohol consumption and the incidence of type 2 diabetes: a 20-year follow-up of the Finnish Twin Cohort Study. Diabetes Care. 2003;26(10):2785–2790. doi: 10.2337/diacare.26.10.2785. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Reed T, Schellenberg GD, Christian JC. The effect of apolipoprotein E ε4 in the relationships of smoking and drinking to cognitive function. Neuroepidemiology. 1999;18(3):125–133. doi: 10.1159/000026204. [DOI] [PubMed] [Google Scholar]
- Cervilla JA, Prince M, Mann A. Smoking, drinking, and incident cognitive impairment: a cohort community based study included in the Gospel Oak project. J Neurol Neurosurg Psychiatry. 2000;68(5):622–626. doi: 10.1136/jnnp.68.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M, Neafsey E. Neuroinflammatory pathways in binge alcohol-induced neuronal degeneration: oxidative stress cascade involving aquaporin, brain edema, and phospholipase A2 activation. Neurotox Res. 2012;21(1):70–78. doi: 10.1007/s12640-011-9276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin EA, Hulshoff Pol HE, Schnack HG, Janssen J, Bijl S, Evans AC, Leon Kenemans J, Kahn RS, Verbaten MN. Focal brain matter differences associated with lifetime alcohol intake and visual attention in male but not in female non-alcohol-dependent drinkers. NeuroImage. 2005;26(2):536–545. doi: 10.1016/j.neuroimage.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Dent OF, Sulway MR, Broe GA, Creasey H, Kos SC, Jorm AF, Tennant C, Fairley MJ. Alcohol consumption and cognitive performance in a random sample of Australian soldiers who served in the second world war. BMJ. 1997;314(7095):1655. doi: 10.1136/bmj.314.7095.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PK, Elias MF, D'Agostino RB, Silbershatz H, Wolf PA. Alcohol consumption and cognitive performance in the Framingham Heart Study. Am J Epidemiol. 1999;150(6):580–589. doi: 10.1093/oxfordjournals.aje.a010056. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR, Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci. 1996;17(4):305–309. doi: 10.1007/BF01997792. [DOI] [PubMed] [Google Scholar]
- Huang W, Qiu C, Winblad B, Fratiflioni L. Alcohol consumption and incidence of dementia in a community sample aged 75 years and older. J Clin Epidemiol. 2002;55(10):959–964. doi: 10.1016/S0895-4356(02)00462-6. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Feskens EJM, Kalmijn S, Kromhout D. Smoking, drinking, and thinking: The Zutphen Elderly Study: the Zutphen Elderly Study. Am J Epidemiol. 1996;143(3):219–227. doi: 10.1093/oxfordjournals.aje.a008732. [DOI] [PubMed] [Google Scholar]
- Leibovici D, Ritchie K, Ledésert B, Touchon J. The effects of wine and tobacco consumption on cognitive performance in the elderly: a longitudinal study of relative risk. Int J Epidemiol. 1999;28(1):77–81. doi: 10.1093/ije/28.1.77. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Lindeman RD, Wayne SJ, Baumgartner RN, Garry PJ. Cognitive function in drinkers compared to abstainers in The New Mexico Elder Health Survey. J Gerontol Ser A Biol Sci Med Sci. 2005;60(8):1065–1070. doi: 10.1093/gerona/60.8.1065. [DOI] [PubMed] [Google Scholar]
- McGuire L, Ajani U, Ford E. Cognitive functioning in late life: the impact of moderate alcohol consumption. Ann Epidemiol. 2007;17(2):93–99. doi: 10.1016/j.annepidem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Moore RD, Pearson TA. Moderate alcohol consumption and coronary artery disease. A review. Medicine. 1986;65(4):242–267. doi: 10.1097/00005792-198607000-00004. [DOI] [PubMed] [Google Scholar]
- Mungas D. In-office mental status testing: a practical guide. Geriatrics. 1991;46(7):54–58. [PubMed] [Google Scholar]
- Neafsey E, Collins M. Moderate alcohol consumption and cognitive risk. Neuropsychiatr Dis Treat. 2011;7:465–484. doi: 10.2147/NDT.S23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngandu T, Helkala EL, Soininen H, Winblad B, Tuomilehto J, Nissinen A, Kivipelto M. Alcohol drinking and cognitive functions: findings from the Cardiovascular Risk Factors Aging and Dementia (CAIDE) Study. Dement Geriatr Cogn Disord. 2007;23(3):140–149. doi: 10.1159/000097995. [DOI] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Seripa D, Logroscino G, Santamato A, Imbimbo BP, Scafato E, Pilotto A, Solfrizzi V. Alcohol consumption in mild cognitive impairment and dementia: harmful or neuroprotective? Int J Geriatr Psychiatr. 2012;27(12):1218–1238. doi: 10.1002/gps.3772. [DOI] [PubMed] [Google Scholar]
- Reid MC, Van Ness PH, Hawkins KA, Towle V, Concato J, Guo Z. Light to moderate alcohol consumption is associated with better cognitive function among older male veterans receiving primary care. J Geriatr Psychiatr Neurol. 2006;19(2):98–105. doi: 10.1177/0891988706286513. [DOI] [PubMed] [Google Scholar]
- Rimm E, Klatsky A, Grobbee D, Stampfer M. Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine, or spirits? BMJ. 1996;312(7033):731–736. doi: 10.1136/bmj.312.7033.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risérus U, Ingelsson E. Alcohol intake, insulin resistance, and abdominal obesity in elderly men. Obesity. 2007;15(7):1766–1773. doi: 10.1038/oby.2007.210. [DOI] [PubMed] [Google Scholar]
- Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg A, Van Swieten J, Witteman J, Mehta K, van Duijn C, Hofman A, Breteler M. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281–286. doi: 10.1016/S0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Elkind M, Boden-Albala B, et al. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281(1):53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Chen X, Wen W, Anstry KJ. Light to moderate alcohol use is associated with increased cortical gray matter in middle-aged men: a voxel-based morphometric study. Psychiatry Res Neuroimaging. 2008;163(1):61–69. doi: 10.1016/j.pscychresns.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F. Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med. 2005;352(3):245–253. doi: 10.1056/NEJMoa041152. [DOI] [PubMed] [Google Scholar]
- Weyerer S, Schäufele M, Wiese B, Maier W, Tebarth F, van den Bussche H, Pentzek M, Bickel H, Luppa M, S.G. Riedel-Heller for the German AgeCoDe Study group Current alcohol consumption and its relationship to incident dementia: results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age Ageing. 2011;40(4):456–463. doi: 10.1093/ageing/afr007. [DOI] [PubMed] [Google Scholar]
- Wiberg B, Lind L, Kilander L, Zethelius B, Sundelöf JE, Sundström J. Cognitive function and risk of stroke in elderly men. Neurology. 2010;74(5):379–385. doi: 10.1212/WNL.0b013e3181ccc516. [DOI] [PubMed] [Google Scholar]